Abstract
Introduction: Computed tomography pulmonary angiography (CTPA) is currently an effective, reliable and widely employed diagnostic test for pulmonary thromboembolism (PT). PT harbors intrinsic clinical and biochemical abnormalities which may be associated with an increased risk of contrast-induced acute kidney injury (CIAKI). Objectives: To assess the incidence and risk factors of CIAKI among patients with PT diagnosed with CTPA. Methods: One hundred and twenty-two consecutive patients who had been diagnosed with PT using CTPA between February 2006 and December 2010 were evaluated retrospectively. In addition to the classical risk factors of CIAKI, arterial blood gases, CTPA and transthoracic echocardiography findings of the patients were also recorded. Results: The incidence of CIAKI was 13.1%. There were statistically important differences with respect to age, the presence of congestive heart failure (CHF), the use of angiotensin converting enzyme inhibitor-angiotensin II receptor blocker drugs (ACEI-ARB), the arterial blood pH (ABpH) and the length of hospitalization between the two groups of patients who developed (n:16) and did not develop (n:106) CIAKI. In the logistic regression analysis, age and ABpH were preserved in the final equation. Conclusion: The incidence of CIAKI among PT patients is significantly higher than the expected average. Older age, the presence of CHF, the use of ACEI-ARB, and additionally, low ABpH are important risk factors of CIAKI in patients with PT. Hypoxemia and low bicarbonate levels intrinsic to PT may contribute to the increased risk of CIAKI in this patient population and their correction may carry a prophylactic potential.
Introduction
Pulmonary thromboembolism (PT) is a frequent and potentially fatal condition with significant morbidity.Citation1,Citation2 Incidence of PT has been rising in recent years mainly due to both the increasing prevalence of the risk factors and the increasingly frequent implementation of the high-risk surgical procedures associated with it. Although the “gold standard” test to diagnose PT is pulmonary angiography, computed tomography pulmonary angiography (CTPA) has currently become an effective, reliable and widely employed diagnostic test for PT.Citation3
PT is characterized by important clinical and biochemical abnormalities. A significant cardiopulmonary alteration is the elevation of mean pulmonary artery pressures potentially causing circulatory compromise. However, a moderate to severe hypoxemia and hypocapnia with low plasma bicarbonate levels are among the prominent biochemical abnormalities occurring in PT.
Contrast-induced acute kidney injury (CIAKI) is a common cause of hospital-acquired acute kidney injury and an important cause of morbidity and mortality.Citation4 The exact pathogenesis of CIAKI is not completely uncovered yet. Nevertheless, main points of emphasis are renal ischemia particularly affecting the renal medulla and formation of reactive oxygen species causing tubular and endothelial cell damage. Knowing this, PT seems to harbor a couple of intrinsic clinical and biochemical abnormalities which may associate CIAKI development. Among them, a low cardiac output due to elevated pulmonary artery pressures, hypoxemia and low plasma bicarbonate are important to note. It may be proposed that, while low cardiac output and hypoxemia worsen renal medullary ischemia, low plasma bicarbonate contributes to formation of reactive oxygen species.
Although the incidence and the potential risk factors of CIAKI in selected patient populations (e.g. coronary angiography with or without coronary angioplasty) are well defined, there is only limited data with regard to CIAKI in PT patients.Citation5–8 In this retrospective study, we assessed the incidence and the potential risk factors of CIAKI among the patients with PT diagnosed using CTPA. We hypothesized that intrinsic clinical and laboratory abnormalities of PT may contribute to the development of CIAKI in this patient population.
Materials and methods
Patients and setting
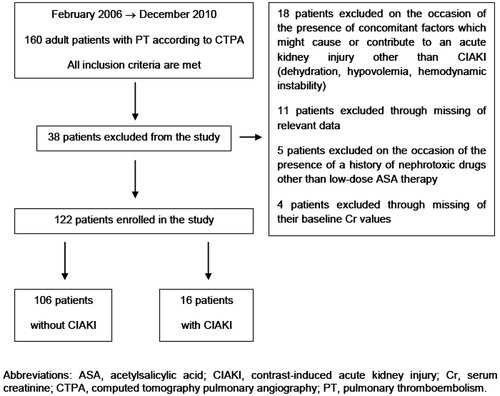
The study was conducted at Ufuk University School of Medicine (Dr. Rıdvan Ege Hospital) and encompassed the period between February 2006 and December 2010. The relevant patient data were retrieved with a systematic search for the diagnostic terms “pulmonary thromboembolism” and “pulmonary embolism” in Dr. Rıdvan Ege Hospital’s electronic database and every consecutive patient was included on a chronological basis. A total of 160 adult patients diagnosed with PT according to their CTPA findings were recruited. The flow diagram of the study is depicted in , while the eligibility criteria for the study are given in .
