Abstract
We aimed to investigate the demographic, clinical, diagnostic, treatment and outcome features of patients with urinary tuberculosis (UTB). Patients with UTB admitted to seven separate centers across Turkey between 1995 and 2013 were retrospectively evaluated. The diagnosis of UTB was made by the presence of any clinical finding plus positivity of one of the following: (1) acid-fast bacilli (AFB) in urine, (2) isolation of Mycobacterium tuberculosis, (3) polymerase chain reaction (PCR) for M. tuberculosis, (4) histopathological evidence for TB. Seventy-nine patients (49.36% male, mean age 50.1 ± 17.4 years) were included. Mean time between onset of symptoms and clinical diagnosis was 9.7 ± 8.9 months. The most common signs and symptoms were hematuria (79.7%), sterile pyuria (67.1%), dysuria (51.9%), weakness (51.9%), fever (43%) and costovertebral tenderness (38%). Cystoscopy was performed in 59 (74.6%), bladder biopsy in 18 (22.8%), kidney biopsy in 1 (1.26%) and nephrectomy in 12 (15.2%) patients. Histopathological verification of UTB was achieved in 12 (63.1%) patients who undergone biopsy and in 100% of those undergone nephrectomy. Mycobacterium tuberculosis was isolated in the urine of 50 (63.3%) cases. Four-drug standard anti-TB treatment was the preferred regimen for 87.3% of the patients. Mean treatment duration was 10.5 ± 2.7 months. Deterioration of renal function occurred in 15 (18.9%) patients two of whom progressed to end-stage renal disease and received hemodialysis. Only one patient died after 74-day medical treatment period. Cases with UTB may present with non-specific clinical features. All diagnostic studies including radiology, cyctoscopy and histopathology are of great importance to exclude UTB and prevent renal failure.
Introduction
Tuberculosis (TB) remains a major global health problem. One-third of the world's population is estimated to have latent TB infection and ∼10% of these individuals will develop active TB in their lifetime.Citation1 In 2012, an estimated 8.6 million people developed TB and 1.3 million died from the disease.Citation1 It is also an intermediate endemic disease in our country (in 2012, n = 14 691, 22/100 000).Citation1 Extra-pulmonary TB accounts for ∼20 % of the reported cases.Citation1–3 Among patients with pulmonary TB, genitourinary involvement occurs in 2–20% of patients.Citation2,Citation4 Hematogenous seeding of the kidney at the time of primary TB infection leads to granuloma formation in proximity to glomeruli, which can caseate and rupture into the tubular lumen. Subsequently, tuberculous bacilli can enter the medullary interstitium, leading to progressive medullary injury. Destruction of renal papilla can lead to calyceal ulceration or abscess formation. Involvement of the collecting system may result in fibrotic scarring and stenosis. The onset of urinary TB (UTB) is usually insidious, presenting with malaise and lower urinary tract symptoms, including dysuria and gross hematuria. Systemic symptoms (fever, weight loss) are relatively rare, since rupture of the glomerular granulomas occurs independently of disease activity at other sites. Pyuria and/or microscopic hematuria are present in more than 90% of cases. Heavy proteinuria and cellular casts are not generally seen, and the plasma creatinine concentration is usually normal. Ureteral stricture can occur and may cause obstructive uropathy. The diagnosis of UTB is established by the demonstration of Mycobacterium tuberculosis in the urine; the constellation of dysuria, sterile pyuria, hematuria and characteristic radiographic findings is highly suggestive of the diagnosis. Three to six first morning midstream specimens should be obtained for acid-fast bacilli (AFB) culture to maximize the likelihood of a positive result. Among patients with active renal TB, 30–40% of single urine specimens will be positive by AFB culture. Polymerase chain reaction (PCR) is also a useful diagnostic tool with 87–100% sensitivity and 93–98% specificity.Citation2
Although uncommon, UTB is an important treatable cause of progressive renal failure. In this study, we aimed to investigate the demographic characteristics, clinical manifestations, laboratory findings, diagnostic features, treatment modalities and outcomes of patients with UTB.
Patients and methods
Patients diagnosed with UTB at 12 referral centers between January 1995 and December 2013 were included in this retrospective observational study. All clinical, microbiological and histopathological data were retrieved from medical records. Patients over 18 years of age were enrolled.
