Abstract
Chronic kidney disease accounts for much of the increased mortality, especially in the elder population. The prevalence of this disease is expected to increase significantly as the society ages. Our aim was to evaluate the kidney function and risk factors of reduced renal function among elderly Chinese patients. This study retrospectively collected clinical data from a total of 1062 inpatients aged 65 years or over. Estimated glomerular filtration rate (eGFR) was calculated with the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Renal function and risk factors were also analyzed. For all 1062 subjects, the mean eGFR was 71.0 ± 24.8 mL/min/1.73 m2, and the incidence rates of reduced renal function, proteinuria, hematuria and leukocyturia were 31.1%, 11.8%, 6.6% and 8.7%, respectively. The eGFR values were 83.4 ± 28.4, 72.2 ± 22.9, 67.8 ± 24.3 and 58.8 ± 29.1 mL/min/1.73 m2 in the groups of 60–69, 70–79, 80–89 and ≥90 years age group (F = 15.101, p = 0.000), respectively; while the incidences of reduced renal function were 12.8%, 27.0%, 37.8% and 51.7% (χ2 = 36.143, p = 0.000). Binary logistic regression analysis showed that hyperuricemia (OR = 4.62, p = 0.000), proteinuria (OR = 3.96, p = 0.000), urinary tumor (OR = 2.92, p = 0.015), anemia (OR = 2.45, p = 0.000), stroke (OR = 1.96, p = 0.000), hypertension (OR = 1.83, p = 0.006), renal cyst (OR = 1.64, p = 0.018), female (OR = 1.54, p = 0.015), coronary artery disease (OR = 1.53, p = 0.008) and age (OR = 1.05, p = 0.000) were the risk factors of reduced renal function. In conclusion, eGFR values decreased by age, while the incidence of reduced renal function, proteinuria, hematuria and leukocyturia increased with age. Treatment and control of comorbidities may slow the decline of renal function in elderly patients.
Introduction
Chronic kidney disease (CKD) is an important public health problem characterized by poor health outcomes and very high health-care costs.Citation1 According to the Global Burden of Disease Study 2010, CKD was ranked 27th in the list of causes of total number of global deaths in 1990 (age-standardized annual death rate of 15.7 per 100,000), but rose to 18th in 2010 (annual death rate 16.3 per 100,000).Citation2
CKD is common in older people and its prevalence increases in parallel with age.Citation3 In the Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES), the prevalence of CKD was 43.6% and 44%, respectively.Citation4 The increasing prevalence particularly in older individuals seems to be partly because of an increasing prevalence of diabetes and hypertension.Citation5
CKD is defined as abnormalities of kidney structure (i.e. albuminuria) or function [i.e. glomerular filtration rate (GFR) <60 mL/min/1.73 m2] present for 3 months or more with implications for health.Citation6 The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation based on serum creatinine was recommended to estimate GFR in adults.Citation6 In healthy Chinese adults, measured GFR decreased with age after 30 years.Citation7 In the elderly, Kilbride et al.Citation8 found that the CKD-EPI equation based on serum creatinine appeared less biased and was more accurate than the Modification of Diet in Renal Disease (MDRD) Study equation. Thus, this study was performed to analyze the kidney function with CKD-EPI equation in elderly Chinese inpatients.
According to the Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines 2002, lower baseline level of kidney function, male gender, older age, proteinuria, lower serum albumin concentration, hypertension, poor glycemic control and smoking were associated with acute GFR decline.Citation9 The 2012 KDIGO guideline also noted that cause of CKD, level of GFR, level of albuminuria, age, gender, race, elevated blood pressure, hyperglycemia, dyslipidemia, smoking, obesity, history of cardiovascular disease and ongoing exposure to nephrotoxic agents are associated with CKD progression.Citation6 However, the role of these factors was not well characterized in the development and progression of CKD among elderly Chinese inpatients. Therefore, this study was conducted to analyze the risk factors of decreased GFR in elderly Chinese inpatients.
Material and methods
Study population
This retrospective study included a total of 1062 individuals (inpatients hospitalized between January 2010 and June 2014 at Guangdong General Hospital) who were eligible for inclusion in this study. All participants were older than 65 years. In this study, repeated hospitalization patients and subjects with missing data for essential variables were excluded.
Study measurements and definitions
The electronic record form consisted of three main parts, namely, participants’ general information and anthropometric measurements files (name, age, gender, body height, body weight, systolic BP and diastolic BP), laboratory files [blood and urine tests results including blood glucose (Glu), glycated hemoglobin, urea nitrogen, creatinine, uric acid, albumin, triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) or high-density lipoprotein cholesterol (HDL-C) values, hemoglobin and urine albumin–creatinine ratio], and medical history files [personal history of hypertension, diabetics, coronary heart disease (CHD), stroke, peripheral vascular disease (PVD), smoking, hyperlipidemia, hyperuricemia, tumor, urinary tract tumors, kidney stones and renal cyst].
