Abstract
Background: It has been shown that single nucleotide polymorphisms (SNPs) near the interleukin 28B (IL28B) gene were associated with sustained virological response following standard antivirological treatment of chronic hepatitis C. Objectives: The aim of the study was to evaluate the association between SNPs near the IL28B gene and response to the treatment of chronic hepatitis C in hemodialysis patients. Patients and methods: The study group included 24 hemodialysis patients with chronic hepatitis C routinely treated with pegylated interferon α-2 a. HCV genotype 1 was the cause of chronic hepatitis C in all study participants. Sustained virological response was determined by an assay with a sensitivity of 20 IU/mL, 6 months after completion of the antivirological treatment. The genotyping of the three most widely studied IL28B gene polymorphisms (rs12979860, rs8099917, and rs12980275) was performed in all study participants. Results: Sustained virological response was achieved in 50% of the treated patients. The treatment response was significantly associated with the CC genotype of rs12979860, TT genotype of rs8099917, and AA genotype of rs12980275 (p = 0.003, p = 0.009, and p = 0.012, respectively). Conclusions: The three most widely studied SNPs near the IL28B gene were associated with sustained virological response following antivirological treatment of chronic hepatitis C in hemodialysis patients.
Introduction
Hepatitis C virus (HCV) infection is prevalent among patients on maintenance hemodialysis, both in developed and less-developed countries. HCV infection is the most common cause of liver disease in hemodialysis patients.Citation1,Citation2 HCV-infected patients on maintenance dialysis have impaired health-related quality of life, increased liver disease-related mortality risk, and a higher cardiovascular mortality risk.Citation3,Citation4 The most important reasons for conducting antivirological treatment in the HCV-infected hemodialysis patients are: to slow the progression of the liver disease and to prevent transmission of the virus within the dialysis unit.
The HCV infection treatment is strongly recommended in renal transplant candidates because interferon-based antivirological regimen is contraindicated after renal transplantation due to the increased risk of graft dysfunction.Citation5,Citation6 HCV eradication before renal transplantation improves patient and graft survival, reduces the occurrence of chronic graft nephropathy, and HCV-associated glomerulonephritis.Citation7,Citation8
Monotherapy with a reduced dose of pegylated interferon α (PEGIFNα) is a standard treatment of chronic hepatitis C in hemodialysis patients. Ribavirin (RBV) was considered to be contraindicated in hemodialysis patients because of the possible development of hemolytic anemia.Citation9,Citation10 However, several studies reported the use of low-dose ribavirin in combination with interferon, carefully controlling for anemia and monitoring serum concentration of the drug during treatment in hemodialysis patients.Citation11–13
The primary goal of the antivirological treatment is eradication of the HCV infection, which is synonymous with sustained virological response (SVR). The antivirological treatment is prolonged and expensive, complicated by frequent and sometimes serious side effects resulting in treatment discontinuation, and about one-third of the treated hemodialysis patients achieve sustained virological response.Citation14–18 The HCV genotype 1 is the most common genotype and one of the most difficult to treat despite the need for 48 weeks of treatment.Citation19 For all these reasons, it is very important to have a possibility to predict which patients are more likely to respond to antivirological therapy before starting the treatment of chronic hepatitis C. Host genetic factors may predict the outcome of the HCV infection treatment. Four genome wide association studies (GWAS) had independently linked treatment-induced HCV clearance following PEGIFN/RBV therapy with the single nucleotide polymorphisms (SNPs) near the interleukin 28B (IL28B) gene on chromosome 19.Citation20–23 The IL28B gene encodes interferon lambda 3 (IFNλ3), a type III interferon, recognized as the predominant interferon produced in humans during HCV infection with antivirological, anti-proliferative, and immune-modulatory activities.Citation24,Citation25 The GWAS showed that favorable genotypes of SNPs near the IL28B gene (CC for rs12979860, TT for rs8099917, and AA for rs12980275) were associated with SVR in patients with chronic hepatitis C treated with PEGIFN/RBV. The favorable CC genotype of rs12979860 may affect treatment outcome through improved innate immune response to IFN therapy and more rapid virological kinetics.Citation26
The aim of the study was to evaluate the association between single nucleotide polymorphisms near the IL28B gene and response to treatment of chronic hepatitis C in hemodialysis patients.
