Abstract
Background: The association between admission serum magnesium (Mg) levels and risk of in-hospital acute kidney injury (AKI) is limited. The aim of this study was to assess the risk of developing AKI in all hospitalized patients with various admission Mg levels. Methods: This is a single-center retrospective study conducted at a tertiary referral hospital. All hospitalized adult patients who had admission Mg available from January to December 2013 were analyzed in this study. Admission Mg was categorized based on its distribution into six groups (less than 1.5, 1.5–1.7, 1.7–1.9, 1.9–2.1, 2.1–2.3 and greater than 2.3 mg/dL). The primary outcome was in-hospital AKI occurring after hospital admission. Logistic regression analysis was performed to obtain the odds ratio of AKI of various admission Mg levels using Mg with lowest AKI incidence (1.9–2.1 mg/dL) as the reference group. Results: Of 9241 patients enrolled, AKI occurred in 1124 patients (12.2%). The lowest incidence of AKI was when serum Mg was within 1.7–1.9 and 1.9–2.1 mg/dL. A U-shaped curve emerged demonstrating higher incidences of AKI associated with both hypoMg (<1.7) and hyperMg (>2.1). After adjusting for potential confounders, both hypoMg (<1.5 mg/dL) and hyperMg (>2.3 mg/dL) were associated with an increased risk of developing AKI with odds ratios of 1.70 (95% CI 1.31–2.18) and 1.42 (95% CI 1.11–1.81), respectively. Conclusion: Both admission hypoMg and hyperMg were associated with an increased risk for in-hospital AKI.
Introduction
Acute kidney injury (AKI) is a common clinical syndrome in hospitalized patients, independently associated with in-hospital morbidity and mortality.Citation1 Although, previous studies have attempted to identify effective interventions to prevent AKI events,Citation2–4 most were unsuccessful, and the mortality rate in patients with AKI remains very high. Therefore, further studies are required to identify patients at high risk of developing AKI during hospitalization.
Magnesium (Mg) serves as a catalyst for greater than 300 intracellular reactions and provides various functions in areas of energy generation, neurotransmitters release, intracellular calcium regulation and protein synthesis and degradation.Citation5 Experimentally, Mg reduces the arteriolar tone and counteracts the vasoconstriction, resulting in an increase in the renal blood flow by stimulating nitric oxide release.Citation6–9 Moreover, recent studies have demonstrated that hypoMg is associated with non-recovery of renal function after an AKI episode.Citation10,Citation11 Therefore, it is possible that Mg provides protective effects on AKI. However, the effect of admission Mg levels on the risk of in-hospital AKI in the general hospital population has not been examined. The objective of this study was to evaluate the risk of developing AKI in all hospitalized patients across a spectrum of Mg levels.
Materials and methods
Study population
All research authorized adult (age 18 year or older) patients admitted to Mayo Clinic Rochester – a tertiary referral hospital – from 01/01/2013 through 12/31/2013 were enrolled. Exclusion criteria were patients with a history of end-stage renal disease (ESRD), patients who presented with AKI at the time of admission and patients who did not have serum creatinine (SCr) measurement during hospitalization. For patients with multiple admissions during this period, only the first hospital admission was analyzed. ESRD was identified based on ICD-9 (International Classification of Diseases, 9th) code assignment (Supplementary Table 1) or an eGFR of less than 15 mL/min/1.73 m2. The local Institutional Review Board approved this study.
Data collection
Clinical characteristics, demographic information and laboratory data were collected using manual and automated retrieval from the institutional electronic medical record system. The admission serum Mg level, defined as the first serum Mg level within 24 h of hospital admission, was collected. eGFR was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.Citation12 Chronic kidney disease (CKD) was defined as a calculated eGFR less than 60 mL/min/1.73 m2. The Charlson Comorbidity scoreCitation13 was computed for co-morbidities at the time of admission. Principal diagnoses were grouped based on ICD-9 codes at admission (Supplementary Table 2).