Table 1. The eligibility criteria for the study.
Data collected
The following data were collected and recorded for the study; (1) demographics (age, sex), (2) associated comorbidities [i.e., chronic kidney disease (CKD), diabetes mellitus (DM), diabetic nephropathy (DN), congestive heart failure (CHF), systemic hypertension (HT), coronary heart disease (CAD), cirrhosis, nephrotic syndrome (NS), multiple myeloma (MM)], (3) detailed survey of nephrotoxic drugs, (4) length of hospital stay, (5) need for renal replacement therapy, (6) mortality, (7) vital signs on admission [i.e., heart rate (HR), blood pressure (BP), respiratory rate (RR)], (8) baseline routine laboratory values [i.e., hemoglobin (Hb), hematocrit (Hct), urine density (UrD), urine pH (UrpH), urine protein (UrPr), blood urea nitrogen (BUN), serum creatinine (Cr), serum albumin (Alb)], (9) d-dimer level, (10) baseline arterial blood gas analysis (ABG) (i.e., pH, PaO2, PaCO2, , SaO2), (11) control Cr and BUN values (at 24, 48 and 72 h), (12) baseline and control estimated glomerular filtration rates [according to the “Modification of Diet in Renal Disease” (MDRD) formula] (MDRD-eGFR),Citation9 (13) volume and specifications of the contrast media used during CTPA, (14) CTPA findings (i.e., right/left lung involvement, size of the vessel(s) involved) and (15) transthoracic echocardiography (TTE) findings [i.e., ejection fraction (EF), mean pulmonary artery pressure (mPAP)].
Definition of CIAKI
CIAKI was defined as an elevation of Cr of more than 25% or ≥0.5 mg/dL (44 μmol/L) from baseline within 48 h following CTPA.Citation10,Citation11
CTPA protocol
In adult cases of suspected PT, the standardized protocol of CTPA included the administration of a 90-mL amount of iohexol, which is a non-ionic, low-osmolar, water soluble contrast agent. The preferred formulation of iohexol was iohexol 647 mg/mL (equivalent to 300 mg of iodine per milliliter) with an osmolality of 672 mOsm/kg.
With regard to the size of the vessel(s) involved, for the main and lobar arteries “large pulmonary artery” and for the segmental and subsegmental branches “small pulmonary artery” denotations were implemented.
CIAKI prophylaxis
All the CTPA procedures included in this study were performed as emergency imaging studies. During the study period, no routine pre- and/or post-procedure CIAKI prophylaxis was implemented in emergency contrast-enhanced imaging studies. Thus, none of the patients included in this study received a pre- and/or post-procedure CIAKI prophylaxis.
Statistical analysis
Data were analyzed using SPSS 17 (SPSS Statistics for Windows, Version 17.0, SPSS Inc., Chicago, IL). A p-value of less than 0.05 was considered to be statistically significant. For comparing the means of two independent groups, non-parametric Mann–Whitney U test was used, whereas comparison of the proportions of two independent groups was performed by chi-square (χCitation2) test. Finally, the defined potential risk factors of CIAKI were investigated using logistic regression analysis in order to determine the independent nature of each variable.
Results
During the period February 2006–December 2010, 160 patients were diagnosed as PT according to their CTPA findings. Among these 160 patients, 38 were excluded on the basis of the exclusion criteria and 122 were enrolled in the study ().
summarizes the demographics, basal renal function and classical CIAKI risk factors of the 122 patients. The average length of stay in hospital was 10.6 ± 8.1 days (mean ± SD) for these 122 patients and no mortalities were observed. Baseline ABG, TTE and CTPA findings of the study population which might be relevant to the development of CIAKI are summarized in .