Decreased GFR was defined as eGFR <60 mL/min/1.73 m2.Citation6 Estimating GFR (eGFR) was calculated by CKD-EPI equation which is as follows: eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 (if female), where Scr is serum creatinine expressed as mg/dL, κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1 and max indicates the maximum of Scr/κ or 1.
Proteinuria was diagnosed if urine protein–creatinine ratio was ≥150 mg/g creatinine, albuminuria was diagnosed if the urine albumin–creatinine ratio was ≥30 mg/g,Citation10 or total protein exceeds 150 mg/24 h urine collection or albumin exceeds 30 mg/24 h urine collection.
Morning random urine samples were collected from the participants of one of the three communities (the urban community). Urine albumin was measured by an immunoturbidimetric method (Dako A/S, Glostrup, Denmark), and urine creatinine urine was measured by the Hitachi 7170 autoanalyzer (Hitachi, Tokyo, Japan). The urine albumin–creatinine ratio (UACR) was used as an expression for albumin excretion. The UACR was calculated according to the following equation: UACR (mg/g) = urine albumin concentration (mg/L)/urine creatinine concentration (g/L). Albuminuria was diagnosed if the UACR was ≥30 mg/g.Citation10
Hematuria is defined as the presence of three or more red blood cells per high-power field, excluding urinary tract infection as the cause. White blood cells in urine are defined as the presence of five or more white blood cells per high-power field, excluding urinary tract infection as the cause.
A subject was considered to have hypertension if: (i) systolic blood pressure ≥140 mmHg, (ii) diastolic blood pressure ≥90 mmHg or (iii) the subject was taking anti-hypertensive drug.Citation11
A subject was considered to have diabetes if: (i) fasting venous blood Glu ≥7.0 mmol/L, (ii) 2 h venous blood Glu ≥11.1 mmol/L, (iii) HbA1c ≥6.5% or (iv) the subject was taking a hypoglycemic drug or insulin.Citation12
CHD is diagnosed with a CHD medical history or by coronary angiography. Stroke includes transient ischemic attack, cerebral infarction and hemorrhagic cerebrovascular disease.
Serum creatinine normal range is 62–115 µmol/L (male) and 53–97 µmol/L (female). Hyperuricemia is defined if serum uric acid is >420 µmol/L (male) and >360 µmol/L (female) or if the subject is taking a hyperuricemia drug. Hyperlipidemia is defined if TC > 6.22 mmol/L or TG > 2.26 mmol/L, or if the subject is taking a hyperlipidemia drug. Anemia is defined if hemoglobin is <120 g/L (male) and <110 g/L (female). Tumor and urinary tract tumors were confirmed by X-ray or computerized tomography and pathology tests. Kidney stones and renal cyst was confirmed by X-ray or computerized tomography.
Statistical analysis
Statistical analysis was performed by SPSS 16.0 (Chicago, IL). Continuous variables were expressed as mean ± SD and compared using Student’s t test. Categorical variables were described as proportions and compared by the chi-squared test. Risk factors for decreased GFR were analyzed using logistic regression models. All tests were two sided with p < 0.05 which was considered statistically significant.
Results
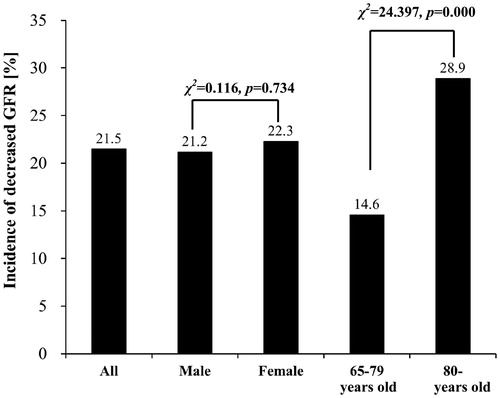
A total of 1062 subjects were included in the current analysis. There were 721 males (67.9%) and 341 females (32.1%). The age range is 65–100 years, with an average of 79.6 ± 5.6 years. The average eGFR of all the patients is 62.9 ± 20.5 mL/min/1.73 m2. The average eGFR in male and female patients is 63.2 ± 18.8 and 62.2 ± 23.5 mL/min/1.73 m2, respectively (F = 0.690, p = 0.491) (). The incidence rate of reduced GFR is 40.7%. In male and female patients, it is 40.2% and 41.6% individually (χ2 = 0.194, p = 0.660) ().
