Abstract
Background: The reported risk of hypomagnesemia in patients with proton pump inhibitor (PPI) use is conflicting. The objective of this meta-analysis was to assess the association between the use of PPIs and the risk of hypomagnesemia. Methods: A literature search of observational studies was performed using MEDLINE, EMBASE and Cochrane Database of Systematic Reviews from inception through September 2014. Studies that reported odd ratios or hazard ratios comparing the risk of hypomagnesemia in patients with PPI use were included. Pooled risk ratios (RRs) and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method. Results: Nine observational studies (three cohort studies, five cross-sectional studies and a case-control study) with a total of 109,798 patients were identified and included in the data analysis. The pooled RR of hypomagnesemia in patients with PPI use was 1.43 (95% CI, 1.08–1.88). The association between the use of PPIs and hypomagnesemia remained significant after the sensitivity analysis including only studies with high quality score (Newcastle–Ottawa scale score ≥ 8) with a pooled RR of 1.63 (95% CI, 1.14–2.23). Conclusions: Our study demonstrates a statistically significant increased risk of hypomagnesemia in patients with PPI use. The finding of this meta-analysis of observational studies suggests that PPI use is associated with hypomagnesemia and may impact clinical management of patients who are taking PPIs and at risk for hypomagnesemia related cardiovascular events.
Introduction
Hypomagnesemia is a common clinical problem with a prevalence of ∼10% in hospitalized patients and up to 65% in critically ill patients.Citation1 The clinical consequences of severe hypomagnesemia include muscle weakness, tetany, convulsions, cardiac arrhythmias and hypotension.Citation2–5 Studies have also demonstrated an association between hypomagnesemia and both cardiovascular and non-cardiovascular mortality.Citation6,Citation7 Magnesium (Mg) has been found to lower inflammation, decrease oxidative stress and diminish endothelial dysfunction – all factors that underlie cardiovascular disease. Further, magnesium helps to reduce platelet aggregation, which could help to prevent the formation of dangerous blood clots.Citation8 Serious arrhythmias and sudden death have been reported in patients with hypomagnesemia and cardiovascular disease. Several prospective epidemiological studies have also shown a relationship between hypomagnesemia and the risk of recurrent coronary heart disease.Citation9,Citation10 Moreover, hypomagnesemia was demonstrated to be associated with nonrecovery of renal function after acute kidney injury episodes.Citation11
Proton pump inhibitors (PPIs) are commonly used worldwide, with or without a prescription, for the treatment of acid-related disorders. In the United States, PPIs have been increasingly prescribed; over 100 million times a year for the past five years.Citation5 Studies, however, have reported the adverse effects of PPIs including the risk of acute interstitial nephritis,Citation12 Clostridium difficile colitis,Citation13 hospital-acquired pneumoniaCitation14 and hip fractures.Citation15 Recently, severe hypomagnesemia has been described in more than 30 cases with PPI therapy in a systematic review.Citation16 Regarding this potential adverse effect, the US Food and Drug Administration (FDA) issued a warning that PPIs may reduce Mg levels in March 2011.Citation17 However, studies have subsequently demonstrated conflicting results on PPI use and the risk of hypomagnesemia. Several studies have shown that hypomagnesemia is associated with the use of PPIs.Citation18–21 Conversely, a number of studies have demonstrated no associations between PPI use and hypomagnesemia.Citation5,Citation22–24
The objective of this meta-analysis was to assess the association between the use of PPIs and the risk of hypomagnesemia.
Methods
Search strategy
Two investigators (WC and CT) independently searched published studies indexed in MEDLINE, EMBASE and the Cochrane database from inception through September 2014 using the search strategy described in online Supplementary Material. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (1) observational studies (cohort studies, case-control or cross-sectional) published as original studies or conference abstracts to evaluate the risk of hypomagnesemia in patients with PPI use, (2) odds ratios, relative risks or hazard ratios with 95% confidence intervals (CIs) were presented and (3) a reference group composed of participants who did not use PPIs.
Study eligibility was independently determined by the two investigators noted above. Differing decisions were resolved by mutual consensus. The quality of each study was independently assessed by each individual investigator using the Newcastle–Ottawa quality assessment scale.Citation25
Data extraction
A standardized data collection form was used to extract the following information: last name of the first author, study design, year of study, country of origin, year of publication, sample size, characteristics of included participants, definition of PPI use, method used to diagnose hypomagnesemia and adjusted effect estimates with 95% CI. The two investigators independently performed this data extraction.