Demographic features and clinical findings
Demographic characteristics of the patients, personal and family history for TB, comorbidities, use of immunosuppressive drugs, data regarding clinical symptoms and signs, time elapsed between the onset of symptoms and the diagnosis of UTB, weight, height were obtained from medical records. Fever was defined as axillary temperature over 37.2 °C or equivalents. Weight loss was defined as a loss of 10% or more of actual body weight within 3 months. Body mass index (BMI) and mean arterial pressure (MAP) were calculated using the following equations:
Laboratory and imaging findings
Laboratory findings including blood count, biochemical tests, acute phase reactants, results of urinalyses with dipstick method and microscopic findings of urine sediment (pH, glycosuria, hematuria and pyuria) and levels of 24-h urinary protein excretion were recorded. Microscopic hematuria was accepted as the excretion of more than two red blood cells per high-power field in a centrifuged urine specimen and pyuria as the excretion of more than five white blood cells. Acidic urine was accepted as the pH of urine below 6.0.Citation5 Renal function was measured by glomerular filtration rate (GFR). GFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula for all patients.Citation6
The diagnosis of hepatotoxicity from anti-TB drugs was established by the presence of at least one of the followings:Citation7,Citation8 (1) a ≥ 5-fold increase in ALT and/or AST levels (upper limit of normal = 40 IU/L), (2) an increase of total bilirubin level >1.5 mg/dL and (3) presence of clinical signs and symptoms suggesting acute hepatitis, such as anorexia, nausea, vomiting, and jaundice, associated with a 3-fold increase in ALT and/or AST levels.
Abnormal imaging findings of urinary system on direct urinary tract radiography (extensive dystrophic calcification in kidney), intravenous pyelography (erosion of the tip of the calyx, incomplete filling, distortion, etc.), ultrasonography (USG) (mucosal thickening of calyces and pelvis, calyceal irregularity, granulomas, etc.), computerized tomography (CT), magnetic resonance, static renal scintigraphy and chest radiography were recorded. Abnormal cystoscopic findings detected in the patients who underwent cystoscopy were recorded.
Skin test for TB (purified protein derivative, PPD) results was recorded. An induration of 5 or more millimeters was evaluated as positive in patients who were immunosuppressed for any reason and ≥10 mL in all other patients.
Histopathological findings
Histopathological findings were recorded. Histological diagnosis of UTB was made by identifying the pathologic triad of caseating necrosis, loose aggregates of epithelioid histiocytes and Langhans giant cells in kidney and bladder tissue samples.Citation9
Diagnosis
The UTB diagnosis was carried out in the presence of clinical findings plus one of the following four criteria: (1) Positivity of AFB in any of the three consecutive early-morning mid-stream samples of urine by Ehrlich–Ziehl–Neelsen (EZN) method, (2) positive urine culture for M. tuberculosis complex in Löwenstein Jensen medium or Mycobacteria Growth Indicator Tube (MGIT) detection system, (3) positivity of PCR for M. tuberculosis complex, (4) histopathological evidence of TB in any relevant tissue specimen. All data were obtained from patients' files and laboratory database. Concurrent involvement of any body site other than urinary tract by TB was recorded.
Treatment and outcome
Data regarding treatment modalities, total treatment durations, surgical interventions, duration of hospitalization, drug side effects, clinical outcomes (death or survival, causes of mortality) and renal outcomes (recovery, stability or deterioration of renal function) were retrieved from patients' files and pharmacy databases. Estimated GFR (eGFR) values at the onset and end of the treatment were recorded. “Recovery” of renal function was defined as an increase of ≥10%, “stability” was defined as a decrease or an increase of 0–9% and “deterioration” was defined as a decrease of ≥10% at the end of treatment when compared to the initial eGFR values.Citation10
Statistical analyses and ethics
SPSS 17.0 Software (Chicago, IL) programme was used for descriptive statistical analyses. All relevant data were expressed as mean + standard deviation (SD). This study was approved by the Ethics Committee of Istanbul University Cerrahpasa Medical School (Approval no: A – 24/05 June 2012).
Results
Demographic features and clinical findings
During the 18 years period of the study, a total of 96 cases were recorded as UTB. After exclusion of those not meeting the diagnostic criteria (n = 7), repeating records (n = 7) and patients below 18 years (n = 3), 79 separate patients with UTB were included.