Table 1. Clinical characteristics of the study patients.
presents the comorbidities among these patients: 264 proteinuria (24.9%), 77 hematuria (7.3%), 104 white blood cells in urine (9.8%), 852 hypertension (80.2%), 410 diabetes (38.6%), 388 CHD (36.5%), 266 stroke (25.0%), 342 PVD (32.2%), 126 hyperlipidemia (11.9%), 337 hyperuricemia (31.7%), 173 tumor (16.3%), 420 anemia (39.5%), 38 urinary tract tumors (3.6%), 79 kidney stones (7.4%), 193 renal cyst (18.2%) and 257 smoker (24.2%).
Table 2. Commobidities of the study patients.
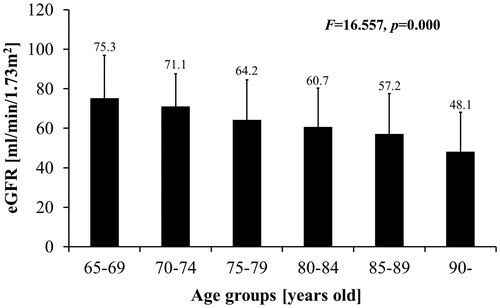
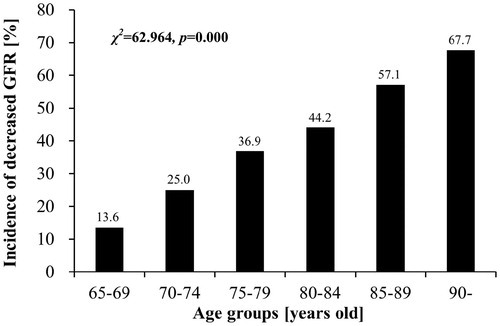
As the patients’ age, GFR decreases and incidence rate of reduced GFR increases. eGFR from 65–69-year old, 70–74-year old, 75–79-year old, 80–84-year old, 85–89-year old and >90-year old is 75.3 ± 21.6, 71.1 ± 16.5, 64.2 ± 20.4, 60.7 ± 19.6, 57.2 ± 20.3 and 48.1 ± 20.0 mL/min/1.73 m2, respectively (F = 16.557, p = 0.000) (). Incidence rate of reduced GFR is 13.6%, 25.0%, 36.9%, 44.2%, 57.2% and 67.7% (χ2 = 62.964, p = 0.000) ().
Among the patients who had creatinine in normal range, the incidence rate of reduced GFR is 21.5%, including 21.2% in male and 22.3% in female patients (χ2 = 0.116, p = 0.734). Whereas, the incidence rate of reduced GFR in 65 - to 79-year-old group is lower than >80-year-old group, which is 14.6% and 28.9% (χ2 = 24.397, p = 0.000) ().
The result of univariate logistic regression analysis showed that hyperuricemia (OR = 5.00, p = 0.000), proteinuria (OR = 2.77, p = 0.000), urinary tract tumors (OR = 2.59, p = 0.033), anemia (OR = 1.76, p = 0.000), stroke (OR = 1.55, p = 0.008), CHD (OR = 1.58, p = 0.003) and age (OR = 1.09, p = 0.000) were risk factors that are responsible for the reduced GFR in elderly patients ().
Table 3. Risk factors of decreased GFR.
Risk factors that are associated with reduced GFR were slightly different between male and female. For male, the risk factors were hyperuricemia (OR = 5.49, p = 0.000), proteinuria (OR = 3.04, p = 0.000), CHD (OR = 1.61, p = 0.010), anemia (OR = 1.53, p = 0.022) and age (OR = 1.09, p = 0.000); for female, the risk factors were hyperuricemia (OR = 3.86, p = 0.000), stroke (OR = 2.85, p = 0.001), anemia (OR = 2.57, p = 0.001), proteinuria (OR = 2.19, p = 0.014) and age (OR = 1.09, p = 0.002) ().
Risk factors were also different among different age groups. For 65–79-year-old group, hyperuricemia (OR = 5.15, p = 0.000), proteinuria (OR = 3.46, p = 0.000), anemia (OR = 2.15, p = 0.001), CHD (OR = 2.00, p = 0.004) and age (OR = 1.15, p = 0.000), while for >80-year-old group, hyperuricemia (OR = 4.91, p = 0.000), urinary tract tumor (OR = 3.15, p = 0.037), proteinuria (OR = 2.67, p = 0.000), stroke (OR = 1.93, p = 0.002), anemia (OR = 1.52, p = 0.038) and age (OR = 1.07, p = 0.022) () were the risk factors.