Clinical outcomes
The primary outcome was AKI, based on the SCr criterion of the KDIGO definition.Citation14 AKI was defined as an increase in SCr, within 7 days after the admission date, of either ≥0.3 mg/dL or a relative change of ≥50% from the baseline. The baseline SCr was defined as the minimum SCr measured within one year before admission. If outpatient SCr was not available, the Modification of Diet in Renal Disease equationCitation15 was used to estimate baseline SCr level, assuming normal baseline GFR of 75 mL/min/1.73 m2, in accordance with this guideline.Citation14 Pre-specified subgroup analysis for patients with and without CKD was also performed.
Statistical analysis
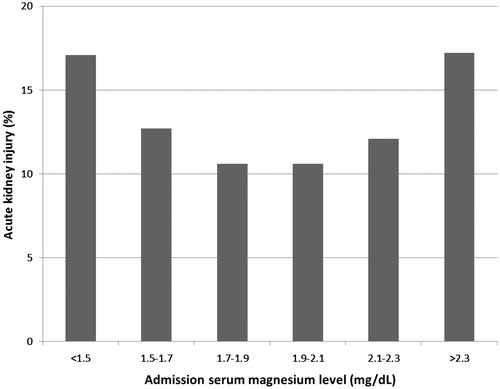
Continuous variables are reported as mean ± SD for normally-distributed data and median (IQR) for non-normally distributed data. All categorical variables are reported as count with percentage. Baseline demographics and clinical characteristics were compared among the admission Mg group, using ANOVA for continuous variables and the Chi-square test for categorical variables. We categorized admission Mg levels, based on 6-quantile percentiles (10% | 25% | 50% | 75% | 90%): less than 1.5, 1.5–1.7, 1.7–1.9, 1.9–2.1, 2.1–2.3 and greater than 2.3 mg/dL. The Mg level of 1.9–2.1 mg/dL was selected as the reference group for outcome comparison since it was associated with the lowest incidence of AKI () and within normal plasma Mg concentration (1.7–2.1 mg/dL).Citation16 We performed univariate analysis and then multivariate logistic regression analysis to evaluate the independent association between admission Mg levels and AKI. Odds ratio (OR) with 95% confidence interval (CI) is reported. OR was adjusted for variables with statistically significant (p value less than 0.05) differences between groups in univariate analysis. The adjusting variables were age, sex, race, Charlson score, baseline GFR, principal diagnosis, comorbidities, medications and the need for vasopressor and mechanical ventilator at hospital admission. Comorbidities were coronary artery disease (CAD), hypertension (HTN), diabetes mellitus (DM) and congestive heart failure (CHF). Medications were angiotensin converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), nonsteroidal anti-inflammatory drugs (NSAIDs) and diuretics. A two-tailed p value of less than 0.05 was considered statistically significant. All analyses were performed using JMP statistical software, version 10 (SAS Institute, Cary, NC).
Table 1. Outcomes.
Results
A total of 11,734 hospital admissions with available Mg levels within 24 h were identified. After excluding 363 patients with ESRD, 2106 patients with AKI at presentation and 24 patients who lacked SCr measurement during hospitalization, 9241 unique patients were enrolled.
Baseline characteristics
Of 9241 patients, 8535 (92%) patients were Caucasian and 5206 (56%) were male (). Mean age was 61 ± 17 years. Patient age was positively correlated with admission Mg levels, while eGFR was inversely correlated with admission Mg levels. Patient comorbidities included HTN (49%), DM (19%), CAD (20%) and CHF (7%). Thirty-seven percent of the patients were taking diuretics, 35% were taking ACEIs or ARBs and 22% were taking NSAIDs before admission.
Table 2. Baseline clinical characteristics.
Admission Mg and the incidence of AKI
Of 9241 patients enrolled, AKI occurred in 1124 patients (12.2%). The lowest incidence of AKI (10.6%) was when serum Mg was within 1.7–1.9 and 1.9–2.1 mg/dL (). A U-shaped curve emerged demonstrating higher incidences of AKI associated with both hypoMg (<1.7) and hyperMg (>2.1), p < 0.001. The incidences of AKI in patients with serum Mg <1.5 mg/dL and >2.3 were 17.1% and 17.2%, respectively.
