Abstract
Background: Functional and morphological renal lesions have been widely described in leprosy for decades. Nevertheless few studies have assessed renal function pre- and during treatment after the advent of multidrug therapy (MDT). Methods: This is a prospective study involving 189 consecutive patients, with all forms of leprosy (Ridley–Jopling scale). Laboratory (serum urea and creatinine, estimated GFR, urinalysis, microalbuminuria, urinary RBP) and clinical features of renal disease were evaluated previously and after onset (3 and 8 months later) of MDT. Results: One hundred and eighty-nine patients (M 1.8: F 1; mean age 44 ± 16 years) were included just after diagnosis of leprosy and before the introduction of MDT. Mean time until manifestation of symptoms and/or signs of leprosy was 29 ± 56 months (25 days–480 months). Microhematuria and microalbuminuria were detected in 7.5% and 9.6% of the cases, respectively. Elevated serum creatinine was detected in 34% pre-MDT; this was statistically more frequent in males, hypertensive and frequent users of non-steroidal anti-inflammatory drugs (NSAID), as well as in patients with erythema nodosum lepromatosum, 45.5% by the time of diagnosis, 18% after 3 months and 9% after 8 months of MDT (p = 0.039). Conclusions: Our results suggest that functional renal lesions in leprosy are currently mild and predominantly of glomerular origin, in opposition to the severe involvement described in the past. This improved outcome of renal disease secondary to leprosy is possibly due to the advent of MDT and effective treatment of episodes of reaction, leading to shorter periods of active infectious disease.
Introduction
Functional and morphological renal lesions in leprosy were widely described in the past.Citation1–6 Mitsuda and OgawaCitation1 were the first to report renal lesions related to this disease, in an autopsy analysis. Afterwards several types of histopathological lesions were demonstrated, with a variable incidence, according to different studies.Citation6–11
Most series report renal lesions in patients with lepromatous leprosy (LL), but these may also occur in other types of this disease.Citation5 Functional and urinary abnormalities in leprosy were commonly reported in association with lepromatous reactive episodes and erythema nodosum leprosum (ENL) development.Citation3,Citation5–7,Citation12–17
The exact pathogenesis of kidney injury in leprosy is still uncertain.Citation18 In fact, Mycobacterium leprae does not seem to be directly involved in renal lesions,Citation19 although it has been detected in the renal parenchyma of some patients, including in the glomeruli.Citation18,Citation20–22 The glomerular lesion is probably mediated by immunocomplexes, which develop during reaction episodes mainly in cases of ENL. This mechanism is supported by clinical and laboratory findings, such as the visualization of immunocomplexes in histological analysis of kidneys and decrease of serum complement in some cases.Citation18
In the present work, we have evaluated clinical and laboratory manifestations of renal disease in patients with leprosy, before the onset of specific treatment with the multidrug therapy (MDT) regimen currently adopted worldwide.
Patients and methods
Patients
This is a prospective study of 189 consecutive patients with clinical and laboratory diagnoses of leprosy, undergoing disease control in a public health center in the Midwest of Brazil, from March to July 2008. The patients who fulfilled inclusion criteria should not have other concurrent disorders, as diabetes mellitus, recurrent urinary tract infection, HIV infection and nephrolithiasis, nor an abusive use of non-steroidal anti-inflammatory drugs (NSAID), characterized as the use of at least one tablet by month for five years. It is of note that since the first visits of this study it was clear that such medications were prescribed for some patients by their physicians, especially for the treatment of acute neuritis. Thus, whether this use occurred for more than a month this condition was classified as “frequent use of NSAID”. All patients were submitted to specific leprosy treatment with MDT soon after diagnosis. The MDT, as recommended by Word Healthy Organization (WHO), consisted of rifampicin and dapsone for paucibacillary patients and rifampicin, dapsone and clofazimine for the multibacillary ones.Citation23 The episodes of reaction were treated with corticosteroids and/or thalidomide.