Patients and methods
Patients
A group of 24 hemodialysis patients with chronic hepatitis C routinely treated with antivirological therapy was investigated in the study. All patients were Caucasians. The study was approved by the local Ethics Committee and written informed consent was obtained from each participant. The patients were on maintenance hemodialysis (HD) program with dialysis sessions of four to five hours, three times per week, on high-flux synthetic membranes and bicarbonate bath. HCV genotype 1 was the cause of chronic hepatitis C in all study participants. The patients received pegylated interferon α-2a (PEG IFNα-2a) given as monotherapy. It was administered subcutaneously, once a week, after dialysis session, in a reduced dose of 135 μg. The treatment duration was 48 weeks. All patients finished the antivirological treatment at least 6 months before enrollment in the study. Sustained virological response, defined as an absence of detectable HCV RNA in the serum, was tested by an assay with a sensitivity of at least 50 IU/mL, 6 months after completion of the antivirological treatment.Citation27 The demographic data and etiology of renal disease of the hemodialysis patients are presented in .
Table 1. The demographic data and etiology of renal disease of the hemodialysis patients.
Methods
Peripheral blood samples in EDTA as an anticoagulant were obtained from each patient enrolled in the study.
HCV quantification in plasma
Hepatitis C virus RNA was extracted from plasma using QIAamp Virological RNA kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. A reverse transcriptase-polymerase chain reaction (RT-PCR) assay for HCV quantification was done with the HCV Real-TM Quant (Sacace Biotechnologies, Como, Italy) on Stratagene MX3005P real-time PCR system (Agilent Technologies, Edinburgh, UK) according to manufacturer’s instructions. Detection limit of the assay was 20 IU/mL.
IL28B genotyping
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation using Histopaque-1077 (Sigma-Aldrich, Munich, Germany) and homogenized in Tri Reagent Solution (Ambion, Life Technologies, Carlsbad, CA). Genomic DNA was extracted from PBMCs homogenized in Tri Reagent Solution (Ambion) according to manufacturer’s instructions and genotyped for three IL28B polymorphisms: rs8099917, rs12979860, and rs12980275. The rs8099917 polymorphism was genotyped using TaqMan predesigned SNP genotyping assay (reference C_11710096_10, Applied Biosystems) according manufacturer’s recommended protocol in a total volume of 25 µL. The two latter SNPs were genotyped using custom-designed TaqMan assays with the following primes and probes: TCTACTGAACCAGGGAGCTC, GCGCGGAGTGCAATTCAAC, 6Fam-TGGTTCACGCCTTC, Vic-TGGTTCGCGCCTTC for rs12979860, and GTGCTGAGAGAAGTCAAATTCC, CCGCTACCCGGCAAATATT, 6Fam-ACACGTCCGTTTCTA, Vic-AGACACGTCTGTTTCTA for rs12980275.Citation28 For both assays, 20 ng of DNA was used in a total volume of 25 µL including 12.5 µL TaqMan Universal PCR master Mix (2×), 1 µM of each primer and 200 nM of each probe. The PCR reaction conditions were as follows: initial denaturing at 95 °C for 10 min; 40 cycles of 15 s at 92 °C and 1 min at 64 °C to reduce miss priming. Thermal cycling was performed using a Stratagene MX3005P real-time PCR system (Agilent Technologies). Both positive and negative controls were included in every genotyping assay.
Statistical analysis
A statistical analysis of the data was performed using SPSS Statistics 17.0 (Chicago, IL). The parametric variables are presented as the mean and standard deviation. Fisher’s exact test was used to compare proportions. A p value of less than or equal to 0.05 was considered statistically significant.