Admission Mg and risk of AKI
To assess whether admission Mg levels contributed to the AKI development, logistic regression models were built, using 1.9–2.1 mg/dL as a reference range. Unadjusted, admission Mg levels of less than 1.5 mg/dL, 1.5–1.7 mg/dL, and greater than 2.3 mg/dL were associated with an increased risk of AKI with ORs of 1.75 (95% CI 1.37–2.21), 1.24 (95% CI 1.0–1.52) and 1.76 (95% CI 1.0–2.21), respectively (). When adjusted for all variables including age, sex, Charlson score, baseline GFR, comorbidities and medications, these associations remained statistically significant in Mg less than 1.5 mg/dL and greater than 2.3 mg/dL. Admission hyperMg (>2.3 mg/dL) was associated with increased risk of developing AKI (OR 1.42; 1.11–1.81). Admission hypoMg (<1.5 mg/dL) was higher associated with an increased AKI (OR 1.70; 1.31–2.18) ().
Table 3. Odds ratios for the association between admission serum magnesium levels and in-hospital acute kidney injury occurrence within 7 days.
Risk of AKI based on baseline GFR
Subgroup analysis was performed to assess the risk of AKI in patients with various admission Mg levels based on baseline GFR as shown in . In multivariate logistic regression analysis for patients with CKD, admission hypoMg <1.5 mg/dL was significantly associated with increased risk of developing in-hospital AKI (OR 1.93; 1.05–3.53). Admission hyperMg (2.1–2.3 mg/dL and >2.3 mg/dL) was not significantly associated with an increased AKI in patients with CKD.
Table 4. Odds ratios for the association between admission serum magnesium levels and in-hospital acute kidney injury occurrence within 7 days in subgroups of patients based on baseline GFR.
Discussion
In the present study, we demonstrated that admission Mg level was correlated with the incidence of AKI during hospitalization. The lowest incidence of AKI was when serum Mg was within 1.7–1.9 and 1.9–2.1 mg/dL and there was a U-shaped curve demonstrating higher incidences of AKI associated with both hypoMg (<1.7) and hyperMg (>2.1). Patients with both hypo (<1.5 mg/dL) and hyperMg (>2.3 mg/dL) at the time of admission had increased risk of developing AKI during hospitalization. In patients with CKD, admission hypoMg (<1.5 mg/dL) was associated with a 1.70-fold increased AKI risk.
There are several plausible explanations for the increased AKI risk in patients with dysmagnesemia at admission. Mg has been known to play an important role in the regulation of cardiovascular homeostasis.Citation17 It counteracts the vasoconstriction by endogenous catecholamines and potentiates the action of endogenous vasodilators.Citation6,Citation7,Citation18 In rats, hypoMg was shown to potentiate the post-ischemic renal injury.Citation19 In addition, an infusion of Mg has been shown to increase the renal blood flow via an endothelium dependent release of nitric oxideCitation8 and via its ability as a calcium channel antagonist.Citation9 Therefore, it is possible that dysmagnesemia, both hypoMg and hyperMg, may cause dysregulation of vascular tonicity homeostasis and result in higher risk of developing AKI via overstimulated vasoconstriction and vasodilation effects, respectively. In CKD patients, renal vasoconstriction can predispose patients to higher risk of AKICitation20 as also demonstrated in our patients with hypoMg.
Although, previous reports have shown that hypoMg is a risk factor for non-recovery of renal function after an AKI episode in critically ill patients,Citation10,Citation11 the investigators found no difference in Mg levels in patients with or without AKI. In the study by Alves et el.,Citation10 hypoMg was defined from any detected low Mg value during intensive care unit (ICU) stay. However, hospitalized Mg levels might not have represented the real patients’ Mg status and patients might receive treatment to normalize Mg level, but they can still be Mg deficient.Citation21 In our present study, we used only the admission serum Mg for analysis, which is a reasonable indication of the patient’s Mg status, and has been demonstrated to correlate best with Mg concentration in the bone.Citation22,Citation23 Moreover, we studied the effects of admission Mg on the risk of developing AKI in the general hospitalized patients, not only in ICUs. Therefore, the results presented in our study are the first to demonstrate that both hypoMg and hyperMg at time of admission is an important predictor of developing in-hospital AKI in the overall hospitalized patients.