The present study protocol was reviewed and approved by the Committees on Ethics of the Federal University of Sao Paulo and Federal University of Goiania, Brazil. Patients were included in the study only after signing the informed consent form.
The following items were evaluated: age, gender, race, job, time since the onset of symptoms and/or signs of leprosy, familial history, laboratory findings before specific treatment and after three and eight months of MDT.
Leprosy diagnosis
The diagnosis of leprosy was based on clinical and laboratory findings, such as skin lesions, peripheral nerve injury, positive bacilloscopy analysis of nasal mucus, lymph from the ear lobe or of skin lesions. Leprosy was classified according to Ridley and Jopling scale,Citation24 as lepromatous (LL), borderline-lepromatous (BL), borderline-borderline (BB), borderline-tuberculoid (BT) and tuberculoid (TT).
Renal function was evaluated by urinalysis (proteinuria, hematuria, glucosuria and leukocyturia were analyzed by using reagent strips, Labstix, Lab. Ames, Miles do Brazil), serum urea and creatinine, estimated glomerular filtration rates based on the formulas of Cockcroft–Gault and Modification of Diet in Renal Disease (MDRD), microalbuminuria (this test was carried out to determine albumin levels in random urine samples in the range of 1–200 mg/L) and urinary (ur) RBP. Blood sugar determinations were made in all cases to exclude diabetes mellitus. Renal failure was defined as a serum level of creatinine ≥1.2 mg/dL for women and ≥1.3 mg/dL for men.
Statistical analysis
In order to describe the population profile tables of frequency the categorical variables were elaborated (n, %), numerical variables were described as means, standard deviation, median and quartiles. For comparison of the categorical variables Chi-square and Fisher’s exact tests were utilized. A p value <0.05 was considered as statistically significant.
Results
General aspects
This study involved 189 patients, 103 (54.5%) males, with mean of age 44 ± 16 years (10–78 years). The mean time of symptoms and/or signs was 29 ± 56 months (25 days to 480 months) (). According to the Ridley–Jopling classification, 33 (17.5%) patients had BL and 26 (13%) had LL (). Among the patients with BL and LL forms, 24 (40%) developed type II reaction, 18 (9.5%) developed ENL; five (2.7%) patients were treated with prednisone and/or thalidomide. Among the 189 patients, 39 (20.6%) had type I reaction, 5 (2.6%) had chronic neurotrophic ulcers, 40 (21.2%) had dapsone definitely interrupted due to adverse events and 63 (33.3%) used NSAID along the whole follow-up.
Table 1. Clinical and laboratory data of 189 patients with leprosy.
Table 2. The distribution of the different forms of leprosy according to the Ridley–Jopling scale.
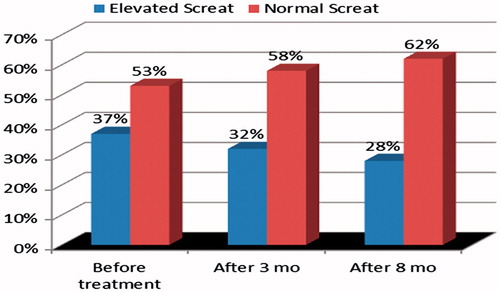
Abnormal levels of serum creatinine were detected in 65 (34.4%) patients, a finding significantly more common among males, hypertensive patients and those with frequent use of NSAID. Creatinine clearance below 60 mL/min, estimated by the Cockcroft–Gault formula, and estimated GFR by MDRD <60 mL/min were more frequent when patients were 45 years-old or older (p < 0.001). The latter test was also more frequently altered in hypertensive patients (p = 0.022). Abnormal serum creatinine (pre-treatment) improved significantly after MDT, steroid and thalidomide, when evaluated after 3 and 8 months in patients who developed ENL (p = 0.039) (). On the other hand, in 90 patients without ENL, elevated serum creatinine (pre-treatment) did not improve significantly after MDT (p = 0.140) ().
