Abstract
Objective: The objective of the present study was to examine the changes in the expression profile of certain genes in rat model of gentamicin-induced acute kidney injury (AKI) and to see whether time period and routes of administration affect their expression levels. Methods: Rat AKI model was established with gentamicin injection using two different routes of administration and two different time periods. The models were evaluated through histopathological observations. Renal specific genes were selected on the basis of their role during kidney injury. These genes were analyzed through reverse transcriptase (RT) PCR. Results: Marked disorganization of normal structure of proximal and distal tubules was observed in all the gentamicin-treated groups. Many tubules showed loss of brush border and presence of intratubular protein casts. Changes in gene expression levels were observed for kidney injury molecule (KIM-1), osteopontin, bone morphogenic protein-7 (BMP-7), extracellular signal-regulated kinases (ERK), stem cell factor (SCF) and IL-7 receptor with different levels of significance in the renal injury groups studied depending on the time period and route of administration. Conclusion: Gene expression seems to be dependent partly on the type of injury, route of administration and time period after induction of injury. An improved mechanistic understanding of gene regulation pathways in AKI may provide basis for potential therapeutic development.
Introduction
Acute kidney injury (AKI) has a profound impact on morbidity and mortality. The pathogenesis of human AKI is complex and is only partially understood. AKI is characterized by impairment in kidney function that can be identified by the accumulation of metabolic nitrogen wastes like urea and creatinine. A rapid elevation occurs in serum creatinine levels due to AKI between 36 and 48 h. No specific remedies have been developed that can diminish injury or speed up the recovery process. Evidence suggests high risks for the development of chronic kidney injury in AKI patients.Citation1 The delayed diagnostic methods for AKI have directed to explore early and precise substitute biomarkers of the disease. Kidney injury molecule-1 (KIM-1), interleukin (IL)-18 and neutrophil gelatinase-associated lipocalin (NGAL) have been identified and presented as effective biomarkers in the diagnosis of several forms of AKI, especially when applied in combination. It has been proposed that new AKI biomarkers along with the implementation of novel treatment modalities may markedly improve early and late outcomes.Citation2
For the growth of new therapeutic strategies for AKI, understanding the process of endogenous kidney regeneration is important. After injury, surviving tubular cells are able to dedifferentiate, expressing mesenchymal (vimentin) and embryonic (Pax-2) markers; they can proliferate and migrate to injured basement membrane and finally may provide epithelial integrity following redifferentiation.Citation3 A series of growth factors orchestrate these mechanisms by promoting tubular cell proliferation. Renal tubular cells, in particular proximal tubule cells, are vulnerable to the toxic effects of drugs because their role in concentrating and reabsorbing glomerular filtrate exposes them to high levels of circulating toxins.Citation4 Drugs that cause tubular cell toxicity do so by impairing mitochondrial function, interfering with tubular transport, increasing oxidative stress and producing free radicals.Citation5,Citation6 Significant advances have recently been made in defining the biological consequences of ischemic renal injury. The morphological response of renal tubular cells depends on the intensity and severity of ischemia and includes loss of cell polarity, cell death, dedifferentiation of viable cells, proliferation, differentiation and restoration of normal epithelium.Citation7
The molecular mechanisms underlying in each of these phenomena are under active investigation and hold significant promise for novel therapeutic interventions aimed at ameliorating cell death and/or accelerating the recovery process. It has been documented that AKI has considerable influence on expression of renal specific genes.Citation8 Study of the outcomes of genetic changes after renal cell damage may help in understanding the mechanism of injury. Despite the advancement in the understanding of the mechanisms of AKI, still hardly any report is available for the gene expression variability in nephrotoxic kidney injury models. Most of the AKI studies done earlier have reported variations in the expression levels of renal genes in ischemia- or hypoxia-induced kidney injury models. However, in normal clinical practice, drug-induced kidney injury is of prime significance and changes in the gene expression levels in nephrotoxic kidney injury model are crucial. Nephrotoxic models are usually induced by gentamicin, cisplatin or mercury compounds because of their predictability and consistency. This may provide a useful tool to investigate the pathophysiological mechanisms and protection strategies against AKI and reveal better repair options.