Table 2. Demographics, basal renal function and classical CIAKI risk factors of the study population.
Table 3. Baseline ABG, transthoracic echocardiography, and CTPA findings of the study population.
During follow-up of the 122 patients enrolled in the study, 16 patients (13.1%) were found to develop CIAKI (CIAKI (+)), while the remaining 106 patients (86.9%) were free of this complication (CIAKI (−)) (). None of the patients in CIAKI (+) group required renal replacement therapy of any form.
Table 4. Comparison of CIAKI (+) and CIAKI (−) groups with respect to their demographics, renal functions and classical CIAKI risk factors.
The comparison of CIAKI (+) and CIAKI (−) groups with respect to their demographics, basal renal functions and classical CIAKI risk factors is summarized in . As can be seen from the table, the mean age of the patients in CIAKI (+) group was significantly higher than the mean age of the patients in CIAKI (−) group (79.5 ± 10.2 and 68.5 ± 13.8 years, respectively, p = 0.004). Also, CHF and angiotensin converting enzyme inhibitor-angiotensin II receptor blocker drugs (ACEI-ARB) use were more prevalent in CIAKI (+) group than CIAKI (−) group (CHF, 56.3% and 22.6% respectively, p = 0.012; ACEI-ARB use, 87.5% and 56.6%, respectively, p = 0.026). With respect to their average length of stay in hospital, CIAKI (+) group displayed a significantly longer duration compared to CIAKI (−) group (12.8 ± 3.22 and 6.8 ± 0.66 days, respectively, p < 0.05).
Results of the comparison of CIAKI (+) and CIAKI (−) groups with respect to their ABG, TTE and CTPA findings are given in . Patients in CIAKI (+) group demonstrated significantly lower ABG pH values than patients in CIAKI (−) group (7.40 ± 0.74 and 7.45 ± 0.55, respectively, p = 0.024). Additionally, although not reaching statistically significant levels, and
values were also lower in CIAKI (+) group with respect to CIAKI (−) group (
, 55.8 ± 17.4 and 67.8 ± 17.9, respectively, p = 0.062;
, 22.9 ± 4.5 and 24.2 ± 4.0, respectively, p = 0.347).
Table 5. Comparison of CIAKI (+) and CIAKI (−) groups with respect to their baseline ABG, transthoracic echocardiography, and CTPA findings.
The four identified potential risk factors for CIAKI (i.e., age, presence of CHF, ACEI-ARB use and ABG pH) were investigated using logistic regression. While age (p = 0.024) and ABG pH (p = 0.027) were shown to have the greatest impact on the development of CIAKI, presence of CHF and ACEI-ARB use (p > 0.05 for both) were excluded from the final logistic regression equation.
Discussion
CIAKI is a frequent cause of hospital-acquired acute kidney injury with significant morbidity and mortality. Although the clinical characteristics and major risk factors of CIAKI are well-defined, its exact pathogenesis is yet not completely understood.Citation12,Citation13 Main points of emphasis with regard to CIAKI development are renal ischemia resulting in severe hypoxia (particularly in the renal medulla), formation of reactive oxygen species (ROS) causing tubular and endothelial cell damage and direct cytotoxic effect of the iodinated contrast media on endothelial and tubular cells.Citation14–17
PT harbors a couple of intrinsic clinical and biochemical abnormalities which may be associated with an increased risk of CIAKI. Among them, right heart failure and low cardiac output state due to elevated mean pulmonary artery pressures, hypoxemia and low plasma bicarbonate levels are noteworthy.Citation18,Citation19 The present study not only documented an increased incidence of CIAKI among patients with PT but also pointed to a new potential risk factor [i.e., low arterial blood pH (ABpH)] of CIAKI in this patient population. The findings are in accordance with our hypothesis stating that intrinsic clinical and laboratory abnormalities of PT may contribute to the development of CIAKI.