Demographic characteristics, history for TB and co-morbid conditions of the patients are shown in . Mean duration between the onset of symptoms and the diagnosis of UTB was 9.7 ± 8.9 months (range 1–47 months). The most common symptoms during the course of disease were dysuria, weakness, fever and macroscopic hematuria. Initial symptoms, physical examination (PE) and laboratory findings of the patients are shown in .
Table 1. Demographic characteristics, history for tuberculosis, comorbid diseases and usage of immunosuppressive drugs in patients with UTB.
Table 2. Symptoms and findings in the patients with UTB.
Frequencies of abnormal imaging findings are shown in .
Table 3. Rates of abnormal imaging findings in the patients with UTB.
Histopathological findings
Cystoscopy was performed in 59 patients of whom 18 had abnormal cystoscopic findings and underwent bladder biopsy. The histopathology revealed pathological findings suggestive of TB in 11 patients (61.1%). Kidney biopsy was performed in only one patient who was confirmed to have renal TB with the histopathology. Nephrectomy was performed in 12 patients all of whom were confirmed as renal TB with histopathology.
Diagnostic modalities
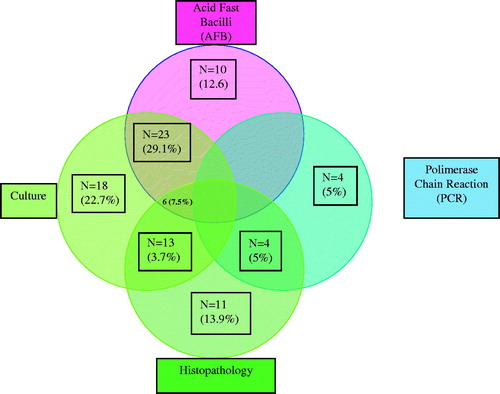
The distribution of cases according to diagnostic methods used to define UTB is shown in . Mycobacterium tuberculosis was isolated in 50 patients, AFB was positive in 39, PCR was positive in 8 and histopathology was positive in 24 cases.
Extra-urinary involvements
Other systems and organs affected by TB, concurrently with urinary tract, were as follows: lung in eight patients, testis in five patients, tuba-ovarian in one patient, prostate in one patient, vertebra in one patient, adrenal gland in one patient and lymph node in one patient.
Treatment and outcome
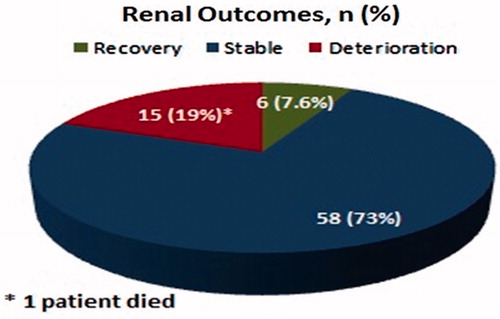
For 87.3% of the patients, the preferred treatment regimen consisted of an initial 2-month phase of four drugs, followed by a 7-month maintenance phase of isoniazid (INH) and rifampicin (RIF). Treatment characteristics of the patients are shown in . Four patients developed hepatotoxicity due to anti-TB drugs between the 3rd and 10th days of treatment and improved within 1–2 weeks of discontinuation. Liver enzymes did not increase after re-initiation. In this study, deterioration of renal function occurred in 15 patients, two of whom progressed to end-stage renal disease and hemodialysis was started. Only one patient died on the 74th day of anti-TB treatment. The cause of death was respiratory failure due to pulmonary TB. Renal outcomes of the patients with UTB are shown in .
Table 4. Treatment characteristics of the patients with UTB.