Results
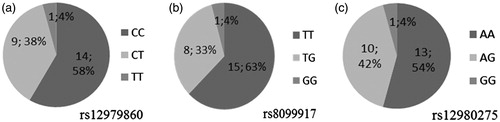
Sustained virological response was achieved in 50% (12/24) of the treated patients, confirmed by RT-PCR assay with detection limit of 20 IU/mL. The distribution of the frequencies of rs12979860 genotypes in the study group was as follows: 14 (58%) patients with CC genotype, 9 (38%) patients with CT genotype, and 1 (4%) patients with TT genotype (). The distribution of the frequencies of rs8099917 genotypes was: 15 (63%) patients with TT genotype, 8 (33%) patients with TG genotype, and 1 (4%) patients with GG genotype (). The distribution of the frequencies of rs12980275 genotypes was: 13 (54%) patients with AA genotype, 10 (42%) patients with AG genotype, and 1 (4%) patients with GG genotype ().
Figure 1. Distribution of the frequencies of rs12979860 (a), rs8099917 (b), and rs12980275 (c) genotypes in the study participants.
The distribution of genotypes of the three SNPs near the IL28B gene (rs12979860, rs8099917, and rs12980275) was significantly different between the patients with SVR and those without SVR. The achievement of SVR was significantly higher in the HD patients with CC genotype of rs12979860 than in the HD patients with non CC genotypes (91.7% vs. 8.3%, p = 0.003) (). The SVR was achieved in significantly more patients with TT genotype of rs8099917 than in patients with non-TT genotypes (91.7% vs. 8.3%, p = 0.009) (). There was also a significant association between SVR and rs12980275: it was achieved in 83.3% of the patients with AA genotype versus 16.7% of the patients with non AA genotypes (p = 0.012) ().
Table 2. Sustained virological response and the SNPs near IL28B gene in HD patients.
Disscusion
For the first time, the three most widely studied SNPs near the IL28B gene were identified in hemodialysis patients to evaluate their association with sustained virological response after HCV infection treatment. The homozygosis for the C allele of rs12979860, the homozygosis for the T allele of rs8099917, and the homozygosis for the A allele of rs12980275 were genotypes associated with achievement of sustained virological response. Four genome wide association studies identified that the same genotypes of SNPs near the IL28B gene predicted favorable response to HCV infection treatment also in patients without renal disease.Citation20–23
Most of the studies investigated the effect of the SNPs near the IL28B gene on sustained virological response in patients with HCV genotype 1.Citation20–22 All of the treated patients in our study were infected with HCV genotype 1. They were renal transplant candidates treated only with PEGIFNα-2a because of a concern for development of ribavirin-induced hemolytic anemia. The treatment response rate was 50%. The same treatment response rate of 50% was reported by Kose et al. PEG IFNα-2a as a monotherapy (135 μg/weekly) was given to 38 hemodialysis patients with chronic hepatitis C for 48 weeks.Citation29
There was no other published study about the association between the IL28B gene polymorphisms and response to HCV infection treatment in hemodialysis patients. Yu et al. identified association of one polymorphism (rs8099917) and HCV infection clearance in hemodialysis patients, but the clearance of HCV infection was spontaneous, and not treatment induced as it was in our study.Citation30 Consistent to our findings, but in patients without renal disease, were the results from the study of Domagalski et al.Citation31 The predictability of the three most widely studied SNPs on the treatment response was determined in a cohort of 174 Caucasian (Polish) patients infected with HCV genotype 1 (81%) and 4 (19%), treated with PEGIFN/RBV. The CC genotype of rs12979860, TT genotype of rs8099917, and AA genotype of rs12980275 were significantly associated with the successful treatment (p = 0.001, p = 0.016, and p = 0.002, respectively). Similar associations between SVR and IL28B gene polymorphisms were evaluated in the studies of Sporea et al.Citation32 and Tolmane et al.,Citation33 although they identified only one polymorphism (rs12979860). The study of Sporea I et al. included a cohort of 107 Caucasian (Romanian) patients infected with HCV genotype 1 treated with PEGIFN/RBV.Citation32 The SVR rate was 50.5% and there was a significant association between the SVR and CC genotype of rs12979860 (p = 0.012). The study of Tolmane I et al. included 142 Caucasian (Latvian) patients infected with HCV genotype 1 (61%) and HCV genotype 2 or 3 (39%) treated with PEGIFN/RBV.Citation33 The SVR rate was 59%, and it was significantly associated with the CC genotype of rs12979860 (p = 0.002).
