Abstract
The hepatoprotective and nephroprotective activity of a polyphenol-rich fraction (BHPF) obtained from Bauhinia hookeri was investigated against CCl4-induced acute hepatorenal toxicity in mice. BHPF was administered (100, 200 and 400 mg/kg/day) for 5 days, then CCl4 was administered. BHPF pretreatment significantly (p < 0.001) inhibited the CCl4-induced increase in ALT, AST, ALP, LDH, total bilirubin, cholesterol, creatinine, uric acid, urea and malondialdehyde in a dose-dependent manner. In contrast, BHPF pretreatment markedly increased the contents of glutathione and superoxide dismutase in the liver and kidney tissues, indicating the strong in vivo antioxidant activity of BHPF. Pretreatment with BHPF preserved the hepatic architecture and conferred marked protection against necrosis and ballooning degeneration. Pretreatment with BHPF reduced the inflammatory cell aggregation and degenerative changes in the lining epithelium of the kidney tubules. It can be concluded that BHPF has a remarkable hepato- and nephroprotective activity by enhancing the antioxidant defense status, reducing lipid peroxidation and protecting against the histopathological changes induced by CCl4 in the liver and kidney tissues.
Introduction
The kidney is the main organ involved in the excretion of xenobiotics.Citation1,Citation2 The liver is the primary organ of metabolism; therefore, the toxic effects of chemicals usually appear primarily in the liver and kidney tissues.Citation3,Citation4 Oxidative stress has a crucial role in many diseases including renal failure, liver damage, atherosclerosis, inflammation and carcinogenesis.Citation5,Citation6 Oxidative stress is caused by cellular excess of reactive oxygen species (ROS). Lipid peroxidation of cell membranes induces cell injury.Citation7–9 Under normal homeostasis, the cells maintain the ROS levels with endogenous non-enzymatic and enzymatic-antioxidants.Citation10–12 The risk of cell injury may also be prevented by natural antioxidants including dietary polyphenols. Dietary polyphenolic compounds have received a great deal of attention because of their beneficial effects on health, including protection against oxidative stress and degenerative diseases.Citation13 Dietary polyphenolic compounds are known to restore the balance between the natural antioxidants and free radicals by direct scavenging of ROS and by enhancing the activity of natural antioxidant enzymes.Citation13 Proanthocyanidins are naturally occurring antioxidants that are abundant in many plant foods such as fruits, vegetables and legumes.Citation14 Proanthocyanidins exert a wide spectrum of pharmacological and therapeutic activities against oxidative stress. Grape seed proanthocyanidin extract (GSPE) is marked as a dietary supplement due to its health benefits, including hepatoprotective, cardioprotective, anti-fibrogenic and chemopreventive activities.Citation15
The genus Bauhinia (family Fabaceae) comprises 300 species, and is commonly known as “cow's paw” tree, because of the shape of their leaves.Citation16 Different species are used in folk medicine worldwide.Citation16 Diverse range of pharmacological activities has been reported for many Bauhinia species, including hepatoprotective, antioxidant, anti-inflammatory and anti-hyperlipoidemic effects.Citation17,Citation18 Bauhinia hookeri F. Mull, is a small ornamental tree native to Australia.Citation19 The current study was designed to investigate the possible mechanisms of the hepato- and nephroprotective action of a proanthocyanidin-rich fraction obtained from B. hookeri (BHPF) against the acute toxicity of CCl4 in mice. Hepatoprotection was determined by assaying the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), bilirubin, cholesterol and protein in the serum of control and treated mice in an acute model of CCl4 intoxication. Nephroprotection was determined by estimating the serum level of uric acid, urea and creatinine. The lipid peroxidation and antioxidant parameters [glutathione (GSH) and superoxide dismutase (SOD)] were estimated in the liver and kidney homogenates to determine the possible mechanisms of the hepato- and nephroprotective activity. A histopathological examination of liver and kidney sections was conducted to confirm the hepato- and nephroprotective effects. The identification of B. hookeri polyphenols was achieved utilizing the high-performance liquid chromatography coupled with diode array detection-electrospray ionization tandem mass spectrometry (HPLC-PDA-ESI/MS/MS) technique.
Material and methods
Chemicals
Carbon tetrachloride (CCl4) was obtained from El Nasr Pharmaceutical & Chemical Company (Cairo, Egypt). Silymarin was purchased from Sedico Pharmaceutical Company (6th October City, Egypt). All the assay kits were purchased from Biodiagnostics Company (Cairo, Egypt). The assay kit of LDH was purchased from Randox Laboratories Ltd (Crumlin, UK). All other chemicals were of analytical grade. LC–MS grade solvents were used in HPLC-PDA-ESI/MS/MS analysis. Sephadex LH-20 was obtained from Amersham Biosciences (Uppsala, Sweden), RP-C18 from Sigma-Aldrich GmbH (Darmstadt, Germany) and pre-coated silica gel TLC GF254 was obtained from Riedel-De Häen-AG (Seelze, Germany).
