Abstract
A multidisciplinary approach represents the best method to interact with patients. Neoplastic and renal diseases are closely related to each other because of an increased risk of cancer among individuals with end-stage renal disease and because of the high prevalence of renal failure in cancer patients. Physicians should be able to know how to prevent and treat the possible complications which may appear during the course of neoplastic disease that may lead to kidney damage such as the Acute Tumor Lysis Syndrome, disorders of hydroelectrolitic balance, metabolic alterations in the calcium-phosphorus, anemia, interstitial and glomerular impairment due to chemotherapy. It is very important to know patients’ renal function and directly monitor it, before and during treatment, using formulas for estimating glomerular filtration rate (GFR) and above all, specific biomarkers are more early and sensitive than the increase of creatinine, like neutrophil gelatinase-associated lipocalin. Additionally, physician should consider that alteration of GFR or substitutive renal treatments severely influence dosage of tumor markers and it could lead to wrong diagnosis of cancer. The aim of this article is to provide a review of problems related to cancer relevant in the development of renal failure and try to define the best therapeutic strategies to cope with possible kidney imbalances induced by cancer or its treatment.
Introduction
Occurrence of renal function and tumors are strictly correlated. It should be considered in two distinct situations: (1) kidney disease caused by the presence of a cancer and/or its treatment particularly nephrotoxicity of antineoplastic drugs and (2) kidney disease as a risk factor for the development of neoplasias. The occurrence of renal failure is an additional morbidity and mortality risk factor in the course of neoplasia.Citation1 Several data suggest a role for chronic renal failure (CRF) as an independent risk factor for cancer development, with high incidence rates.Citation2,Citation3 According to the Michigan Kidney Registry, collected prostate cancer, renal cell carcinoma and cervical carcinoma are the most common tumoral diseases occurring in CRF patients.Citation4 Even neoplasms arising in other organs such as liver, thyroid and tongue resulted are more frequent in CRF patients than in the general population. Among oncohematologic diseases, multiple myeloma and non-Hodgkin lymphoma are most commonly associated with CRF, especially, in patients with glomerulonephritis.Citation5 In this review, we resumed main aspects of the link Kidney-neoplasias.
Renal toxicity of old and new antineoplastics drugs
Acute renal failure (ARF) is the most frequent renal complication induced by chemotherapy treatment occurring in 12–49% of terminal cancer patients. Although a pre-existing renal impairment might influence the outcome, it is estimated that 9–32% of ARF cancer patient needs hemodialysis showing a high mortality rate (72–85%).Citation6 The main mechanism related to renal damage by antineoplastic drugs are the accumulation of some drugs with renal excretion causing the aggravation of a pre-existing renal damage and de novo renal disease induced by drugs.
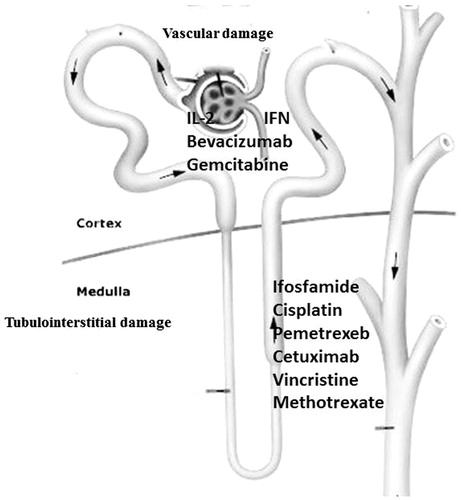
Several drugs are responsible for some pathophysiological mechanisms of renal damage such as the activation of intracellular pathways determining apoptosis or cell necrosis, the initiation of the inflammatory cascade and tubular obstruction by precipitation of cylinders.Citation7 We can also categorize chemotherapy-related kidney lesions based on the nephron sites primarily affected by the drug. Some agents can injure the tubules, the glomeruli and/or renal vasculature ().
Tubular damage
Among drugs that may cause tubular injury, the most important are cisplatin and methotrexate (MTX). The nephrotoxicity of cisplatin occurs in approximately one-third of patients after 10 days of administration, resulting in events attributable to tubulopathy (hypokalemia, hypomagnesemia and Fanconi syndrome) and renal failure. The nephrotoxicity is related to inflammation, apoptosis or cell death, oxidative stress, as well as a vasoconstriction that determines an early ischemic injury.Citation8 Hypomagnesemia may exacerbate cisplatin toxicity. This is important to remember while using other drugs that may interfere with magnesium metabolism, such as cetuximab.