Statistical analysis
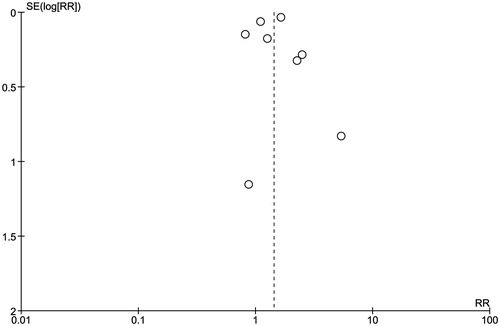
Review Manager 5.2 software (Copenhagen, Denmark) from the Cochrane Collaboration was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.Citation26 Given the high likelihood of between study variances, we used a random-effect model rather than a fixed-effect model. Statistical heterogeneity was assessed using the Cochran's Q test. This statistic is complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0–25% represents insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity and >75% high heterogeneity.Citation27 The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios versus their standard errors.Citation28
Results
Our search strategy yielded 681 potentially relevant articles. Six hundred and sixty articles were excluded based on title and abstract for certainly not fulfilling inclusion criteria on the basis of the type of article, study design, population or outcome of interest. Twenty-one articles underwent full-length article review. Twelve articles were excluded (seven articles were not observational studies and five articles did not report the outcomes of interest). Nine observational studies with a total of 109,798 patients were identified and included in the data analysis. Our search methodology and selection process were included in online Supplementary Material.
The risk of hypomagnesemia in patients with PPI use
Nine observational studiesCitation5,Citation18–24,Citation29 (three cohort studies, five cross-sectional study and a case control study) with a total of 109,798 patients were included in the data analysis for the risk of hypomagnesemia in patients with PPI use. describes the detailed characteristics and quality assessment of the included studies. The pooled RR of hypomagnesemia in patients with PPI use was 1.43 (95% CI, 1.08–1.88, I2 of 87%). shows the forest plot of the included studies. The association between the use of PPIs and hypomagnesemia remained significant after the sensitivity analysis including only studies with high quality scores (Newcastle–Ottawa scale score ≥ 8) with a pooled RR of 1.63 (95% CI, 1.14–2.23, I2 of 91%).
Figure 1. Forest plot of the included studies comparing risk of hypomagnesemia in patients who used PPI and those who did not; square data markers represent risk ratios (RRs); horizontal lines, the 95% CIs with marker size reflecting the statistical weight of the study using random-effects meta-analysis. A diamond data marker represents the overall RR and 95% CI for the outcome of interest. IV, inverse variance; SE, standard error.
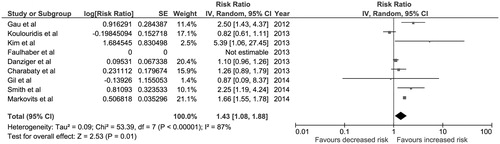
Table 1. Main characteristics of the studies included in this meta-analysis.
Evaluation for publication bias
A funnel plot to evaluate publication bias for the risk of hypomagnesemia in patients with PPI use is summarized in . The plot did not show significant publication bias of included studies.
Discussion
Our meta-analysis results indicate a significant association between hypomagnesemia and the use of PPIs with an overall 1.43-fold increased risk of hypomagnesemia compared to those who did not use PPIs. This association remains significant with the sensitivity analysis including only high-quality studies (Newcastle–Ottawa scale score ≥ 8) with a 1.63-fold increased risk of hypomagnesemia.
The findings of our meta-analysis confirmed the FDA's warning of PPI-related hypomagnesemia. The underlying mechanism of the association of hypomagnesemia in patients with PPI use is likely explained by the disturbance of gastrointestinal (GI) handling of Mg since studies have shown that an increased renal Mg loss is not the only culprit in those patients with significant hypomagnesemia after PPI use.Citation30 Although, GI absorption of Mg occurs by passive movement between enterocytes, it is augmented by an active transport system utilizing transient receptor potential Melastatin 6 (TRMP6) and TRPM7. A decrease in intestinal luminal pH by the use of PPIs may alter TRPM6/TRPM7 channel affinity for Mg and disrupt the active transport system. The passive paracellular Mg absorption across the GI tract, however, is not affected by alteration of pH by PPIs. Therefore, hypomagnesemia does not occur in everyone who uses PPIs due to sufficient passive GI absorption of Mg. The risk of PPI-induced hypomagnesemia should be considered in patients with a low intake of Mg, GI malabsorption disorders or an increased renal loss of Mg due to diuretic use.
Although, almost all included studies were of moderate to high quality (as evaluated by Newcastle–Ottawa scale) and we also confirmed the result by sensitivity analysis in only high quality studies,Citation5,Citation18,Citation20,Citation24 there are some limitations. Firstly, there are statistical heterogeneities in the complete analysis. The potential sources of these heterogeneities include the differences in the exposure definition (types of PPI and duration of PPI use), the differences in confounder adjusted methods and the duration of the follow-up, and the different patient settings (inpatient and outpatient). Unfortunately, the data on types of PPI and duration of PPI use in the included studies of our meta-analysis are limited, so we were unable to investigate these details, and further studies are needed. In addition, although most of the included studies adjusted for diuretic use, GI malabsorption and malnutrition are also very important factors that need to take into consideration. Lastly, this is a meta-analysis of observational studies with its inherent limitations. Therefore, our meta-analysis can at best demonstrate an association but not a causal relationship. However, since epidemiological studies have shown the association between hypomagnesemia and the risk of recurrent coronary heart disease and serious arrhythmias,Citation9,Citation10 PPIs need to be cautiously used in patients with cardiovascular diseases and hypomagnesemia.