Figure 1. The distribution of serum creatinine in 18 patients with erythema nodosum leprosum (ENL) before MDT and 3 and 8 months after the initiation of specific treatment (p = 0.039).
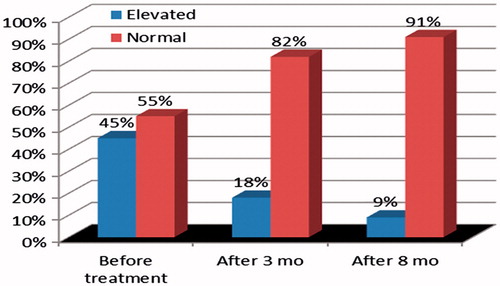
Figure 2. The distribution of serum creatinine (Screat) in patients with leprosy before MDT and 3 and 8 months after the initiation of specific treatment (p = 0.140).
Serum creatinine levels (p = 0.737), estimated creatinine clearance by Cockcroft–Gault (p = 0.344) and eGFR by MDRD formulas (p = 0.552) were not statistically different among all types of leprosy.
Microalbuminuria was more common in hypertensive patients (p < 0.005); microhematuria predominated in females (p = 0.047) with ENL (p = 0.033) and those with signs and/or symptoms for 12 months or more (p = 0.003).
Discussion
Renal involvement has been described in leprosy for decades. In this group of patients, end-stage renal disease used to be a common outcome, frequently related to death.Citation25,Citation26 It is possible that such statements are not true in our days.
In the present study, renal functional involvement characterized by altered levels of serum creatinine occurred in a high proportion of patients with leprosy (34.4%), although such dysfunction was mild, and there was a significant improvement of serum creatinine in patients with ENL submitted to treatment (p = 0.039), but not in the absence of ENL (p = 0.140). Elevated serum creatinine was significantly more common in males, hypertensive patients and frequent users of NSAID. Deficit of renal function characterized by eGFR (Cockcroft–Gault, MDRD) <60 mL/min was predominant in patients with ages equal to or above 45 years, the MDRD was also more frequently altered in hypertensive individuals.
Sritharan et al.,Citation27 in India, evaluated renal function, including distal tubular function, in patients with leprosy without treatment. As in our study, they have observed that renal function is worse in patients without specific treatment and in reaction episodes.Citation28,Citation29 In the present study, functional renal changes in patients who developed ENL improved significantly after MDT (dapsone, rifampicin, clofazimine), steroids and/or thalidomide (p = 0.039). Microhematuria was significantly more frequent in the context of ENL (p = 0.033), when the onset of signs and/or symptoms was equal to or above 12 months (p = 0.003), and marginally more common in females (p = 0.047). In fact it has been previously shown that renal abnormalities in leprosy are associated to ENL.Citation3,Citation5–7,Citation12–17 Waters et al.,Citation30 as well as Drutz and GutmanCitation5 have proposed that the ENL results from accumulation of immune complexes. During the reactional states that lead to the development of ENL, immune complexes have been demonstrated in the skin and blood vessels.Citation31–34 The etiologic role of the reactional state associated to ENL in the nephropathy secondary to leprosy cannot be ignored, but some investigators have previously described glomerulonephritis in patients without reactional episodes associated to ENL (i.e. six cases of mesangioproliferative glomerulonephritis reported by Çologlu).Citation35 In different studies it was also observed that decreases of renal function during reactional episodes can improve in the quiescence phase.Citation3,Citation15
In our leprosy population, microalbuminuria was particularly more frequent among hypertensive patients. In other studies, urinary abnormalities, including albuminuria, microhematuria, casts and leukocytes were demonstrated mainly in LL, especially during reactional phases.Citation3,Citation5–7,Citation12–17 According to Date et al.,Citation36 proteinuria is usually detected in reactional states with ENL and it can be as high as 9 g/day and such patients can manifest nephrotic syndrome. Nevertheless proteinuria was described by others in 45% of patients with quiescent ENL and 45% of those with LL without ENL.Citation3 In an additional study of Date and Johny,Citation14,Citation19 patients were submitted to renal biopsy due to edema, hematuria or proteinuria, five in the subgroup with ENL had glomerulonephritis and among those without history of ENL or LL three patients had diffuse proliferative GN. Similar findings were described by some authorsCitation20,Citation37 but they were not confirmed by others.Citation38 In our study, neither macroproteinuria nor nephrotic syndrome was observed. It is of note that in the past TarabiniCitation39 emphasized that nephrotic syndrome in leprosy is always accompanied by renal amyloidosis. We have not diagnosed amyloidosis in the present study population, but nephrotic syndrome was not detected as well.