Plant material
The leaves of B. hookeri were collected in July 2011 from the botanical garden of the Faculty of Agriculture, Cairo University, Cairo, Egypt. The plant was botanically identified by Eng. Therese Labib, the taxonomy specialist at the herbarium of El-Orman Botanical Garden, Giza, Egypt. A voucher specimen of B. hookeri was deposited at the herbarium of the Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt (ASU BHF2011).
Extract preparation and fractionation
The air-dried powdered leaves of B. hookeri (1700 g) were extracted three times with 80% aqueous ethanol (EtOH). The total extract was concentrated and freeze-dried to obtain a dry powder, which was dissolved in absolute EtOH. The EtOH-soluble portion was concentrated and freeze-dried to obtain a dry powder (140 g). Column fractionation of part of the extract (100 g) was performed using Sephadex LH-20 (5 × 100 cm), eluted with H2O followed by H2O–MeOH mixtures of decreasing polarities. Fractions are combined based on their analytical HPLC profiles to afford eight major fractions. The fraction eluted with 60% aqueous methanol (MeOH) was concentrated and freeze-dried to obtain a dry powder of BHPF (3 g).
HPLC-PDA-ESI/MS/MS method
Part of the fraction was dissolved in 20% MeOH (20 mg/mL) and the solution was filtered through 0.2 μm PTFE membranes. LC–HRESIMS was performed on a Bruker micrOTOF-Q quadrupole time-of-flight mass spectrometer (Bremen, Germany), coupled to a 1200 series HPLC system (Agilent Technologies, Waldbronn, Germany), equipped with an auto sampler, a binary pump and a diode-array detector. Chromatographic separation was performed on an XBridge C18 (2.1 mm × 100 mm; 3.5 μm) column (Waters, Dublin, Ireland). The mobile phase consisted of acetonitrile (A) and 0.1% formic acid (B). The elution profile was 0–3 min, 100% B (isocratic); 3–30 min, 0–30% A in B; 30–45 min, 30–70% A in B; 45–55, 70% A in B (isocratic) with a flow rate of 0.2 mL/min. The HPLC system was controlled by Hystar software (version 3.2; Bruker BioSpin, Rheinstetten, Germany). The mass spectrometer was controlled by the Compass 1.3 for micrOTOF software package (Bruker Daltonics). The ionization technique was an electrospray. The mass spectrometer was operated in negative mode. Mass detection was performed in full scan mode in the mass range m/z 50–2000. The following settings were applied to the instrument: capillary voltage, 4000 V; end plate offset, −500 V. Heated drying gas (N2) flow rate was 12 L/min; the drying gas temperature was 200 °C. The gas flow to the nebulizer was set at a pressure of 1.6 bar. For collision-induced dissociation MS/MS measurements, the voltage over the collision cell varied by collision sweeping mode from 20 to 70 eV. Argon was used as a collision gas. Sodium formate was used for calibration at the end of each LC/MS run. The data were analyzed using Compass Data Analysis Software (version 4.0 SP5; Bruker Daltonics).
Animals
Male Swiss Albino mice weighing 27.5 ± 2.5 g were purchased from the Animal House Facility, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. The mice were housed in cages in a ventilated room under the controlled laboratory conditions of temperature (25 ± 2 °C) and under 12 h light/dark cycles. The mice were fed a standard rodent pellet chow and water ad libitum. The animals were acclimatized for at least l week before use. All the animal experiments were approved by the ethical committee of the Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt (201405).
Acute toxicity study
Male Swiss Albino mice weighing 27.5 ± 2.5 g were used to determine the acute oral toxicity of BHPF according to the reported method.Citation20 BHPF was diluted in saline, and the mice (n = 8 per group) were treated with high doses (500, 1000 and 2000 mg/kg body weight p.o.). The animals were observed for 24 h to record toxicity symptoms and mortality rates. The animals were also observed for possible toxicological or behavioral changes for further 14 days after BHPF treatment.
Experimental design
Forty-eight Swiss Albino mice were divided into six groups (n = 8 per group). Group (I) served as the normal control and received saline only. Group II was treated intraperitoneally with a sublethal dose of CCl4 at a dose of 0.5 mL/kg body weight (20% v/v in corn oil) at the end of the experiment and served as the positive control. The mice in groups (III, IV and V) were treated orally with 100, 200 and 400 mg/kg body weight of BHPF, respectively. Group VI was treated with standard silymarin at a daily dose of 200 mg/kg.Citation21 All tested material and silymarin were administered orally, once daily, for 5 consecutive days. On the sixth day, liver and kidney injury was induced in animals (all groups except the normal group) by a single i.p. injection of 20% CCl4 v/v in corn oil (0.5 mL/kg bw).Citation21 Blood collection was done by direct cardiac puncture under diethyl ether anesthesia 24 h after the last treatment dose. The collected blood was left to clot at room temperature and the sera were separated by centrifugation at 3000 rpm for 15 min, then the sera were stored at −20 °C for biochemical analysis. The animals were then sacrificed by decapitation under diethyl ether anesthesia and the liver and kidney were excised rapidly. One part of each tissue was perfused with a 50 mM ice-cold phosphate buffered saline (pH 7.4), containing 0.1 mM ethylenediaminetetraacetic acid to remove any red blood cells and clots. These tissues were then homogenized ice-cold phosphate buffered saline in (5–10 mL/g tissue), centrifuged at 5000 rpm for 30 min and stored at −80 °C for the estimation of lipid peroxidation, GSH content and SOD activity. Another hepatic and renal tissue part was preserved in 10% formalin for the histopathology.