In conclusion, our study suggests a statistically significant association between PPI use and hypomagnesemia. Physicians should be aware of this potential association which may impact clinical management of patients who are taking PPIs who are at risk for cardiovascular events from hypomagnesemia, especially those who have impairment in gastrointestinal absorptive capacity for magnesium, renal losses of magnesium due to diuretics or poor nutrition.
Supplementary material available online
Supplemental File
Download PDF (43.9 KB)Acknowledgments
All authors had access to the data and a role in writing the manuscript.
Declaration of interest
We wish to confirm that all authors have no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Agus ZS. Hypomagnesemia. J Am Soc Nephrol. 1999;10:1616–1622
- Moore MJ, Flink EB. Magnesium deficiency as a cause of serious arrhythmias. Arch Intern Med. 1978;138:825–826
- Vallee BL, Wacker WE, Ulmer DD. The magnesium-deficiency tetany syndrome in man. N Engl J Med. 1960;262:155–161
- Flink EB. Magnesium deficiency. Etiology and clinical spectrum. Acta Med Scand Suppl. 1981;647:125–137
- Danziger J, William JH, Scott DJ, et al. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–699
- Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85:174–181
- Reffelmann T, Ittermann T, Dorr M, et al. Low serum magnesium concentrations predict cardiovascular and all-cause mortality. Atherosclerosis. 2011;219:280–284
- Bo S, Pisu E. Role of dietary magnesium in cardiovascular disease prevention, insulin sensitivity and diabetes. Curr Opin Lipidol. 2008;19:50–56
- Liao F, Folsom AR, Brancati FL. Is low magnesium concentration a risk factor for coronary heart disease? The atherosclerosis risk in communities (Aric) study. Am Heart J. 1998;136:480–490
- Del Gobbo LC, Imamura F, Wu JH, de Oliveira Otto MC, Chiuve SE, Mozaffarian D. Circulating and dietary magnesium and risk of cardiovascular disease: A systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2013;98:160–173
- Alves SC, Tomasi CD, Constantino L, et al. Hypomagnesemia as a risk factor for the non-recovery of the renal function in critically ill patients with acute kidney injury. Nephrol Dial Transplant. 2013;28:910–916
- Sierra F, Suarez M, Rey M, Vela MF. Systematic review: Proton pump inhibitor-associated acute interstitial nephritis. Aliment Pharmacol Ther. 2007;26:545–553
- Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial clostridium difficile infection. Arch Intern Med. 2010;170:784–790
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301:2120–2128
- Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: A meta-analysis of 11 international studies. Am J Med. 2011;124:519–526
- Hess MW, Hoenderop JG, Bindels RJ, Drenth JP. Systematic review: Hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther. 2012;36:405–413
- US Department of Health and Human Services F DSC. Drug safety communication—Low magnesium levels can be associated with long-term use. Available from: http://www.fda.gov/Drugs/DrugSafety/ucm245011.htm. Accessed March 3, 2011
- Gau JT, Yang YX, Chen R, Kao TC. Uses of proton pump inhibitors and hypomagnesemia. Pharmacoepidemiol Drug Saf. 2012;21:553–559
- Kim S, Lee H, Park CH, et al. Clinical predictors associated with proton pump inhibitor-induced hypomagnesemia. Am J Ther. 2015;22:14--21
- Markovits N, Loebstein R, Halkin H, et al. The association of proton pump inhibitors and hypomagnesemia in the community setting. J Clin Pharmacol. 2014;54:889–895
- Smith K, Gatesman ML, Harpe SE, Clark W. Evaluation of electrolyte repletion in hematopoietic cell transplant patients receiving h-2 receptor antagonists or proton pump inhibitors. Bone Marrow Transplant. 2014;49:1106–1108
- El-Charabaty E, Saifan C, Abdallah M, et al. Effects of proton pump inhibitors and electrolyte disturbances on arrhythmias. Int J Gen Med. 2013;6:515–518
- Gil HW PS, Hong SY. Change in serum magnesium concentration after use of a proton pump inhibitor. 51st ERA-EDTA Congress, Amsterdam, Netherlands: Oxford University Press; 2014;29:iii180–iii181
- Koulouridis I, Alfayez M, Tighiouart H, et al. Out-of-hospital use of proton pump inhibitors and hypomagnesemia at hospital admission: A nested case-control study. Am J Kidney Dis. 2013;62:730–737
- Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872
- Faulhaber GA, Ascoli BM, Lubini A, et al. Serum magnesium and proton-pump inhibitors use: A cross-sectional study. Rev Assoc Med Bras. 2013;59:276–279
- Perazella MA. Proton pump inhibitors and hypomagnesemia. A rare but serious complication. Kidney Int. 2013;83:553–556