The number of study participants is a limitation of the study. The treatment of chronic hepatitis C in hemodialysis patients is associated with poor tolerability and suboptimal efficacy, and it is mainly recommended for renal transplant candidates. Recent studies of hepatitis C treatment in hemodialysis patients, with PEG IFNα-2a, included between 13 and 40 patients.Citation29,Citation34–36
However, it has to be taken into consideration that roughly 20% of the treated patients with favorable CC genotype of rs12979860 did not achieve SVR,Citation37 due to dynamic interactions between genes or between genes and environment.Citation38 Other factors such as ethnicity and HCV genotype should be used together with IL28B genotype as pretreatment predictors for response.
Conclusion
The benefits from a successful antivirological treatment in hemodialysis patients include lowering the liver disease associated complications, preventing nosocomial transmission of the virus within the dialysis unit and improving patient survival during maintenance hemodialysis and after renal transplantation. However, the interferon based therapy can be associated with poor tolerability and lower efficacy. Since the host genetic factors have been proven to affect treatment response rates, their assessment may be necessary for optimal management and therapeutic decisions. IL28B gene polymorphisms can predict which hemodialysis patients are more likely to respond to treatment, suggesting the possibility of personalized medicine for the treatment of HCV infection.
Increased response rates and shorter duration of treatment are the characteristics of the new antivirological drugs. It appears that the IL28B gene polymorphisms will maintain their pretreatment prognostic importance, since interferon remains the backbone of the new antivirological therapy, preventing development of resistance.
Acknowledgements
Authors wish to thank Mihaela Codreanu from Agence Universitaire de la Francophinie (AUF), for her administrative support in organization and realization of the regional multicenter collaboration.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the Agence Universitaire de la Francophinie (AUF). Project Title: Facteurs génétiques prédictifs de la réponse virale sous traitement par interféron pégylé chez des patients infectés par infection le virus de l'hépatite C (VHC), traités ou non par dialyse.
References
- Jadoul M, Barril G. Hepatitis C in hemodialysis: Epidemiology and prevention of hepatitis C virus transmission. Contrib Nephrol. 2012;176:35–41
- Fissell R, Bragg-Gresham J, Woods J, et al. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004;65:2335–2342
- Fabrizi F, Messa P, Martin P. Health-related quality of life in dialysis patients with HCV infection. Int J Artif Organs. 2009;32:473–481
- Fabrizi F, Dixit V, Messa P. Impact of hepatitis C on survival in dialysis patients: A link with cardiovascular mortality? J Viral Hepat. 2012;19:601–607
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–S155
- Carvalho-Filho RJ, Feldner ACCA, Silva AEB, Ferraz MLG. Management of hepatitis C in patients with chronic kidney disease. World J Gastroenterol. 2015;21(2):408–422
- Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11(3):172--182
- Scott DR, Wong JK, Spicer TS, et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90:1165–1171
- Yu YC, Wang Y, He CL, Wang MR, Wang YM. Management of hepatitis C virus infection in hemodialysis patients. World J Hepatol. 2014;6(6):419–425
- Kidney disease: Improving global outcomes. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation and treatment of hepatitis C in chronic kidney disease. Kidney Int. 2008;73(Suppl 109):S1–S99
- Dammacco F, Tucci FA, Lauletta G, et al. Pegylated interferon-alpha, ribavirin, and rituximab combined therapy of hepatitis C virus-related mixed cryoglobulinemia: A long-term study. Blood. 2010;116:343–353
- Liu C, Huang C, Liu C, et al. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: A randomized trial. Ann Intern Med. 2013;159:729–738