Serum biochemical analysis
The already separated sera were used for the estimation of serum liver biomarkers according to the manufacturer's protocol and previously reported methods for ALT, AST,Citation22 ALP,Citation23 LDH,Citation24 total bilirubin,Citation25 cholesterolCitation26,Citation27 and total proteins.Citation28 The biochemical markers of kidney damage were estimated according to reported methods for creatinine,Citation29 ureaCitation30 and uric acid.Citation31
Evaluation of tissue lipid peroxidation and antioxidant parameters
Lipid peroxidation was evaluated by measuring the MDA content in hepatic and renal tissues.Citation32 The antioxidant parameters were assayed according to previous methods for the SOD activityCitation33 and GSH.Citation34
Histopathological examination
Liver and kidney specimens from all experimental groups were fixed in 10% formol saline for 24 h. Washing was done by the tap water followed by serial dilutions of alcohol (methyl, ethyl and absolute ethyl). The specimens were cleared in xylene and embedded in paraffin at 56 °C in a hot-air oven for 24 h. Sections were prepared (4 μm thick) by a sledge microtome and then stained with hematoxylin and eosin. The liver and kidney sections were examined for pathological changes by a light microscope.
Statistical analysis
All the data were expressed as the means ± SEM. The statistical analysis of the data was performed using the one-way ANOVA test followed by Tukey's post hoc test to determine the difference between the mean values of the different groups. All statistical analyses were performed using the GraphPad InStat software (Version 3.06, La Jolla, CA). p-Values < 0.05 were considered statistically significant.
Results
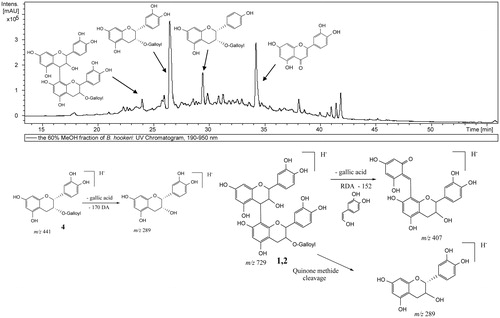
Identification of the constituents of BHPF by HPLC-PDA-ESI/MS/MS
The individual constituents of BHPF were identified using the HPLC instrument coupled to a PDA detector and a mass spectrometer. The combination of the PDA and mass spectrometry (MS/MS) data provided a sensitive method for the characterization of the constituents of BHPF ( and ). Compounds 1–3 were tentatively identified based on their molecular ions [M−H]− at m/z 729.16 and 881.18, respectively, and the MS/MS ions at m/z 407.09, 289.08, 245.08, 169.02, 125.03, of which the first three ones are typical for dimeric procyanidins.Citation35,Citation40 The MS/MS ions at m/z 169.02 indicated the presence of galloyl groups.Citation41 Compounds 1 and 2 produced the MSCitation2 base peak at m/z 407.09 by the loss of a gallic acid moiety (−170 amu) followed by a retro-Diels-Alder fragment (−152 amu).Citation42 The position of the galloyl residue was confirmed to be attached to the C3′ of the base unit of the procyanidin molecule based on the presence of the fragment ion at m/z 407.08, while the fragment ions at m/z 425, 577, 559 were not detected. These fragments are more predominant if the galloyl residue is attached to the C3 of the top unit. The molecule loses the galloyl residue or gallic acid and produces the MSCitation2 base peak at m/z 577 and a secondary peak at m/z 559, respectively.Citation42 Similarly, compounds 4–9 were identified based on their [M−H]− and fragment ions as well as from the distinctive PDA data.Citation36–38 Compounds 10 and 12 were tentatively identified based on their molecular ions and their distinctive PDA of flavonoids.Citation39
Table 1. LC-PDA-ESI/MS/MS Identification of the major constituents of BHPF.
Acute toxicity
No adverse behavioral changes, toxicity symptoms or mortality were observed in mice at doses up to 2000 mg/kg of BHPF. Based on these findings, the oral 50% lethal dose (LD50) value of BHPF is greater than 2000 mg/kg.