The incidence of CIAKI shows great variation depending on patient, procedure and contrast media characteristics, and any prophylactic measures implemented. With respect to the frequency of CIAKI among the patients with PT, data present in the literature are scarce. Among the four studies reviewing CIAKI following CTPA, three pointed to an increased incidence (12–25.8%),Citation5,Citation7,Citation8 whereas only one reported an incidence (8.9%)Citation6 comparable to the range reported in the literature. Important to note, these studies were analyzing all the patients undergoing CTPA for suspected PT, without taking into account the definite diagnoses of the patients.Citation5–8 In our study, “a definite diagnosis of PT with CTPA” was an eligibility criteria and the CIAKI incidence of 13.1% was well above the incidences in the current literature. Moos et al. performed a meta-analysis (time period between 2002 and 2012, 42 articles with 18,790 patients) searching for the incidence and the risk factors of CIAKI in patients undergoing intravenous contrast enhanced computed tomography (CECT).Citation20 The mean incidence found in this meta-analysis was 4.96%.Citation20 In another study, Lee et al. evaluated the prevalence of the risk factors and the actual occurrence of CIAKI in patients undergoing CECT.Citation21 In this multi-institutional study encompassing 140,838 examinations in 101,487 patients, the incidence of CIAKI was reported to be 2.2%.Citation21 The incidence figures revealed in these two large studies may give an approximate estimate of CIAKI incidence for CECT and hence for CTPA.
As previously stated, the classical risk factors of CIAKI are well-defined and reviewed elsewhere.Citation22 However, with few exceptions, most of these studies used to define the risk factors of CIAKI were carried out on specific patient populations (e.g., patients undergoing coronary angiography with or without coronary angioplasty). As such, bulk of CIAKI data is derived from the cardiovascular literature and the patients in these studies display a characteristic profile of comorbidities which clearly puts them at risk for CIAKI.Citation23 Knowing this, it is imperative to design and implement similar studies on different patient populations, as in this case in patients with PT.Citation24
With regard to risk factors of CIAKI among patients with PT diagnosed with CTPA, the aforementioned four studies mainly pointed to the classical risk factors already depicted in the literature.Citation5–8 The risk factors which stood out in these studies were age (older age), DM, use of NSAIDs, and MM.Citation6,Citation8 The present study, which specifically put under the scope the patients with a definite diagnosis of PT, identified four risk factors, namely, age (older age), CHF, ACEI-ARB use and low ABG pH. While older age, CHF and ACEI-ARB use are among the well-known classical risk factors of CIAKI, low ABG pH is a novel risk factor. Furthermore, although not reaching levels of statistical significance, our study pointed to three additional factors which may also have an impact on the development of CIAKI in patients with PT. These three factors were hypoxemia, low ABG and high mean pulmonary artery pressure.
Previously, plasma pH has not been emphasized as a risk factor of CIAKI. Nevertheless, administration of sodium bicarbonate, which apparently induces both plasma and urinary pH changes, has been an important theme for the prophylaxis of CIAKI.Citation25–27 According to the findings of our study, CIAKI (+) group demonstrated a significantly lower ABG pH with respect to CIAKI (−) group (7.40 ± 0.74 vs. 7.45 ± 0.55, respectively, p = 0.024). As the iron-catalyzed Haber–Weiss reaction responsible for the generation of toxic ROS is known to occur much more actively at lower pH levels, the lower ABG pH observed in CIAKI (+) group may result in more abundant production of ROS in this patient group.Citation28 As previously stated, ROS-mediated tubular and endothelial cell damage is an important component of CIAKI pathogenesis.Citation16,Citation17
Hypoxemia is a serious concern in PT. Impairment of oxygenation is apparent in nearly all patients.Citation18 Accordingly, our study population had a mean value of 66.5 ± 18.1 mmHg. In CIAKI (+) group, mean
value dropped to 55.8 ± 17.4 mmHg which is compatible with a moderate degree of hypoxemia. It is rational to think that when compounded by renal ischemia this level of hypoxemia will result in severe renal medullary hypoxia. As is well known, renal medullary hypoxia is a central theme in the pathogenesis of CIAKI.Citation12–15
With respect to the use of bicarbonate for the prophylaxis of CIAKI, the majority of evidence in the literature points to a protective effect.Citation29–32 Even though the mechanisms for this protection are not clearly established, there are some important points frequently mentioned. First, administration of sodium bicarbonate produces a similar effect to systemic and tubular volume expansion which follows physiological sodium chloride solution administration. Hence, increased tubular flow rate results in both a decreased tubular concentration of the contrast agent and a mild increase of tubular pH, both of which seem to generate a protective effect.Citation25,Citation33 With sodium bicarbonate, there are at least two additional prominent protective effects. Initially, by increasing tubular pH, sodium bicarbonate administration decreases the generation of ROS mediated by the Haber–Weiss reaction.Citation25,Citation33 Second, increased tubular bicarbonate concentration provides an increased scavenging capacity for ROS formed in the tubules.Citation33 A well-designed experimental study by Barlak et al. have yielded important findings consistent with the protective effect of bicarbonate in CIAKI through mechanisms acting against ROS-mediated oxidative injury.Citation34 Putting these effects together, it is possible that the higher ABG levels observed in CIAKI (−) group may render protection against CIAKI.