Discussion
UTB can cause end-stage renal failure in 5.7% of the affected patients.Citation4 According to the data obtained from the European Dialysis and Transplant Association registry, 0.65% of new patients had renal failure caused by renal TB.Citation11 Because it is potentially preventable and easily treatable, early diagnosis of UTB is important to prevent complications including renal failure.Citation11
The kidney is usually infected by a hematogenous spread of bacilli from a focus of primary infection in the lung, with subsequent involvement of the renal pelvis, ureters and bladder through descending infection of the collecting system.Citation11,Citation12 Despite hematogenous seeding of both kidneys, clinically significant disease is usually limited to one side.Citation13 Multiple bilateral cortical granulomas can remain asymptomatic and dormant for decades.Citation14 It is estimated that it takes 8–22 years to produce a symptomatic renal lesion.Citation15 The clinical manifestations are variable. The onset of clinically evident UTB is insidious with dysuria and hematuria. Constitutional symptoms, such as weight loss, fever, anorexia and fatigue, are less common.Citation13,Citation16 UTB affects people of all ages, but predominates in men in their fourth or fifth decades.Citation17,Citation18 Similarly, our cohort was consisted of a wide range of ages with a mean age of 50.1 ± 17.4 (range 18–87 years). However, there was no male predominance, probably due to exclusion of primary urogenital TB cases in our cohort, majority of whom are males.
Radiographic evidence of pulmonary TB is shown in less than 50% of patients with UTB; 5% of whom are defined to have active pulmonary disease.Citation19 About 25% of patients who present with urogenital TB have a known history of prior pulmonary TB; an additional 25–50% of patients will have radiographic evidence of prior subclinical pulmonary infection.Citation14 Previous diagnosis of pulmonary TB or evidence of imaging was reported as 36.5% in the review of Figueiredo et al.Citation4 In our cohort, history of any clinical form of TB was present in 17.7% and radiologic evidence of previous TB was detected in only 11.3% of the patients. These lower rates were probably due to the retrospective design of the study. However, the proportion of cases with concurrent active pulmonary TB (10%) was consistent with the literature.
In most active cases of extrapulmonary TB, latent foci were reactivated following a decrease in immunity brought about by older age, malnutrition, diabetes mellitus, use of steroids or other immunosuppressants and immunodeficiency most particularly due to HIV infection.Citation4,Citation20 In our cohort, the total proportion of immunocompromising conditions, such as malignancy, diabetes mellitus, chronic renal failure and immunosuppressive drug use, were found as 46.7%. No single case of UTB had HIV coinfection, not surprisingly given the very low prevalence (9.8/100 000) of HIV infection in our country.Citation21
The proportion of HT in our cohort was relatively higher (39.2%) when compared to the prevalence of HT in the general population (32.7%), probably due to a unilateral or bilateral non-functioning kidney destructed by UTB.Citation22 Renal and ureteric calculi have been reported in up to 19% of the cases with UTB.Citation23
In our study, the renal and urinary tract calculi were detected in 12.6% of the cases. The association of calculi with UTB may depend on the obstructive uropathy due to TB scars in the urinary tract which may precipitate renal calculi.
Patients with UTB typically have local insidious symptoms including frequent voiding, dysuria, hematuria (microscopic or macroscopic), flank or abdominal pain. Constitutional symptoms, such as fever, weight loss, fatigue and anorexia, are less common.Citation13,Citation16 However, fever and weakness were relatively more common in our cohort. Other constitutional symptoms, such as anorexia, weight loss and sweating, were recorded less frequently.
In a series of 105 cases with urogenital TB, the most common symptoms were reported to be flank pain, nocturia, frequent voiding and dysuria.Citation15 Figueiredo et al. reported that storage symptoms (urinary frequency, urgency, urgency incontinence, nocturia) dysuria and hematuria were the most common symptoms on admission, affecting 50.5%, 37.9% and 35.6% of cases, respectively.Citation4 Loin pain and fever were reported less frequently (34.4% and 21.9%, respectively). However, the most common symptoms recorded in our study were dysuria, weakness, fever and macroscopic hematuria. Storage symptoms (pollakiuria and nocturia) and loin pain were less common.