Hepato- and nephroprotective effects
A significant increase in the activity of the serum markers of liver damage ALT, AST, ALP and LDH (p < 0.001) was observed in the CCl4-intoxicated group compared with the negative control group (). The pretreatment of intoxicated mice with BHPF produced a marked hepatoprotective effect and reduced the activity of serum hepatic biomarkers in a dose-dependent manner. The percentage decrease in the liver marker enzymes at the treatment doses (100, 200 and 400 mg/kg/day) was 16%, 42% and 53% for ALT, 20%, 45% and 58% for AST, 21%, 49% and 57% for ALP and 22%, 49% and 60% for LDH, respectively, compared with the CCl4-treated group. Notably, BHPF pretreatment at a dose of 400 mg/kg markedly reduced the levels of ALT and AST, which were comparable and non-significant to those in the silymarin-treated group (). The levels of the ALP and LDH in the group treated with 400 mg/kg of BHPF were comparable and not significantly different from the normal control and silymarin groups (). Similarly, a remarkable and dose-dependent decrease of the serum bilirubin and cholesterol levels was observed in the BHPF pretreated groups (). The percentage decrease in the serum bilirubin and cholesterol of the treated groups compared with the CCl4-treated group is listed in . In contrast, the total protein level was significantly increased in the groups treated with BHPF in a dose-dependent manner. The total protein level in the groups treated with 200 and 400 mg/kg of BHPF were comparable and not significantly different from the normal control and silymarin groups (). The biochemical markers of kidney damage, uric acid, urea and creatinine were markedly reduced in the BHPF treated groups compared with the CCl4-intoxicated group (). These results indicated that BHPF effectively reduced the CCl4-induced hepatorenal toxicity.
Figure 2. Effect of BHPF and silymarin on the hepatic function tests of CCl4-intoxicated mice. Data are expressed as the means ± SEM (n = 8). BHPF 1, 2 and 3: 100, 200 and 400 mg/kg of BHPF, respectively. Values having different superscripts are significantly different at p < 0.05.
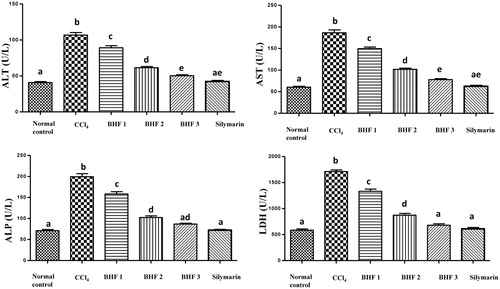
Table 2. Effect of BHPF on biochemical parameters of CCl4-intoxicated mice.
Antioxidant activity and lipid peroxidation
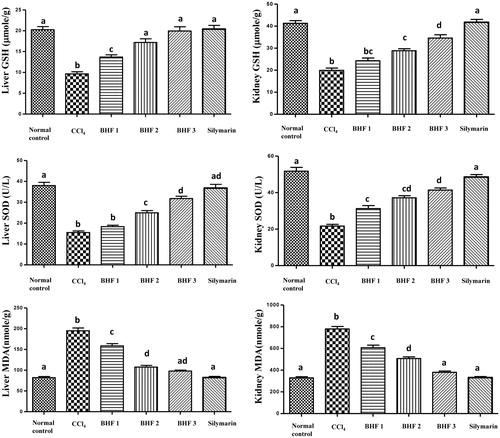
A significant reduction in hepatic and renal GSH and SOD contents was observed in CCl4-intoxicated mice. In contrast, a significant increase in the MDA levels was evident as compared to the normal control group (). Administration of BHPF at the treatment doses (100, 200 and 400 mg/kg/day) produced a marked increase in hepatic GSH (by 42%, 78% and 106%, respectively), as well as in renal GSH (by 22%, 45% and 74%, respectively). In addition, BHPF pretreatment improved the activity of hepatic SOD (by 18%, 61% and 105%, respectively), and the activity of renal SOD (by 44%, 72% and 91%, respectively) relative to the CCl4-intoxicated group. In contrast, the elevated hepatic level of MDA was reduced by 19%, 45% and 50% and the renal MDA by 22%, 35% and 51% at the tested doses, respectively, compared with the CCl4-intoxicated group (). Notably, the liver GSH content in the groups treated with 200 and 400 mg/kg of BHPF was comparable and insignificant to those in the normal control and silymarin groups. BHPF pretreatment at a dose of 400 mg/kg markedly reduced the hepatic and renal MDA levels, which were comparable and non-significant to those in the normal and silymarin-treated groups. These results clearly indicated the strong in vivo antioxidant activity provided by BHPF.
Figure 3. Effect of BHPF and silymarin on lipid peroxidation and antioxidant parameters in the liver and kidney of CCl4-intoxicated mice. Data are expressed as the means ± SEM (n = 8). BHPF 1, 2 and 3: 100, 200 and 400 mg/kg of BHPF, respectively. Values having different superscripts are significantly different at p < 0.05.