Materials and methods
Animals
Animal treatment was conducted in conformity with the international guidelines for the care and experimental use of laboratory animals with the approval from the local ethical committee. Inbred Sprague Dawley (SD) rats weighing 200–260 g were used for the experimental model of AKI. Rats were provided by the animal facility of Dr. Panjwani Center for Molecular Medicine and Drug Research (PCMD), International Center for Chemical and Biological Sciences (ICCBS), University of Karachi. The rats were housed in individual cages in a temperature (21 ± 1 °C) and humidity (55 ± 5%) controlled room with 12-h light:12-h dark cycle. The animals were provided ad libitum with rodent chaw and water.
Development of AKI model
SD rats were randomized into different groups () that received various drug doses of nephrotoxic drug, gentamicin (Bosch Pharmaceuticals Pvt. Ltd., Khi, Pakistan) following 24 h water deprivation, while normal group received equal volume of saline injection. Rats were sacrificed at 24 h after the last dose of drug infusion as well as following 2 weeks (on 14th day of the experiment) to examine renal cell changes.
Table 1. Experimental groups for the generation of gentamicin-induced AKI models.
Sample collection
Kidneys were removed, cut along median longitudinal axis and immediately immersed in Bouins fixative for 6–8 h. A small portion of kidney tissue was preserved in RNA Later solution (Qiagen Inc, Velencia, CA) for RNA isolation.
Histological examination
Kidneys were sectioned and fixed on glass slides. To evaluate renal morphology, fixed kidney sections were dehydrated by immersing tissues in a gradual series of 70%, 90% and 100% alcohol and finally in xylene. Thin sections (5 µm) were cut and stained with Hematoxylin and Eosin (Carl-ROTH, GmbH, Germany) staining kit according to the manufacturer's protocol.
RNA isolation and cDNA synthesis
Total RNA was isolated from normal and treated kidneys using TRIzol reagent (Invitrogen, Life Technologies, Carlsbad, CA). Kidney tissue (<30 mg) was homogenized in 1 mL TRIzol. Chloroform (0.2 mL) was added to the homogenate, incubated at RT for 20 min, followed by centrifugation at 12,000g for 15 min at 4 °C. Mixture was phase separated. To the colorless supernatant, 0.5 mL of isopropanol was added and incubated at RT for 10 min. RNA pellet was recovered by centrifugation at 12,000g for 10 min at 4 °C and washed with 1 mL of 75% ethanol. RNA pellet was dried and dissolved in 50 µL RNase free water to elute RNA. cDNA was synthesized by reverse transcription of 2 µg RNA using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, Thermo Fisher Scientific Inc., Waltham, MA) according to the manufacturer's instructions.
RT-PCR and gel electrophoresis
cDNA was amplified using specific primers corresponding to each gene (). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as internal control. The thermal cycler was programmed for a preheat treatment of 94 °C for 1 min followed by 35 cycles denaturation (94 °C for 1 min), annealing (50–60 °C for 1 min) and extension (72 °C for 1 min) followed by a final extension at 72 °C for 10 min. The reaction was terminated at 4 °C. Amplified products were electrophoresed on 1% agarose gel. Fragment length of loaded sample was estimated by comparison with a co-electrophoresed DNA ladder and integrated density of each band was calibrated by normalizing with the corresponding GAPDH band density.
Table 2. Forward (F) and reverse (R) primer sequences, annealing temperatures and expected product sizes.
Statistical analysis
Data were expressed as means ± standard error of means (SEM). Differences between groups were analyzed using one-way ANOVA, followed by Tucky's post-hoc test. p Value < 0.05 was considered statistically significant.