Eventual divergences between our findings and previous publications are attributed by us to the successful performance of the current therapies adopted in leprosy cases and to earlier diagnosis.
In summary, we have shown that renal functional changes in leprosy occur before the onset of specific therapy and it improves after MDT is initiated, as well as after the treatment of reactional states manifested as ENL. Our results suggest that renal lesions in leprosy are mild and predominantly of glomerular origin, in opposition to the severe involvement described in the past. Microhematuria and microalbuminuria were detected only in 7.5% and 9.6% of our cases, respectively. Decrease of glomerular filtration rate as well as elevated levels of serum creatinine were documented more frequently in the present study population but these abnormalities were not severe, and there was an improvement trend after introduction of MDT and along the first year of follow-up. Such findings suggest that relevant renal disease in leprosy is currently uncommon, and this secondary involvement is self-limited once specific effective treatment is performed.
Declaration of interest
All the authors declare no conflict of interests.
References
- Mitsuda K, Ogawa M. A study of one hundred and fifty autopsies on cases of leprosy. Int J Lepr. 1937;5:53–60
- Kean BH, Childress ME. A summary of 103 autopsies on leprosy patients on the isthmus of Panama. Int J Lepr. 1942;10:51–59
- Thomas G, Karat ABA, Rao PSS, Drathapkumar C. Changes in renal functions during reactive phases of lepromatous leprosy. Int J Lepr Other Mycobact Dis. 1970;38:170–176
- Johny KV, Karat BA. Renal biopsy studies in leprosy (Abst.). J Assoc Physicians India. 1971;19:117–118
- Drutz DJ, Gutman RA. Renal manifestations of leprosy; glomerulonephritis a complication of ENL. Am J Trop Med Hyg. 1973;22:496–502
- Johny KV, Karat AB, Rao PS, Date A. Glomerulonephritis in leprosy: A percutaneous renal biopsy study. Lepr Rev. 1975;46:29–37
- Granells M. Renal lesions in leprosy. Int J Lepr Other Mycobact Dis. 1968;36:645
- Desikan KV, Job CK. A review of postmortem findings of 37 cases of leprosy. Int J Lepr Other Mycobact Dis. 1968;36(1):32–44
- Sachdev DS, Puri D, Bansal S. Secondary amyloidosis in leprosy. Lepr India. 1969; 41:73–76
- Mittal MM, Agarval SC, Maheshwari HB, Kumar S. Renal lesions in leprosy. Arch Pathol. 1972;93:8–12
- Gupta JC, Dewikar R, Singh S, Gupta DK, Panda PK. A histopathologic study of renal biopsies in fifty cases of leprosy. Int J Lepr Other Mycobact Dis. 1977;45(2):167–170
- Bernard JC. Regional and racial differences in leprosy. Lepr Rev. 1948;19:20–22
- Brusco CM, Masanti JG. Causes of death of leprosy patients: Influence of lepra reactions and renal disease. Int J Lepr. 1963; 31:14–25
- Date A, Johny KV. Glomerular subepithelial deposits in lepromatous leprosy. Am J Trop Med Hyg. 1975;24:853–856
- Gokhale BB, Kurkure NB. Phenol red excretion test of kidney function in leprosy patients. Indian J Med Sci. 1958;12:331–333