Finally, with regard to cardiovascular parameters, there were some noteworthy findings in our study. Though not reaching a statistically significant level, the comparison of CIAKI (+) group with respect to CIAKI (−) group documented a higher mean pulmonary artery pressure (44.0 ± 14.1 mmHg and 40.7 ± 10.0 mmHg, respectively, p = 0.326) and a lower ejection fraction (50.0 ± 13.4% and 56.0 ± 10.7%, respectively, p = 0.077). Both of these findings, which are compatible with a lower cardiac output, may contribute to the renal medullary ischemia for a more severe hypoxia and hence carry a greater risk for CIAKI.
According to the findings of our study, the incidence of CIAKI following CTPA in patients with PT is substantially higher with respect to other patient populations (13% vs. 5%, respectively). Low ABpH is a novel risk factor for CIAKI during PT. Intrinsic abnormalities of PT, namely, hypoxemia, low bicarbonate levels, and decline in cardiac function may contribute to the increased risk of CIAKI observed in this patient population and their correction may carry a prophylactic potential. In addition to the substantial amount of data present in the literature, the findings in this study provide additional evidence for the prophylactic role of sodium bicarbonate in CIAKI.
As a concluding remark, large and prospectively designed studies are clearly needed to validate the findings of our study. If validated through carefully designed rigorous studies, we believe that the findings presented in this article will carry important implications for CIAKI in general and specifically in PT. In the interim, in cases of suspected PT undergoing CTPA, bicarbonate administration, effective oxygen supplementation, and the correction of any hemodynamic compromise, may prove to be instrumental against the development of CIAKI.
Declaration of interest
The authors declare no potential conflicts of interests with respect to the authorship and/or publication of this article. No financial support was received for the research and/or authorship of this article.
References
- Nielsen JD. The incidence of pulmonary embolism during deep vein thrombosis. Phlebology. 2013;28:29–33
- Dalen JE. Pulmonary embolism: What have we learned since Virchow? Natural history, pathophysiology, and diagnosis. Chest. 2002;122:1440–1456
- Russo V, Piva T, Lovato L, Fattori R, Gavelli G. Multidetector CT. A new gold standard in the diagnosis of pulmonary embolism? State of the art and diagnostic algorithms. Radiol Med. 2005;109:49–61
- Solomon R, Dauerman HL. Contrast-induced acute kidney injury. Circulation. 2010;122:2451–2455
- Mitchell AM, Kline JA. Contrast nephropathy following computed tomography angiography of the chest for pulmonary embolism in the emergency department. J Thromb Hemost. 2007;5:50–54
- Kooiman J, Klok FA, Mos IC, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Hemost. 2010;8:409–411
- Mitchell AM, Jones AE, Tumlin JA, Kline JA. Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med. 2012;19:618–625
- Reagle Z, Tringali S, Gill N, Peterson MW. Diagnostic yield and renal complications after computed tomography pulmonary angiograms performed in a community-based academic hospital. J Community Hosp Intern Med Perspect. 2012;2. doi: 10.3402/jchimp.v2i2.17722
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470
- Solomon R. Contrast-medium-induced acute renal failure. Kidney Int. 1998;53:230–242
- Goldenberg I, Matetzky S. Nephropathy induced by contrast media: Pathogenesis, risk factors and preventive strategies. CMAJ. 2005;172:1461–1471
- Feldkamp T, Kribben A. Contrast media induced nephropathy: Definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177–196