Results
Histological observations
The microscopic examination of kidney tissues in the control group reveals normal morphology of kidney. The glomeruli as well as the proximal and distal tubules are intact with well-preserved extra cellular matrix (ECM) (). A comparison of the histological parameters is presented in . Marked disorganization of the normal structure of proximal and distal tubules is observed in all the gentamicin-treated groups (). Histological analysis through H&E staining showed epithelial injury characterized by tubular necrosis and desquamation of basement membrane that is quite prominent in the gentamicin group treated with 100 mg/kg i.p. for 6 days (). In addition, many tubules showed loss of brush border and the presence of intratubular protein casts. The animal groups treated with gentamicin 100 mg/kg s.c. for 4 days also displayed prominent tubular injury with better preserved ECM (). Tubular cast became much more prominent on the 14th day of experiment ().
Figure 1. H&E analysis of gentamicin-induced AKI models in SD rats showing (A) saline administered control and (B–D) AKI groups; gentamicin at (B) 100 mg/kg i.p. 6 days showing destruction of brush borders (arrow), tubular necrosis (arrow head), intraluminal cast formation (star) and disorganization of kidney architecture; (C) 100 mg/kg s.c. 4 days showing degeneration, desquamation and necrosis in tubules (arrow) and (D) 100 mg/kg i.p. 2 weeks showing tubuloepithelial changes, flattened and dilated tubules (arrows) and luminal cast (arrow head). Note: Images were taken at 20× magnification.
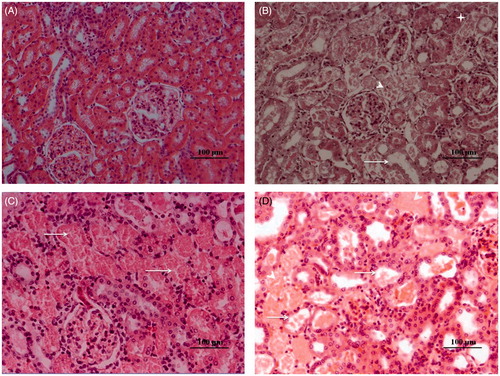
Table 3. Comparison of the histological parameters among different AKI groups.
Gene expression analysis
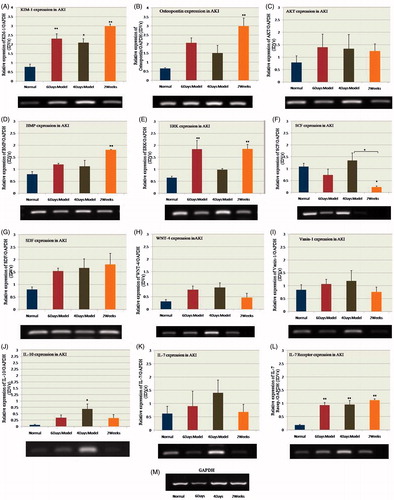
Gentamicin-induced AKI caused changes in the expression levels of various genes (). KIM-1 showed increased expression in all the AKI groups compared with the control group (*p ≤ 0.05; gentamicin 100 mg/kg s.c. for 4 days and **p ≤ 0.01; 100 mg/kg i.p. for 6 days and 2 weeks). Osteopontin and BMP-7 showed significant increase (**p ≤ 0.01) only on the 14th day of the experiment after injury. Although, AKT, stromal cell-derived factor (SDF) and WNT-4 did not show any significant difference, the trend showed increased expression in all the AKI groups compared to the control. IL-10 expression was significantly increased (*p ≤ 0.05) in the 100 mg/kg s.c. group, while extracellular signal-regulated kinases (ERK) expression was significantly increased (**p ≤ 0.01) in the 100 mg/kg i.p. group at both time periods; 24 h after the last drug dose and on the 14th day of injury. The expression of stem cell factor (SCF) became significantly lower (*p ≤ 0.05) in injured tissue on the 14th day while no significant change was observed in case of injury induced by either of the two routes. The IL-7 gene did not show any notable change in expression in any group. However, IL-7 receptor gene showed significant increase (**p ≤ 0.01) in expression in all the renal injury groups. No significant change was observed in Vanin-1 gene expression in any group.