In our study, costovertebral angle tenderness, elevated initial arterial blood pressure and mild fever were the most prominent findings of PE. In the presence of these three findings, UTB should be taken under consideration. The PPD skin test may contribute to the diagnosis of UTB with a positivity of 85–95%.Citation4,Citation13,Citation24 In our cohort, PPD skin test was positive in 76% of the patients. Some urinalysis findings, such as hematuria, sterile pyuria and acidic urine, suggest UTB in clinical practice.Citation4,Citation12,Citation24 In our study, hematuria and pyuria were detected in 79.7% and 67.1% of patients, respectively. Hematuria was the most common laboratory finding suggesting UTB. Also, the percentage of acidic urine (75.9%) and positive urine culture for usual pathogens (24%) was found similar to the literature in our study.Citation4 Imaging findings are crucial for the timely diagnosis of UTB. The most valuable radiological findings of urogenital TB comprise a combination of multiple abnormal images.Citation13,Citation14,Citation25 The sensitivity of imaging techniques for diagnosis of UTB is up to 91.4%.Citation4,Citation26 In our cohort, the most commonly used imaging techniques were direct urinary tract radiography (100%), abdominal USG (93.6%) and CT (35.4%). Pathological findings suggesting UTB were found in 15.1% of direct urinary tract radiographies, in 58.1% of USGs and 92.8% of CT scans.
Cystoscopy with bladder biopsy may be performed in cases with clinical suspicion of UTB and presence of bacillus-negative urine. Sensitivity of bladder biopsy is between 18.5% and 52%.Citation4,Citation26 In our study, cystoscopy was performed in 59 (75%) of the patients and bladder biopsy was performed in 18 (23%) of patients who had abnormal cystoscopic findings. In the histopathological examination of these 18 patients, pathological findings suggestive of UTB were found in 11 (61.1%).
The mean duration between initial symptoms and the definite diagnosis (mean 9.7 months, range 1–47 months) was longer in our cohort when compared to some other reports in the literature, primarily due to the insidious onset with few and non-specific symptoms, technical difficulties to isolate TB bacilli, the long time required to confirm the diagnosis by classical conventional methods of cultivation, lack of awareness of physicians and poor care-seeking behavior.Citation4,Citation12,Citation26–29 Advances in diagnostic techniques (molecular methods, etc.) and improvements in general health infrastructure hopefully will decrease this latency period. As a rapid and sensitive diagnostic method, PCR for M. tuberculosis identification in the urine has become the ideal diagnostic tool in recent years.Citation6 It allows for diagnosis to make even when there are few bacilli and AFB detection is not available. The sensitivity of acid-fast staining with EZN technique is between 42.1% and 52.1%, and the sensitivities of culture methods vary from 10% to 90% while it is 98.1% for PCR.Citation4,Citation12,Citation18,Citation26,Citation30,Citation31
Treatment guidelines for tuberculosis recommend 6 months therapy without meningitis.Citation3,Citation32,Citation33 Pulmonary and extra-pulmonary disease should be treated with the same regimens. In our cohort, 4-drug regimen was used in 87.3% of patients and 3-drug regimen (without EMB) in 12.7% of patients. Mean treatment duration was 10.5 months (range 6–24 months). The preference of 4-drug regimen is due to the standard recommendations based on the relatively high prevalence of TB in our country. The treatment duration was longer than 6 months in most patients may be due to the concern of preventing recurrence, lack of early treatment response and lack of microbiological evidence for drug susceptibility in some centers. A study comprising 174 cases in Turkey demonstrated a relapse rate of 19% even after 12 months of therapy.Citation34
Ureteral and bladder TB are secondary to descending infection through the urinary collecting system. Multiple stenoses of the collecting system from the infundibulum to the ureterovesical junction are the most suggestive findings for UTB, occurring in 60–84% of cases.Citation4,Citation35 For ureteral strictures, timely introduction of stents across the narrow segment may prevent the need for major surgical procedures.Citation9 In this study, double j stent placement was performed in 5% of patients due to ureteral strictures. The issue of removal of a unilateral nonfunctioning kidney for the management of UTB is controversial. Several authors propose that prolonged anti-TB treatment for 18–24 months effectively sterilizes caseous and calcified masses of the involved kidney, whereas others believe that the sequestered, caseous material should be removed to shorten the duration of medical therapy and to prevent late TB reactivation.Citation13,Citation35 Nephrectomy is recommended only in cases of secondary sepsis, bleeding, pain, uncontrollable hypertension and continued positive urinary cultures for TB.Citation12 In our cohort, nephrectomy was performed in 12 (15.1%) of the patients because of a unilateral nonfunctioning kidney. Renal TB was verified in the histopathology of these patients.