Histopathological observations
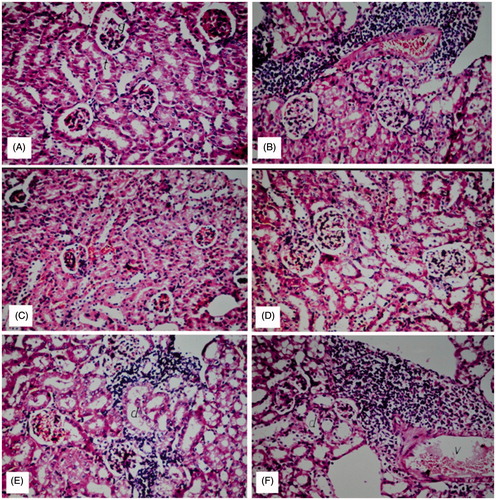
Liver sections of the normal control group showed normal histological structure of the central vein and intact surrounding hepatocytes in hepatic parenchyma. CCl4 induced severe loss of hepatic architecture with multiple focal necrosis, ballooning degeneration in the hepatocytes all over the hepatic parenchyma. The pathological changes induced by CCl4 were markedly ameliorated in the groups treated with BHPF at the three treatment doses. Pretreatment with 200 and 400 mg/kg of BHPF conferred marked protection against liver damage as evidenced by the intact hepatic architecture and absence of histopathological lesions. Diffuse Kupffer cells proliferation in between the hepatocytes was observed in the group treated with 100 mg/kg of BHPF (). Histological examination of kidney sections revealed normal histological structure of the glomeruli and tubules at the cortex with absence of histopathological alterations in the normal control group. CCl4 induced marked inflammatory cell aggregation in between the tubules, associated with marked degeneration in the lining epithelium of all the tubules, along with blood vessel congestion. The aforementioned renal pathological changes induced by CCl4 were markedly reduced in the groups treated with BHPF at the three treatment doses. Notably, pretreatment with BHPF at a dose of 400 mg/kg markedly reduced all the degenerative changes in the lining epithelium of the tubules. Mild inflammatory cell infiltration was noticed in between the tubules in the groups pretreated with 100 and 200 mg/kg of BHPF ().
Figure 4. Hepatoprotective effect of BHPF in CCl4-intoxicated mice. (A) Group I (normal control): showing normal histological structure of the central vein and intact hepatocytes. (B) Group II (CCl4-treated group): showing severe loss of hepatic architecture with multiple focal necrosis, ballooning degeneration in the hepatocytes. (C) Group VI (CCl4 + 200 mg/kg of silymarin): showing absence of histopathological alterations. (D and E) Groups V and IV (CCl4 + 400 mg/kg and CCl4 + 200 mg/kg, respectively of BHPF): showing normal histological structure. (F) Group III (CCl4 + 100 mg/kg of BHPF): showing diffuse Kupffer cell proliferation in between the hepatocytes (H&E, × 20).
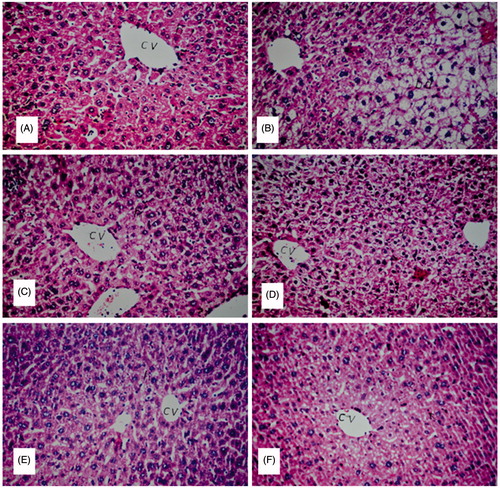
Figure 5. Nephroprotective effect of BHPF in CCl4-intoxicated mice. (A) Group I (normal control): showing normal histological structure of the glomeruli and tubules at the cortex with absence of histopathological alterations. (B) Group II (CCl4-treated group): showing marked inflammatory cell aggregation in between the tubules, marked degeneration in the lining epithelium of all the tubules, and blood vessel congestion. (C) Group VI (CCl4 + 200 mg/kg of silymarin): showing absence of histopathological alterations. (D) Group V (CCl4 + 400 mg/kg of BHPF): showing normal histological structure. (E and F) Groups IV and III (CCl4 + 200 mg/kg and CCl4 + 100 mg/kg of BHPF, respectively): showing mild inflammatory cell infiltration in between the tubules (H&E, × 20).