Figure 2. Gel images and bar diagrams showing quantitative densitometry analyses of (A) KIM-1, (B) osteopontin, (C) AKT, (D) BMP-7, (E) ERK, (F) SCF, (G) SDF, (H) WNT-4, (I) Vanin-1, (J) IL-10, (K) IL-7 and (L) IL-7 receptor expression in normal and injured kidneys. Y-axis shows the relative expression of each gene with respect to GAPDH expression. Notes: Expression of each gene was calculated as fold change with control value taken as 1. Data are presented as means ± standard error of means (SEM); n = 3. Differences between groups were analyzed using one-way ANOVA, followed by Tucky's post-hoc test. p Values < 0.05 were considered statistically significant.
Discussion
In this study, we analyzed variations in gene expression pattern following an optimized dose of gentamicin to induce AKI in rats. The gene expression levels were compared with respect to the effect of the same drug administered either by intra-peritoneal (i.p.) or subcutaneous (s.c.) routes while that of i.p. route was also analyzed both immediately and at 2 weeks (14th day) after injury. The extent of damage was also examined and compared. Tubular necrosis and cellular damage in the basement membrane were observed in the histological sections of kidney tissues in all the groups. Renal damage was recognized by diffuse and extensive necrosis. In AKI, a number of pathophysiological mechanisms contribute after toxic insult.Citation9 These include tubular dysfunction and cell death, desquamation of viable and dead cells contributing to intratubular obstruction and acute inflammatory response, as we observed through H&E staining. The pathological mechanisms comprise a complex interplay of inflammatory cytokines/chemokines, reactive oxygen species, and apoptotic factors.Citation10 Nephrotoxic models, such as those induced by gentamicin, cisplatin or mercury compounds, are usually provoked by transport-mediated accumulation of the nephrotoxin within proximal tubular cells.Citation11 Renal damage and dysfunction are dose-dependent, and are often enhanced by sodium depletion or dehydration, conceivably reflecting enhanced tubular uptake. Nephrotoxic agents differ in their intracellular tubular injury pathways, which may include direct or reactive oxygen species (ROS)-mediated damage to cellular components, or interruption of tubular cell metabolism and energy stores.Citation8
Gene expression pattern showed different trends at different time periods following injury. In some cases, routes of drug administration also had an impact on the expression level. KIM-1 is considered as a major biomarker of the AKI.Citation12 We observed significant increase in its expression after drug induction both in case of i.p. and s.c. models and it remained significantly increased till 2 weeks after injury. KIM-1 is a type 1 transmembrane receptor that plays a role in T-helper cell development and regulation of allergic diseases. It plays an important role in the restoration of morphological integrity. Its mRNA and protein express at a low level in normal kidney but increase dramatically in post-ischemic kidney.Citation13
The expression levels of BMP-7 and osteopontin did not change immediately after drug induction in case of either route. However, their expression significantly increased after 2 weeks of injury. This shows that their expression is time dependent. The BMPs are a family of secreted signaling molecules that can induce ectopic bone growth. Many BMPs are part of the transforming growth factor-beta (TGF-β) superfamily. They have a proposed role in early development and epithelial osteogenesis. BMP-7 is also an integral part of the physiological response of the kidney towards injury. It inhibits epithelial to mesenchymal transition involving adult renal epithelial tubular cells and decreases the secretion of type I collagen by adult renal fibroblasts.Citation14 In the adult kidney, BMP-7 is endogenously expressed in epithelial cells of distal tubules and collecting ducts.Citation15 Over-expression of BMP-7 protects the kidney from injury, confirming its role in facilitating kidney repair.Citation16 BMP-7 is proposed as a possible treatment for reversing end-stage renal disease.Citation17 Osteopontin is involved in the attachment of osteoclasts to the mineralized bone matrix. It acts as cytokine that upregulates expression of interferon-gamma and IL-12. Osteopontin is also a macrophage chemotactic and adhesion molecule and plays a role in glomerular and tubulointerstitial injury in several kidney disease models.Citation18 An up-regulation of osteopontin expression may suggest its role in progressive renal injury.