As a consequence of the delay in the diagnosis and treatment, UTB is associated with significant morbidity (end-stage renal failure, ureteral strictures and shrunken bladder).Citation12,Citation36,Citation37 Renal parenchymal destruction may occur due to progression of a focal lesion with caseous granuloma formation, fibrosis and renal cavitations, or more frequently obstruction of the urinary collecting system.Citation4,Citation23 In this study, deterioration of renal function occurred in 19% of patients and 13.3% of them progressed to end-stage renal disease. Hemodialysis was started in these patients. Due to the low sensitivities of diagnostic methods, UTB cannot definitely be demonstrated in some instances. Empirical anti-TB treatment should promptly be initiated when UTB is not absolutely ruled out, particularly in cases admitted with episodes of cystitis–pyelonephritis concomitant with sterile pyuria and progressive parenchymal damage not related to other clinical diseases (vesicoureteral reflux, urolithiasis, etc.).
There are some limitations of the study, retrospective design being the major. All patient data are limited to those recorded by the relevant primary physician. Although, presence of at least one clinical finding was established in the inclusion criteria, false positive results of urinary TB PCR due to latent infection could not be excluded.
In conclusion, UTB remains to be an important cause of progressive renal failure, despite being potentially preventable and easily treatable unlike many other renal conditions. Insidious and non-specific clinical manifestations of the disease may lead to a delay in diagnosis. UTB should be considered in cases with sterile pyuria and hematuria with storage symptoms, dysuria, weakness, fever and loin pain, particularly when there is no response to the usual antibiotic treatment. Systematic evaluation is crucial to detect initial cases of UTB, regardless of the symptoms. Furthermore, a better definition of patient population at high risk for UTB and rapid diagnostic tools including molecular tests should be ensured.
Declaration of interest
The authors report no conflicts of interest.
References
- World Health Organization (WHO). Global Tuberculosis Report 2013. Geneva: WHO; 2013
- Pais VM, Dionne-Odom J, von Reyn CF, Curhan G, Baron EL, Sheridan AM. Renal disease in tuberculosis. UpToDate. Available at: http://www.uptodate.com/contents/search?search=Renal+disease+in+tuberculosis.&x=15&y=8. Accessed April 2, 2015
- World Health Organization (WHO). Treatment of Tuberculosis: Guidelines. 4th ed. Geneva, Switzerland: WHO
- Figueiredo AA, Lucon AM. Urogenital tuberculosis: Update and review of 8961 cases from the world literature. Rev Urol. 2008;10(3):207–217
- Slack RC. Definition of urinary tract infection and assessment of efficacy in drug trials – A laboratory perspective. Infection. 1992;20(Suppl 3):S155–S156
- Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612
- Tahaoğlu K, Ataç G, Sevim T, et al. The management of anti-tuberculosis drug-induced hepatotoxicity. Int J Tuberc Lung Dis. 2001;5(1):65–69
- Patterson PE, Kimerling ME, Bailey WC, Dunlap NE. Chemotherapy of tuberculosis. In: Schlossberg D, ed. Tuberculosis and Nontuberculous Mycobacterial Infections. 4th ed. Philadelphia: WB Saunders Company; 1999:71–82
- Visweswaran RK, Bhat S. Tuberculosis of the urinary tract. In: Floege J, Johnson RJ, Feehally J, eds. Comprehensive Clinical Nephrology. 4th ed. St. Louis, MO: Elsevier Saunders; 2010:641–648
- Lambers Heerspink HJ, Tighiouart H, Sang Y, et al. GFR decline and subsequent risk of established kidney outcomes: A meta-analysis of 37 randomized controlled trials. Am J Kidney Dis. 2014;64(6):860–866
- Gupta S, R S, Meitei KS, Singh SR. Primary genito-urinary tuberculosis with bilateral urolithiasis and renal failure – An unusual case. J Clin Diagn Res. 2013;7(5):927–929