- Ortega LM, Harmouch I, Nayer A. Contrast-induced nephropathy: Pathogenesis and new therapeutic options for prevention. Am J Ther. 2014. [Epub ahead of print]. doi: 10.1097/MJT.0000000000000163
- Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Sci World J. 2014;2014:823169
- Heyman SN, Rosen S, Rosenberger C. Renal parenchymal hypoxia, hypoxia adaptation, and the pathogenesis of radiocontrast nephropathy. Clin J Am Soc Nephrol. 2008;3:288–296
- Pisani A, Riccio E, Andreucci M, et al. Role of reactive oxygen species in pathogenesis of radiocontrast-induced nephropathy. Biomed Res Int. 2013;2013:868321
- Caiazza A, Russo L, Sabbatini M, Russo D. Hemodynamic and tubular changes induced by contrast media. Biomed Res Int. 2014; 2014:578974
- Sasahara AA, Cannilla JE, Morse RL, Sidd JJ, Tremblay GM. Clinical and physiologic studies in pulmonary thromboembolism. Am J Cardiol. 1967;20:10–20
- Ferrer A, Rodriguez-Roisin R. Ventilation-perfusion distributions in disease. In: Hamid Q, Shannon J, Martin J, eds. Physiologic Basis of Respiratory Disease. Lewiston, NY: BC Decker Inc.; 2005:185–202
- Moos SI, van Vemde DN, Stoker J, Bipat S. Contrast induced nephropathy in patients undergoing intravenous (IV) contrast enhanced computed tomography (CECT) and the relationship with risk factors: A meta-analysis. Eur J Radiol. 2013;82:e387–e399
- Lee J, Cho JY, Lee HJ, et al.; Korean Society of Urogenital Radiology, Korean Society of Radiology. Contrast-induced nephropathy in patients undergoing intravenous contrast-enhanced computed tomography in Korea: A multi-institutional study in 101487 patients. Korean J Radiol. 2014;15:456–463
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399
- McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008;51:1419–1428
- Prasad V, Gandhi D, Stokum C, Miller T, Jindal G. Incidence of contrast material-induced nephropathy after neuroendovascular procedures. Radiology. 2014;273:853–858
- Merten GJ, Burgess WP, Gray LV, et al. Prevention of contrast-induced nephropathy with sodium bicarbonate. A randomized controlled trial. JAMA. 2004;291:2328–2334
- Ozcan EE, Guneri S, Akdeniz B, et al. Sodium bicarbonate, N-acetylcysteine, and saline for prevention of radiocontrast-induced nephropathy. A comparision of 3 regimens for protecting contrast-induced nephropathy in patients undergoing coronary procedures. A single-center prospective controlled trial. Am Heart J. 2007;154:539–544
- Recio-Mayoral A, Chaparro M, Prado B, et al. The reno-protective effect of hydration with sodium bicarbonate plus N-acetylcysteine in patients undergoing emergency percutaneous coronary intervention. The RENO Study. J Am Coll Cardiol. 2007;49:1283–1288
- Kehrer JP. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology. 2000;149:43–50
- Navaneethan SD, Singh S, Appasamy S, Wing RE, Sehqal AR. Sodium bicarbonate therapy for prevention of contrast-induced nephropathy: A systematic review and meta-analysis. Am J Kidney Dis. 2009;53:617–627
- Meier P, Ko DT, Tamura A, Tamhane U, Gurm HS. Sodium bicarbonate-based hydration prevents contrast-induced nephropathy: A meta-analysis. BMC Med. 2009;7:23
- Jang JS, Jin HY, Seo JS, et al. Sodium bicarbonate therapy for the prevention of contrast-induced acute kidney injury – A systematic review and meta-analysis. Circ J. 2012;76:2255–2265
- Dabare D, Banihani M, Gibbs P, Grewal P. Does bicarbonate prevent contrast-induced nephropathy in cardiovascular patients undergoing contrast imaging? Interact Cardiovasc Thorac Surg. 2013;17:1028–1035
- Burgess WP, Walker PJ. Mechanisms of contrast-induced nephropathy reduction for saline (NaCl) and sodium bicarbonate (NaHCO3). Biomed Res Int. 2014;2014:510385
- Barlak A, Akar H, Yenicerioglu Y, Yenisey C, Meteoğlu I, Yilmaz O. Effect of sodium bicarbonate in an experimental model of radiocontrast nephropathy. Ren Fail. 2010;32(8):992–999