Discussion
After 30 years of age, GFR decreased at 1 mL/min annually. Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) indicated that in the population older than 65 years, GFR decreased significantly with aging.Citation4 Our study also confirmed that eGFR estimated by CKD-EPI decreased ∼3.5–9.1 mL/min/1.73 m2 every 5 years. As human ages, structure and physiological functions of kidney develops age-related changes.Citation13,Citation14 Changes including vascular intima thickening, vascular wall stiffening, vascular lumen dilation, compliance reduction, glomerulosclerosis, tubular atrophy and interstitial fibrosis, all contribute to the decrease of GFR with aging.
eGFR <60 mL/min/1.73 m2 was an independent indicator of mortality risk in the general population.Citation10 According to guideline KDIGO 2012, GFR <60 mL/min/1.73 m2 is the standard of reduced GFR.Citation6 In western countries, the prevalence of decreased GFR was ∼10% at the age of 65 years and increased to 60% in individuals aged 80 years and older.Citation15 In the 2009 Health Survey for England, the prevalence of decreased GFR was 50% in people aged >75 years, while 25% in people aged 65–74 years.Citation12
According to epidemiologic studies conducted in China, the incidence rate of reduced GFR observed in elder populations is high, 4.2–12.4% and 7.8–28.4% for age group between 60–69 and >70 years, respectively.Citation16,Citation17 Our study showed that incidence rate of reduced GFR in >65-year-old hospitalized patients is 40.7%. There was no significant difference between male and female patients. The incidence rate decreased with age. The incidence rate observed in this study was higher than the studies mentioned above. This could be explained by the high percentage of complication rates in the subjects in this study. The percentages of subjects with high BP, diabetes and proteinuria were 80.2%, 38.6% and 24.9%, respectively. All these were influential risk factors for CKD progression.
Serum creatinine was the most commonly used method to evaluate renal function in the past, but it was insensitive in elderly due to its relationship with muscle mass. 2002 K/DOQI guideline recommended using Cockcroft–Gault (CG) equation and Modification of Diet in Renal Disease (MDRD) equation to estimate adult GFR.Citation9 Levey et al.Citation11 reported a CKD-EPI equation to calculate eGFR based on serum creatinine, age and gender. This calculation is more accurate and precise than the MDRM. LiCitation18 and Liao et al.Citation19 also found that in adult CKD patients, compared to the simplified MDRD, CKD-EPI equation produced a smaller bias, higher precision and accuracy, suggesting its advantages over MDRD in eGFR. Therefore, 2012 KDOQI guideline recommended CKD-EPI equation for calculating eGFR in adults.Citation6
Study from Kilbride et al.Citation8 showed that among the population aged between 74 and 97 years (median age is 80 years), CKD-EPI equation resulted smaller deviation and more precision, suggesting in elder population CKD-EPI equation is superior to MDRD equation. Thus, in this study, we adopted the serum creatinine-based CKD-EPI equation for eGFR in elderly patients. We also found in this study that, among the elder patients that have serum creatinine within normal range, the incidence rate of reduced GFR is 21.5% in general, 21.2% for male and 22.3% for female with no significance in between. The incidence rate of reduced GFR among 65–79 year group is 14.6%. This is lower than 28.9% which is found in >80-year group. This suggests that it is not enough to use only serum creatinine to indicate the reduced GFR with the misdiagnosis rate at 21.5%, which also increases with age.
Logistic regression analysis from this study suggested that risk factors for GFR reduction in elder patients include hyperuricemia, proteinuria, urinary tract tumors, anemia, stroke, CHD and aging. Previous studies and guideline also indicated aging, male, proteinuria, high BP, high blood sugar, lipid metabolic abnormalities, CVD disease history are risk factors for CKD progression.Citation6 Consistent with previous study, no matter in male or female, age and proteinuria were all risk factors for reduced GFR in both aged and elder patients. Lower urinary protein level could slow down the progression of kidney function reduction. However, in the elderly patients from this study, we did not find hypertension, diabetes and hyperlipidemia as risk factors for reduced GFR. Further analysis showed that, in patients that suffered from above diseases, percentage of medication is 88.4%, 75.1% and 72.2%, respectively. We speculated that due to the well-monitored blood pressure, sugar and lipid level, there was no correlation found between these diseases and progressive kidney function loss.