We did not find significant change in the gene expression levels of SDF, WNT-4 and protein kinase B or AKT. As the trend shows increase in expression levels, it may be possible that more time period is required for these genes to reach significant levels. The levels of SDF have been reported to be elevated after ischemia-induced AKI.Citation19,Citation20 The possibility of increase in the SDF expression may be related to the type of insult used to induce injury. SDF is thought to promote the migration of bone marrow stem or progenitor cells to the site of injury and likely to be influenced by the hypoxic environment. Similar results were reported previously for WNT-4Citation21 and AKT,Citation22 which showed up-regulation in their expression levels after ischemia/hypoxia. WNT-4 and AKT also possess roles in the developmental process and are involved in cell proliferation and growth; thus hypoxia-induced injury may influence their expression levels.
The route of administration seems to influence the expression levels of IL-10 and ERK. While, IL-10 is over-expressed significantly in case of injury induced only by gentamicin administered through subcutaneous route, ERK expression was increased in groups in which the drug was administered intraperitoneally with persistent increase till 2 weeks. These molecules play important roles in many cellular processes. IL-10 is produced primarily by monocytes and to a lesser extent by lymphocytes and has pleiotropic effects in immunoregulation and inflammation.Citation23 It also enhances B cell survival, proliferation and antibody production, and is involved in the regulation of the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) signaling pathway.Citation24 IL-10 gene product has also been successfully used as a treatment option to repair the damaged kidney tissue.Citation25 ERKs are members of the mitogen-activated protein (MAP) kinase family and act as an integration point for multiple biochemical signals. They are involved in a variety of cellular processes, such as proliferation, differentiation, transcription regulation and development.Citation26 In vitro studies suggest that activation of the ERK pathway plays a critical role in the proliferation of tubular epithelial and myofibroblast-like cells.Citation27
SCF level was found to be decreased in i.p. administered models and increased in that of s.c. but in both cases the difference remained non-significant in the first week of injury. However, after 2 weeks, the expression of SCF was significantly reduced in i.p. administered models. Again the route of drug administration seems to play a role in its regulation and release. SCF is a ligand of tyrosine-kinase receptor, and is a pleiotropic factor that acts in germ and neural cell development.Citation28 It also has an essential role in the stem cell maintenance, gametogenesis, mast cell development, migration and function and in melanogenesis.Citation29 SCF has been reported to inhibit apoptosis of kidney tubular cells in vitroCitation30 and the severe damage in the kidney tissue induced by gentamicin may have resulted in decrease in their expression level.
Previous reports showed a significant reduction in the expression of Vanin-1 in nephrotoxicity induced kidney injury models.Citation31 However, in the present study, we did not find any significant change in their expression levels. Further experiments using higher doses of gentamicin should be carried out to analyze the expression levels of Vanin-1.
We observed no significant change in the expression level of IL-7; however, its receptor showed significant increase in gene expression in all the injury groups. It may be possible that more time period is required for IL-7 expression following injury. IL-7 is a hematopoietic growth factor released by many cell types. It mediates its action through non-receptor tyrosine kinase which interlinks with other pathways including JAK/STAT, PI3 kinase and Src family kinase. IL-7 signaling brings about the stimulation of many downstream pathways that result in the induction of cell survival, proliferation and differentiation signals.Citation32
Conclusion
Drug-induced rat kidney injury model may provide a useful tool to investigate pathophysiological mechanisms and protection strategies against AKI. The present study has utilized a simple strategy to correlate the expression levels of certain genes with the drug-induced kidney injury at various time points using two different routes of drug administration. We conclude that the degree of change in the expression profile is mainly dependent on the type of injury that can be categorized as ischemia-induced and nephrotoxicant-induced injuries. Furthermore, the route of drug administration also plays a role in regulating the expression levels of certain genes modulated in response to injury. These genes play central roles in several key cellular pathways. Targeting these pathways may turn out to be effective therapeutic option for kidney diseases.