- Ghaleb K, Afifi M, El-Gohary M. Assessment of diagnostic techniques of urinary tuberculosis. Mediterr J Hematol Infect Dis. 2013;5(1): e2013034. doi: 10.4084/MJHID.2013.034
- Gibson MS, Puckett ML, Shelly ME. Renal tuberculosis. Radiographics. 2004;24(1):251–256
- Pasternak MS, Rubin RH. Urinary tract tuberculosis. In: Schrier RW, ed. Diseases of The Kidney and Urinary Tract. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2001:1017–1037
- Fanning A. Tuberculosis: 6. Extrapulmonary disease. CMAJ. 1999;160(11):1597–1603
- Narayana A. Overview of renal tuberculosis. Urology. 1982;19(3):231–237
- Schubert GE, Haltaufderheide T, Golz R. Frequency of urogenital tuberculosis in an unselected autopsy series from 1928 to 1949 and 1976 to 1989. Eur Urol. 1992;21(3):216–223
- Ferrie BG, Rundle JS. Genito-urinary tuberculosis in Glasgow 1970 to 1979: A review of 230 patients. Scott Med J. 1985;30(1):30–34
- Burrill J, Williams CJ, Bain G, Conder G, Hine AL, Misra RR. Tuberculosis: A radiologic review. Radiographics. 2007;27(5):1255–1273
- Elliott AM, Luo N, Tembo G, et al. Impact of HIV on tuberculosis in Zambia: A cross sectional study. BMJ. 1990;301(6749):412–415
- Turkish Public Health Institute, HIV Report, Ankara; 2013. Available at: http://thsk.saglik.gov.tr/Dosya/bulasici-hastaliklar-db/Dnya_AIDS_Gn_02122013.pdf
- Süleymanlar G, Utaş C, Arinsoy T, et al. A population-based survey of chronic renal disease in Turkey – The CREDIT study. Nephrol Dial Transplant. 2011;26(6):1862–1871
- Eastwood JB, Corbishley CM, Grange JM. Tuberculosis and the kidney. J Am Soc Nephrol. 2001;12(6):1307–1314
- Psihramis KE, Donahoe PK. Primary genitourinary tuberculosis: Rapid progression and tissue destruction during treatment. J Urol. 1986;135(5):1033–1036
- Goldman SM, Fishman EK, Hartman DS, Kim YC, Siegelman SS. Computed tomography of renal tuberculosis and its pathological correlates. J Comput Assist Tomogr. 1985;9(4):771–776
- Hemal AK, Gupta NP, Rajeev TP, Kumar R, Dar L, Seth P. Polymerase chain reaction in clinically suspected genitourinary tuberculosis: Comparison with intravenous urography, bladder biopsy, and urine acid fast bacilli culture. Urology. 2000;56(4):570–574
- Ramanathan R, Kumar A, Kapoor R, Bhandari M. Relief of urinary tract obstruction in tuberculosis to improve renal function: Analysis of predictive factors. Br J Urol. 1998;81(2):199–205
- Merchant S, Bharati A, Merchant N. Tuberculosis of the genitourinary system – Urinary tract tuberculosis: Renal tuberculosis – Part I. Indian J Radiol Imaging. 2013;23(1):46–63
- Bacci MR, Namura JJ, Lera AT. Complicated urinary infection and extrapulmonary tuberculosis. BMJ Case Rep. 2012 . [Epub ahead of print]. doi: 10.1136/bcr-2012-007553
- Mortier E, Pouchot J, Girard L, Boussougant Y, Vinceneux P. Assessment of urine analysis for the diagnosis of tuberculosis. BMJ. 1996;312(7022):27–28
- Moussa OM, Eraky I, El-Far MA, Osman HG, Ghoneim MA. Rapid diagnosis of genitourinary tuberculosis by polymerase chain reaction and non-radioactive DNA hybridization. J Urol. 2000;164(2):584–588
- Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662
- Joint Tuberculosis Committee of the British Thoracic Society. Chemotherapy and management of tuberculosis in the United Kingdom: Recommendations 1998. Thorax. 1998;53:536–548
- Gokce G, Kilicarslan H, Ayan S, et al. Genitourinary tuberculosis: A review of 174 cases. Scand J Infect Dis. 2002;34(5):338–340
- Wang LJ, Wu CF, Wong YC, Chuang CK, Chu SH, Chen CJ. Imaging findings of urinary tuberculosis on excretory urography and computerized tomography. J Urol. 2003;169(2):524–528
- Wise GJ, Marella VK. Genitourinary manifestations of tuberculosis. Urol Clin North Am. 2003;30(1):111–121
- Zarrabi AD, Heyns CF. Clinical features of confirmed versus suspected urogenital tuberculosis in region with extremely high prevalence of pulmonary tuberculosis. Urology. 2009;74(1):41–45