Although the guidelines indicated that there is not enough evidence to support or to deny the relationship between lower blood uric acid and decelerated CKD progression or to determine if anemia is related to dramatic loss of kidney function,Citation6,Citation9 in this study, we found hyperuricemia and anemia were the risk factors for reduced GFR in elderly patients, for both male and female in all the ages. Hyperuricemia and anemia are not only complications for chronic renal insufficiency. Hyperuricemia itself is an independent risk factor for CKD,Citation20 which accelerate the thickening afferent arterioles and atherosclerosis further damage kidney function; anemia could also accelerate the deterioration of renal function through ischemic damage. Thus, for elderly patients, decreasing serum uric acid and improving anemia could delay the decline of GFR to some extent.
Proteinuria, high BP and aging are risk factors for reduced kidney functions in elder people, which is consistent with literature. Although diabetes is not considered the risk factor for reduced kidney functions, further comparative analysis suggested that the incidence rate in patients with reduced kidney function and diabetes is higher in patients without diabetes, suggesting diabetes could be an important factor for reducing kidney function.
In addition, our study also found that stroke, CHD and urinary tract tumors were also risk factors for reduced GFR in elder people. Atherosclerosis can affect any artery in the body, including arteries in the heart, brain, which causes stroke and CHD; in the kidney, it causes ischemia in kidney arteries, GFR reduction and loss of kidney function slowly. GFR reduction could also be caused by urinary tract obstruction due to urinary tract tumor, dehydration medicine used in patients with stroke or large dose of dye in CHD patients. Further loss of nephron due to kidney tumor and surgical removal will further decrease GFR.
In conclusion, the incidence rate of reduced GFR increased with age, possibly due to the complications in elder hospitalized patients. In aging population, especially in elderly patients, serum creatinine as an indicator could increase the misdiagnosis rate based on GFR when used to evaluate kidney function. Treatment of primary disease and risk factors, like lowering urine albumin and serum uric acid, controlling blood pressure, sugar and improving anemia would help slow down the reduction of kidney function in elderly patients with complications.
Declaration of interest
The authors declared no conflicts of interests. This study was supported by grants from the National Natural Science Foundation (81300608) and the National Clinical Key Specialty Construction Preparatory Projects, China.
References
- Tonelli M, Riella MC. Chronic kidney disease and the ageing population. Nat Rev Nephrol. 2014;10:127–128
- Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260–272
- Tonelli M, Riella MC. Chronic kidney disease and the aging population. Kidney Int. 2014;85:487–491
- Stevens LA, Li S, Wang C, et al. Prevalence of CKD and comorbid illness in elderly patients in the United States: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2010;55:S23–S33
- Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;S3:1–150
- Ma YC, Zuo L, Chen L, et al. Distribution of measured GFR in apparently healthy Chinese adults. Am J Kidney Dis. 2010;56:420–421
- Kilbride HS, Stevens PE, Eaglestone G, et al. Accuracy of the MDRD (Modification of Diet in Renal Disease) study and CKD-EPI (CKD Epidemiology Collaboration) equations for estimation of GFR in the elderly. Am J Kidney Dis. 2013;61:57–66
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1–S266
- Matshushita K, van de Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet. 2010;375:2073–2081
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612
- Roth M, Roderick P, Mindell J. Kidney Disease and Renal Function. Health Survey for England 2009. London: NHS Information Centre; 2010
- Abdelhafiz AH, Brown SH, Bello A, et al. Chronic kidney disease in older people: Physiology, pathology or both? Nephron Clin Pract. 2010;116:c19–c24
- Pannarale G, Carbone R, Del Mastro G, et al. The aging kidney: Structural changes. J Nephrol. 2010;23:S37–S40
- Van Pottelbergh G, Vaes B, Adriaensen W, et al. The glomerular filtration rate estimated by new and old equations as a predictor of important outcomes in elderly patients. BMC Med. 2014;12:27
- Zhang L, Zuo L, Xu G, et al. Community-based screening for chronic kidney disease among population older than 40 years in Beijing. Chin J Nephrol. 2006;22:67–71
- Chen W, Wang H, Dong X, et al. Epidemiologic study of chronic kidney disease in Guangzhou urban area. Chin J Nephrol. 2007;23:147–151
- Li JT, Xun C, Cui CL, et al. Relative performance of two equations for estimation of glomerular filtration rate in a Chinese population having chronic kidney disease. Chin Med J (Engl). 2012;125:599–603
- Liao Y, Liao W, Liu J, Xu G, Zeng R. Assessment of the CKD-EPI equation to estimate glomerular filtration rate in adults from a Chinese CKD population. J Int Med Res. 2011;39:2273–2280
- Chang HY, Tung CW, Lee PH, et al. Hyperuricemia as an independent risk factor of chronic kidney disease in middle-aged and elderly population. Am J Med Sci. 2010;339:509–515