Declaration of interest
The authors report no conflicts of interest.
References
- Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766
- Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58:680–689
- Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36:S187–S192
- Perazella MA. Drug-induced nephropathy: An update. Expert Opin Drug Saf. 2005;4:689–706
- Markowitz GS, Perazella MA. Drug-induced renal failure: A focus on tubulointerstitial disease. Clin Chem Acta. 2005;351:31–47
- Zager RA. Pathogenetic mechanisms in nephrotoxic acute renal failure. Semin Nephrol. 1997;17:3–14
- Supavekin S, Weijia Z, Raju K, et al. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003;63:1714–1724
- de Broe ME, Porter GA, eds. Clinical nephrotoxins. In: Renal Injury from Drugs and Chemicals. The Netherlands: Springer/Kluwer Acad Pub; 2009:29–42
- Vanmassenhove J, Raymond V, Evi N, et al. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273
- Sahu BD, Reddy KKR, Putcha UK, et al. Carnosic acid attenuates renal injury in an experimental model of rat cisplatin-induced nephrotoxicity. Food Chem Toxicol. 2011;49:3090–3097
- Vishal S, Vaidya M, Ferguson A, et al. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493
- Won KH, Veronique B, Rekha A, et al. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2004;62:237–244
- Takaharu I, Joseph VB, Véronique B, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142
- Zeisberg M, Amish AS, Raghu K. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. J Biol Chem. 2005;280:8094–8100
- Wetzel P, Haag J, Campean V, et al. Bone morphogenetic protein-7 expression and activity in the human adult normal kidney is predominantly localized to the distal nephron. Kidney Int. 2006;70:717–723
- Zeisberg M, Kalluri R. Reversal of experimental renal fibrosis by BMP7 provides insights into novel therapeutic strategies for chronic kidney disease. Pediatr Nephrol. 2008;23:1395–1398
- Tanaka M, Endo S, Okuda T, et al. Expression of BMP-7 and USAG-1 (a BMP antagonist) in kidney development and injury. Kidney Int. 2008;73:181–191
- Xue Q, Yu L, Xiao LW, et al. Osteopontin expression in progressive renal injury in remnant kidney: Role of angiotensin II. Kidney Int. 2000;58:1469–1480
- Florian TG, Jorge I, Zhuma H, et al. Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int. 2005;67:1772–1784
- Stokman G, Ingrid S, Nike C, et al. SDF-1 provides morphological and functional protection against renal ischemia/reperfusion injury. Nephrol Dial Transplant. 2010;25:3852–3859
- Kameswaran S, Sean PM, Theodore CS. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;282:F431–F441
- Edward J, Sharples N, Patel PB, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124
- deVries JE. Immunosuppressive and anti-inflammatory properties of interleukin 10. Ann Med. 1995;27:537–541
- Eppinger MJ, Ward PA, Bolling SF. Regulatory effects of interleukin-10 on lung ischemia–reperfusion injury. J Thorac Cardiovasc Surg. 1996;112:1301–1305
- Jiangping D, Yukimasa K, His C, et al. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128
- Tian W, Zhang Z, Cohen DM. MAPK signaling and the kidney. Am J Physiol Renal Physiol. 2000;279:F593–F604
- Masaki T, Rita F, Prudence A, et al. Activation of the ERK pathway precedes tubular proliferation in the obstructed rat kidney. Kidney Int. 2003;63:1256–1264
- Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637
- McNiece IK, Briddell RA. Stem cell factor. J Leukoc Biol. 1995;58:14–22
- Bengatta S, Arnould C, Letavernier E, et al. MMP9 and SCF protect from apoptosis in acute kidney injury. J Am Soc Nephrol. 2009;20:787–797
- Hosohata K, Ando H, Fujimura A. Urinary vanin-1 as a novel biomarker for early detection of drug-induced acute kidney injury. J Pharmacol Exp Ther. 2012;341:656–662
- Fry TJ, Mackall CL. Interleukin-7: From bench to clinic. Blood. 2002;99:3892–3904