- Fabrizi F, Dixit V, Messa P, Martin P. Antivirological therapy (pegylated interferon plus ribavirin) of hepatitis C in dialysis patients: Meta-analysis of clinical studies. J Viral Hepat. 2014;21:681–689
- Kikuchi K, Akiba T, Nitta K, et al. Multicenter study of pegylated interferon α-2a monotherapy for hepatitis C virus-infected patients on hemodialysis: REACH study. Ther Apher Dial. 2014;18(6):603–611
- Fabrizio F, Messa P, Martin P. Recent advances on hepatitis C virus in dialysis population. Kidney Blood Press Res. 2014;39:260–271
- Carvalho-Filho RJ, Dalgard O. Individualized treatment of chronic hepatitis C with pegylated interferon and ribavirin. Pharmgenomics Pers Med. 2010;3:1–13
- Sulkowski MS, Cooper C, Hunyady B, et al. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011;8:212–223
- Sikole A, Dzekova P, Selja N, et al. Treatment of hepatitis C in hemodialysis patients with pegylated interferon alpha-2a as monotherapy. Ren Fail. 2007;29(8):961–966
- Hayes CN, Imamura M, Aikata H, Chayama K. Genetics of IL28B and HCV response to infection and treatment. Nat Rev Gastroenterol Hepatol. 2012;9:406–417
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced virological clearance. Nature. 2009;461:399–401
- Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104
- Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109
- Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: A genome-wide association study. Gastroenterology. 2010;138:1338–1345
- Marcello T, Grakoui A, Barba-Spaeth G, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898
- Thomas E, Gonzalez VD, Li Q, et al. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142(4):978–988
- Naggie S, Osinusi A, Katsounas A, et al. Dysregulation of innate immunity in hepatitis C virus genotype 1 IL28B-unfavorable genotype patients: Impaired virological kinetics and therapeutic response. Hepatology. 2012;56:444–454
- Pearlman BL, Traub N. Sustained virological response to antivirological therapy for chronic hepatitis C virus infection: A cure and so much more. Clin Infect Dis. 2011;52:889–900
- Lagging M, Askarieh G, Negro F, et al. Response prediction in chronic hepatitis C by assessment of IP-10 and IL28B-related single nucleotide polymorphisms. PLoS One. 2011;6(2):e17232
- Köse S, Senger SS, Ersan G, Cavdar G. Virological responses of pegylated interferon alpha-2a treatment in hemodialysis patients infected with hepatitis C. Clin Exp Nephrol. 2013;17:115–119
- Yu ML, Dai CY, Huang CF, et al. High hepatitis B virus surface antigen levels and favorable interleukin 28B genotype predict spontaneous hepatitis C virus clearance in uremic patients. J Hepatol. 2014;60(2):253–259
- Domagalski K, Pawlowska M, Tretyn A, et al. Association of IL28B polymorphisms with the response to peginterferon plus ribavirin combined therapy in Polish patients infected with HCV genotype 1 and 4. Hepat Mon. 2013;13(11):e13678
- Sporea I, Popescu A, Curescu M, et al. The correlation of Il28B genotype with sustained virological response in Romanian patients with chronic hepatitis C. Hepat Mon. 2011;11(12):975–979
- Tolmane I, Rozentale B, Keiss J, et al. Interleukin 28B gene polymorphism and association with chronic hepatitis C therapy results in Latvia. Hepat Res Treat. 2012;2012:ID324090
- Alsaran K, Sabry A, Shaheen N. Pegylated interferon alpha-2a for treatment of chronic HCV infection in hemodialysis patients: A single Saudi center experience. Int Urol Nephrol. 2011;43:865–873
- Khedmat H, Amini M, Ghamar-Chehreh ME, Agah S. Hepatitis C virus infection in dialysis patients. Saudi J Kidney Dis Transpl. 2014;25(1):1–8
- Ahn SB, Jun DW, Kim SG, et al. Efficacy and safety of pegylated interferon base treatment in patients with chronic hepatitis C on dialysis. Eur J Intern Med. 2015;26(4):292–296
- Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009, 461:798–801
- Hardy J, Singleton A. Genome wide association studies and human disease. N Engl J Med. 2009;360:1759–1768