Discussion
CCl4 is a potent environmental hepatotoxin. CCl4 also causes disorders in kidneys, lungs and brain. In addition, it may lead to acute and chronic renal injuries.Citation43 CCl4 is widely used in experimental models to induce liver and kidney damage.Citation44,Citation45 The highly reactive trichloroethyl radicals (CCl3. and CCl3O2.) are formed from CCl4 by cytochrome P-450. These radicals initiate lipid peroxidation, necrosis and fatty changes of the liver. The trichloroethyl radicals also change the cellular antioxidant capacity by deactivating GSH and the defense antioxidant enzymes.Citation21,Citation44 In this study, the acute model of CCl4-intoxication was used because it resembles the acute intoxication in human.Citation43 The present study indicated that CCl4 administration significantly increased the serum levels of ALP, AST, ALT, LDH, cholesterol and total bilirubin. Moreover, it reduced the total protein level, which indicated severe loss of liver function. Histopathological examination revealed severe loss of hepatic architecture associated with multiple necrosis, marked ballooning degeneration of hepatocytes along with marked inflammatory cell infiltration and degeneration of the kidney tissues. CCl4 intoxication also induced severe oxidative stress as evidenced by the marked increase in hepatic and renal MDA levels. The SOD and GSH levels in the liver and kidney tissues were markedly reduced in response to CCl4 intoxication. The results of the present study indicated that pretreatment with BHPF restored the increased MDA levels to their normal values. The inhibitory effect against lipid peroxidation suggested that BHPF could prevent the hepatorenal damage induced by free radicals, along with the subsequent histopathological alterations in the liver and kidney. In contrast, BHPF pretreatment significantly enhanced the GSH and SOD levels as compared to the CCl4-intoxicated group. Modulation of these antioxidant defenses clearly contributed to the hepato- and nephroprotective activity of BHPF. Based on the results of this study, the hepato- and nephroprotective effect of BHPF is attributed to its ability to reduce the rate of lipid peroxidation, to enhance the antioxidant defense status and to guard against the pathological changes of the liver and kidney induced by CCl4 administration. The remarkable hepato- and nephroprotective effect of BHPF may, at least partly, be due to the antioxidant effect of its bioactive constituents. The HPLC-PDA-ESI/MS/MS analysis of BHPF revealed the presence of proanthocyanidins and epicatechin gallate (ECG) as the major components. Experimental evidence suggests that whole plant fractions usually have much better pharmacological activities than their single isolated ingredients due to synergistic interactions between the individual components.Citation46 It is also proved that mixtures of antioxidant compounds are more active than the individual components of these mixtures.Citation47
Proanthocyanidins are complex polymers of polyhydroxy flavan-3-ol constitutive units, and are classified according to the hydroxylation pattern of their constitutive units and the linkages between them.Citation35 The most common units are (+)-catechin, (−)-epicatechin in the case of procyanidin type, or (+) gallocatechin and (−)-epigallocatechin, for the prodelphinidin structure.Citation35 Previous studies demonstrated that proanthocyanidins confer greater protection against free radicals and lipid peroxidation than vitamins C, E and β-carotene.Citation14 Many studies confirmed the protective effect of GSPE against chemical assaults in various organs using different models. GSPE exhibited a strong protective effect on thioacetamide-induced hepatic fibrosis in mice,Citation15 and produced hepatoprotective as well as anti-fibrogenic effects against dimethylnitrosamine-induced liver injury in rats.Citation48 In addition, GSPE significantly protected against acetaminophen-induced liver and kidney injury by reducing oxidative stress, ALT activity and by inhibiting apoptotic and necrotic cell death.Citation14 GSPE also conferred protection against cyclosporine A- and cisplatin-induced nephropathy in rats and recovered the kidney functions. The nephroprotective activity of GSPE was attributed to its potent antioxidant activity, the attenuation of renal tubular damage and the enhancement of the regeneration response.Citation49,Citation50 The remarkable hepato- and nephroprotective effect of BHPF may be related to the presence of different proanthocyanidins, which may confer a synergistic action. ECG, a major component in GSPE, exhibited a protective role against renal failure, along with the ability to reduce serum uric acid, urea, and creatinine concentrations.Citation51 Diverse pharmacological activities have been attributed to ECG, including potent antioxidant, anti-fibrogenic and hepatoprotective activity.Citation13,Citation52 Besides, it was proved that ECG has more effective antioxidant activity over vitamin C.Citation53 All these attributes might contribute to the hepato- and nephroprotective effect of BHPF. Based on the results of this study, the potent hepatoprotective effect previously reported for the total extract of B. hookeriCitation44 could be attributed to its proanthocyanidin and ECG contents. Furthermore, the results of this study confirmed that B. hookeri proanthocyanidin has a potent nephroprotective effect.