- Gutman RA, Lu WH, Drutz DJ. Renal manifestations of leprosy: Impaired acidification and concentration of urine in patients with leprosy. Am J Trop Med Hyg. 1973;22:223–228
- McAdam KJ, Anders RF, Smith SR, Russell DA, Price MA. Association of amyloidosis with erythema nodosum leprosum reaction and recurrent neutrophil leukocytosis in leprosy. Lancet. 1975;2:572–575
- Date A. The immunological basis of glomerular disease in leprosy – A brief review. Int J Lepr Other Mycobact Dis. 1982;50:351–353
- Kanwar AJ, Bharija SC, Belhaj MS. Renal functional status in leprosy. Indian J Lepr. 1984;56(3):595–599
- Nigam P, Pant KC, Kapoor KK, et al. Histo-functional status of kidney in leprosy. Indian J Lepr. 1986;58:567–575
- Sainani GS, Rao KV. Renal changes in leprosy. J Assoc Physicians India. 1974;22:659–664
- Drutz DJ, Gutman RA. Renal manifestation of leprosy: glomerulonephritis, a complication of erythema nodosum leprosum. Am J Trop Med Hyg. 1973;22:496–502
- Bhattacharya SN, Sehgal VN. Reappraisal of the drifting scenario of leprosy multi-drug therapy: New approach proposed for the new millennium. Int J Dermatol. 2002;41:321–326
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–273
- Bernard JC, Vasquez CJ. Visceral lesions in lepromatous leprosy, study of sixty necropsies. Int J Lepr Other Mycobact Dis. 1973;41:94–101
- Powell SC, Swan LL. Leprosy: Pathologic changes observed in fifty consecutive necropsies. Am J Pathol. 1955;31:1131–1147
- Sritharan V, Venkatesan K, Bharadwaj P, Girdhar BK. Renal functions in lepromatous leprosy patients. Lepr India. 1981;53:437–442
- Cochrane RG. Complicating conditions due to leprosy. In: Cockrane RG, Davey TF, eds.Leprosy in Theory and Practive. 2nd ed. Bristol: John Wright and Sons Ltd; 1984:331
- Ramanujam K, Ramu G, Balakrishnan S, Desikan KV. Nephrotic syndrome complicating lepromatous leprosy. Int J Med Res. 1973;61:548–556
- Waters MFR, Turk JL, Wemambu SNC. Mechanisms of reactions in leprosy. Int J Lepr. 1971;39:417–428
- Bullock WE, Callerame ML, Panner BJ. Immunohistologic alteration of skin and ultrastructural changes of glomerular basement membranes in leprosy. Am J Trop Med Hyg. 1974;23:81–86
- Turk JL, Bryceson ADM. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266
- Quismorio FP, Rea TH, Levan NE, Friou GT. Immunoglobulin deposits in lepromatous leprosy skin. Arch Dermatol. 1975;111:331–334
- Wemambu SCN, Turk JL, Waters MFR, Rees RJW. Erythema nodosum leprosum. A clinical manifestation of arthus phenomenon. Lancet. 1969;2:933–935
- Çologlu AS. Immune complex glomerulonephritis in leprosy. Lepr Rev. 1979;50:213–222
- Date A, Harihar S, Jeyavarthini SE. Renal lesions and other major findings in necropsies of 133 patients with leprosy. Int J Lepr Other Mycobact Dis. 1985;53:455–460
- Bajaj AK, Gupta SC, Sinha SN, Govil DC, Gaur UC, Kumar R. Renal function status in lepromatous leprosy. Int J Lepr. 1981;49:294–301
- Redley CRRM, Sulochana G, Naidu PS, Dev SS. Amyloidosis in leprosy. Med Surg. 1970;10:45–46
- Tarabini CG. Uroproteins in leprous nephrosis. Int J Lepr. 1959;27:292