This study has several limitations. Firstly, this is a single-center, retrospective study. Secondly, the patient population in this study is relatively homogeneous (predominantly Caucasian). Further studies with more heterogeneous population are desirable to ascertain the clinical effects of admission Mg on AKI in a broad patient population. Thirdly, there is potential selection bias, as those patients who had admission Mg measurements may have had different clinical characteristics from others who did not have admission Mg measurement. A multi-center, prospective study is ultimately required to address these limitations. Lastly, the effects of normalizing Mg levels and the risk of AKI were not the focus of our present study. Ritter and colleagues have recently completed an RCT comparing the incidence of AKI of Mg therapy to placebo in critically ill patients with hypoMg (ClinicalTrials.gov identifier – NCT01700998), which will elucidate if this intervention is effective to prevent AKI or shorten AKI recovery.
In conclusion, this study demonstrates that both admission hypoMg and hyperMg are associated with an increased risk for in-hospital AKI.
Supplementary material available online
Supplementary Tables 1 and 2.
supplemental document.pdf
Download PDF (11.5 KB)Declaration of interest
We do not have any financial or non-financial potential conflicts of interest.
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
All authors had access to the data and a role in writing the manuscript.
References
- Thongprayoon C, Cheungpasitporn W, Akhoundi A, Ahmed AH, Kashani KB. Actual versus ideal body weight for acute kidney injury diagnosis and classification in critically ill patients. BMC Nephrol. 2014;15:176
- Cheng X, Tong J, Hu Q, Chen S, Yin Y, Liu Z. Meta-analysis of the effects of preoperative renin-angiotensin system inhibitor therapy on major adverse cardiac events in patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2015;47:958--966
- Yacoub R, Patel N, Lohr JW, Rajagopalan S, Nader N, Arora P. Acute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: A meta-analysis of observational studies. Am J Kidney Dis. 2013;62:1077–1086
- Garg AX, Kurz A, Sessler DI, et al. Perioperative aspirin and clonidine and risk of acute kidney injury: A randomized clinical trial. JAMA. 2014;312:2254--2264
- Altura BM. Basic biochemistry and physiology of magnesium: A brief review. Magnes Trace Elem. 1991;10:167–171
- Altura BM, Altura BT. Vascular smooth muscle and prostaglandins. Fed Proc. 1976;35:2360–2366
- Altura BM, Altura BT. New perspectives on the role of magnesium in the pathophysiology of the cardiovascular system. II. Experimental aspects. Magnesium. 1985;4:245–271
- Nadler JL, Goodson S, Rude RK. Evidence that prostacyclin mediates the vascular action of magnesium in humans. Hypertension. 1987;9:379–383
- Agus ZS. Hypomagnesemia. J Am Soc Nephrol. 1999;10:1616–1622
- Alves SC, Tomasi CD, Constantino L, et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2013;28:910–916
- Santos MS, Seguro AC, Andrade L. Hypomagnesemia is a risk factor for nonrecovery of renal function and mortality in aids patients with acute kidney injury. Braz J Med Biol Res. 2010;43:316–323
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251
- KDIGO AKI Work Group. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Kidney Int. 2012;Suppl 2:1–138
- Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for rifle classification. Nephrol Dial Transplant. 2010;25:3911–3918
- Collignon FP, Friedman JA, Piepgras DG, et al. Serum magnesium levels as related to symptomatic vasospasm and outcome following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2004;1:441–448
- Backlund M, Pere P, Lepantalo M, Lehtola A, Lindgren L. Effect of intra-aortic magnesium on renal function during and after abdominal aortic surgery: A pilot study. Acta Anaesthesiol Scand. 2000;44:605–611
- Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation. 2000;102:2353–2358
- Campos SB, Silva JC, Seguro AC. Hypomagnesemia potentiates postischemic acute renal failure. J Am Soc Nephrol. 2001;12:A4057
- Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031–3041
- Ayuk J, Gittoes NJ. Treatment of hypomagnesemia. Am J Kidney Dis. 2014;63:691–695
- Alfrey AC, Miller NL, Trow R. Effect of age and magnesium depletion on bone magnesium pools in rats. J Clin Invest. 1974;54:1074–1081
- Alfrey AC, Miller NL, Butkus D. Evaluation of body magnesium stores. J Lab Clin Med. 1974;84:153–162