Experimental evidence indicates that oxidative stress is a major link between liver injury and hepatic fibrosis. Persistent hepatocellular damage disrupts the regeneration process of damaged tissues and overwhelms the liver's defensive mechanisms. The injured hepatocytes are potent sources of ROS and lipid peroxidation products that stimulate the transformation of hepatic stellate cells (HSCs) to fibrogenic myofibroblast-like cells, which produce collagen.Citation15,Citation48 An excessive extracellular matrix is accumulated in liver tissues, which may progress to hepatic fibrosis or cirrhosis. Inactivation of HSCs represents a promising approach to block the progression of hepatic fibrosis.Citation15 The protective effect of BHPF against infiltration by inflammatory cells and other CCl4-induced pathological changes in the liver, along with the protective effect against oxidative stress, indicated that a dietary supplement of BHPF has hepatoprotective and antifibrotic therapeutic potential.
Acknowledgments
The authors would like to thank Eng. Therese Labib, the taxonomy specialist at the herbarium of El-Orman Botanical Garden, Giza, Egypt for his kind help regard to identification of B. hookeri leaves used in our study.
Declaration of interest
The authors have declared no conflicts of interest. The current research received no fund from any organization.
References
- Abdel-Daim MM. Synergistic protective role of ceftriaxone and ascorbic acid against subacute diazinon-induced nephrotoxicity in rats. Cytotechnology. 2014. [Epub ahead of print]. doi: 10.1007/s10616-014-9779-z
- Abdel-Daim MM, El-Ghoneimy A. Synergistic protective effects of ceftriaxone and ascorbic acid against subacute deltamethrin-induced nephrotoxicity in rats. Renal Fail. 2015;37(2):297–304
- Abdel-Daim MM, Abuzead SMM, Halawa SM. Protective role of Spirulina platensis against acute deltamethrin-induced toxicity in rats. PLoS One. 2013;8(9):e72991
- Al-Sayed E, Abdel-Daim MM. Protective role of cupressuflavone from cupressus macrocarpa against carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Planta Med. 2014;80(18):1665–1671
- Abdel-Daim MM, Abd Eldaim MA, Mahmoud MM. Trigonella foenum-graecum protection against deltamethrin-induced toxic effects on hematological, biochemical, and oxidative stress parameters in rats. Can J Physiol Pharmacol. 2014;92(8):679–685
- Abdel-Daim MM, Ghazy EW. Effects of Nigella sativa oil and ascorbic acid against oxytetracycline-induced hepato-renal toxicity in rabbits. Iran J Basic Med Sci. 2015;18(3):221–227
- Abdou RH, Abdel-Daim MM. Alpha-lipoic acid improves acute deltamethrin-induced toxicity in rats. Can J Physiol Pharmacol. 2014;92(9):773–779
- Abdel-Daim MM, Ghazy EW, Fayez M. Synergistic protective role of mirazid (Commiphora molmol) and ascorbic acid against tilmicosin-induced cardiotoxicity in mice. Can J Physiol Pharmacol. 2014;93(1):45–51
- Ibrahim AE, Abdel-Daim MM. Modulating effects of Spirulina platensis against tilmicosin-induced cardiotoxicity in mice. Cell J. 2015;17(1):137–144
- Abdel-Daim MM, Abdelkhalek NK, Hassan AM. Antagonistic activity of dietary allicin against deltamethrin-induced oxidative damage in freshwater Nile tilapia; Oreochromis niloticus. Ecotoxicol Environ Saf. 2015;111:146–152
- Abdelkhalek NK, Ghazy EW, Abdel-Daim MM. Pharmacodynamic interaction of Spirulina platensis and deltamethrin in freshwater fish Nile tilapia, Oreochromis niloticus: Impact on lipid peroxidation and oxidative stress. Environ Sci Pollut Res Int. 2015;22(4):3023–3031
- Abdel-Daim MM. Pharmacodynamic interaction of Spirulina platensis with erythromycin in Egyptian Baladi bucks (Capra hircus). Small Ruminant Res. 2014;120(2–3):234–241
- Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–988
- Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology. 2000;148(2–3):187–197
- Li J, Li J, Li S, et al. Ameliorative effect of grape seed proanthocyanidin extract on thioacetamide-induced mouse hepatic fibrosis. Toxicol Lett. 2012;213(3):353–360
- Filho VC. Chemical composition and biological potential of plants from the genus Bauhinia. Phytother Res. 2009;23(10):1347–1354
- Bodakhe SH, Ram A. Hepatoprotective properties of Bauhinia variegata bark extract. Yakugaku Zasshi. 2007;127(9):1503–1507
- Sosa S, Braca A, Altinier G, Della Loggia R, Morelli I, Tubaro A. Topical anti-inflammatory activity of Bauhinia tarapotensis leaves. Phytomedicine. 2002;9(7):646–653
- Maddigan L, Allan R, Reid R. Coastal Plants of the Burdekin Dry Tropics. Queensland, Australia: NQ Dry Tropics, Burdekin Solutions Ltd. & Townsville and Coastal Dry Tropics Landcare Incorporated; 2008
- Bruce RD. An up-and-down procedure for acute toxicity testing. Fundam Appl Toxicol. 1985;5(1):151–157
- Park SW, Lee CH, Kim YS, et al. Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. J Pharmacol Sci. 2008;106(1):136–143
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28(1):56–63
- Tietz NW, Burtis CA, Duncan P, et al. A reference method for measurement of alkaline phosphatase activity in human serum. Clin Chem. 1983;29(5):751–761
- Babson SR, Babson AL. An improved amylase assay using dyed amylopectin. Clin Chim Acta. 1973;44(2):193–197
- Schattmann K. A spectrophotometric quantitative caffein-free method for determination of the serum bilirubin index with the Lange universal colorimeter. Arztl Wochensch. 1952;7(49):1154–1156
- Richmond W. Preparation and properties of a cholesterol oxidase from Nocardia sp. and its application to the enzymatic assay of total cholesterol in serum. Clin Chem. 1973;19(12):1350–1356
- Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275
- Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clin Chim Acta. 1972;38(2):475–476
- Coulombe JJ, Favreau L. A new simple semimicro method for colorimetric determination of urea. Clin Chem. 1963;9:102–108
- Whitehead TP, Bevan EA, Miano L, Leonardi A. Defects in diagnostic kits for determination of urate in serum. Clin Chem. 1991;37(6):879–881
- Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86(1):271–278
- Nishikimi M, Appaji N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 1972;46(2):849–854
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888
- Hellström J, Sinkkonen J, Karonen M, Mattila P. Isolation and structure elucidation of procyanidin oligomers from Saskatoon berries (Amelanchier alnifolia). J Agric Food Chem. 2007;55(1):157–164
- Khallouki F, Haubner R, Hull WE, et al. Isolation, purification and identification of ellagic acid derivatives, catechins, and procyanidins from the root bark of Anisophyllea dichostyla R. Br. Food Chem Toxicol 2007;45(3):472–485
- de Souza LM, Cipriani TR, Iacomini M, Gorin PA, Sassaki GL. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J Pharm Biomed Anal. 2008;47(1):59–67
- Hartisch C, Kolodziej H. Galloylhamameloses and proanthocyanidins from Hamamelis virginiana. Phytochemistry. 1996;42(1):191–198
- Mabry TJ, Markham KR, Thomas MB. The Systematic Identification of Flavonoids. New York, Heidelberg, Berlin: Springer-Verlag; 1970
- Verardo V, Arráez-Román D, Segura-Carretero A, Marconi E, Fernández-Gutiérrez A, Caboni MF. Identification of buckwheat phenolic compounds by reverse phase high performance liquid chromatography–electrospray ionization-time of flight-mass spectrometry (RP-HPLC–ESI-TOF-MS). J Cereal Sci. 2010;52(2):170–176
- Reed JD, Krueger CG, Vestling MM. MALDI-TOF mass spectrometry of oligomeric food polyphenols. Phytochemistry. 2005;66(18):2248–2263
- Jaiswal R, Jayasinghe L, Kuhnert N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC–MS. J Mass Spectrom. 2012;47(4):502–515
- Manna P, Sinha M, Sil P. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complementary Altern Med. 2006;6(1):33
- Al-Sayed E, Martiskainen O, Seif el-Din SH, et al. Hepatoprotective and antioxidant effect of Bauhinia hookeri extract against carbon tetrachloride-induced hepatotoxicity in mice and characterization of Its bioactive compounds by HPLC-PDA-ESI-MS/MS. BioMed Res Int. 2014;2014:1–9
- Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenethyl ester protects kidneys against carbon tetrachloride toxicity in rats. J Ethnopharmacol. 2005;97(2):273–280
- Wagner H, Ulrich-Merzenich G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine. 2009;16(2–3):97–110
- Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011;82(4):513–523
- Shin MO, Yoon S, Moon JO. The proanthocyanidins inhibit dimethylnitrosamine-induced liver damage in rats. Arch Pharm Res. 2010;33(1):167–173
- Ulusoy S, Ozkan G, Yucesan FB, et al. Anti-apoptotic and anti-oxidant effects of grape seed proanthocyanidin extract in preventing cyclosporine A-induced nephropathy. Nephrology. 2012;17(4):372–379
- Saad AA, Youssef MI, El-Shennawy LK. Cisplatin induced damage in kidney genomic DNA and nephrotoxicity in male rats: The protective effect of grape seed proanthocyanidin extract. Food Chem Toxicol. 2009;47(7):1499–1506
- El-Adawi H, El-Azhary D, Abd El-Wahab A, El-Shafeey M, Abdel-Mohsen M. Protective effect of milk thistle and grape seed extracts on fumonisin B1 induced hepato-and nephro-toxicity in rats. J Med Plant Res. 2011;5(27):6316–6327
- Tipoe GL, Leung TM, Liong EC, Lau TYH, Fung ML, Nanji AA. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273(1–3):45–52
- Sajilata MG, Bajaj PR, Singhal RS. Tea polyphenols as nutraceuticals. Compr Rev Food Sci Food Safe. 2008;7(3):229–254