Several other drugs such as nitric oxide synthase inhibitors, spironolactone and gemcitabine increase the nephrotoxicity of cisplatin. The combination of a diuretic is not advantageous. The administration protocols involve the use of cisplatin in patients with creatinine clearance >60 mL/min or creatinine <2 mg/dL. In case of necessity of the use of this drug, the dosage adjustment is necessary, with 25% reduction if creatinine clearance is between 46 and 60 mL/min and a reduction of 50% for a creatinine clearance between 31 and 45 mL/min. In case of occurrence of nephrotoxicity, cisplatin should be suspended.Citation9
MTX represents another drug responsible for nephrotoxicity. The renal damage is due to a direct tubular toxicity and intratubular precipitation of crystals of MTX, determined by an acid urinary pH. Other risk factors are a previous renal disease, a folate deficiency, advanced age, alcohol intake and hypoalbuminemia. ARF induced by MTX is reversible in most of the cases.Citation10
In order to avoid renal dysfunction caused by cisplatin, hydration (1000 mL of saline solution 2–3 h before and 500 mL in the 2 h following infusion) and the addition of KCl and MgSO4 are essential. As regards, MTX, an adequate volume of infusion and a urinary pH >7, that improves drug’s solubility, are essential for the prevention of nephrotoxicity.
Glomerular damage
Many medications can cause glomerular injury through several mechanisms ranging from Acute Thrombotic Microangiopathy (TMA) to podocyte damage. TMA may be caused by gemcitabine or antiangiogenesis agents, such as Bevacizumab and Aflibercept. Microangiopathic hemolytic anemia, thrombocytopenia, hypertension, schistocytosis, acute kidney injury (AKI) with hematuria and proteinuria are the most important symptoms. Interestingly, the onset of hypertension in patients treated with bevacizumab is a predictor of response to therapy.
Podocyte injury that may occur after chronic interferon therapy (IFN-a, IFN-b) is used to treat various malignancies. Renal histopathology reveals minimal change disease or focal segmental glomerulosclerosis (FSGS). Clinically, one could have to face symptoms ranging from nephrotic syndrome to AKI.Citation11
Other drugs may cause renal impairment. We report a list of these medicaments that require dose adjustment in case of pre-existing CKD and their mechanism of kidney damage ().
Table 1. Medicaments that require dose adjustment (%) in case of pre-existing CKD.
Contrast-induced acute kidney injury
Cancer patients should be subjected to periodic instrumental examinations in order to stage the disease and use an appropriate therapeutic dose at any disease phase. For this reason, cancer patients have a higher risk of developing CI-AKI (Contrast-induced Acute Kidney Injury) when compared with the general population. CI-AKI has become the third leading cause of hospital-acquired acute kidney injury.Citation12
In the most recent guidelines of the European Society of Radiology (ESUR) and KDIGO, CI-AKI is defined as an absolute increase in serum creatinine ≥0.5 mg/dL or relative ≥25% from baseline after 48–72 h by the use of contrast substance, not correlated to other causes.Citation13
The definition of CI-AKI based on creatinine, however, has limitations because it only considers the effect on renal function, whereas it would be desirable to use the markers of renal cell damage equivalent to troponins in cardiology. Some biomarkers of AKI are currently under study in both plasma [neutrophil gelatinase-associated lipocalin (NGAL), cystatin C] and urine [NGAL, Kidney Injury Molecule-1 (KIM-1), interleukin-18 and N-acetylglucosamine (NAG)]. These substances are more early and sensitive biomarkers of AKI than the increase of creatinine. Our group evaluated, in particular, the diagnostic power of serum and urinary NGAL in depicting an event of CI-AKI in patients who received iodinated contrast material, gadoterate meglumine, or radiopharmaceutical technetium-99 m (99mTc). We found that serum creatinine levels showed a statistically significant increase 48 h after iodinated contrast material administration, whereas NGAL was an early marker, with its elevations observed only after 8 h.Citation14 Risk factors for CI-AKI are listed in .
Table 2. Risk factor for CI-AKI.
We report an algorithm for therapeutic strategies that should be carried out in accordance with the KDIGO guidelines ().
Table 3. Prevention strategy in patients at risk for CI-AKI.
Disorders of hydroelectrolitic balance during neoplasia
Hyponatremia
Hyponatremia, defined as a level of serum sodium (Na) <135 mEq/L, has a frequency of 47% in cancer patients. Chemotherapy or other drugs such as opioids or antidepressants can cause the deficiency of Na. Peripheral edema or ascites are able to stimulate vasopressin (AVP) secretion through a volume depletion.Citation15 Moreover, pneumonia, active tuberculosis, asthma, infections of the central nervous system, hypothyroidism, adrenal insufficiency and trauma represent other important causes of hyponatremia. AVP could also be produced by the tumor determining a syndrome of inappropriate antidiuretic hormone secretion (SIADH).Citation16
The prevention of hyponatremia includes control of gastrointestinal losses and hydration before chemotherapy. In certain circumstances, it is essential to remove water but not sodium: treatment consists of water restriction and of receptor antagonists of the vasopressin V1a, V1b and V2 (aquaretic drugs). Tolvaptan increases the diuresis without natriuretic effects. Several studies have demonstrated the effectiveness in the treatment of pathological conditions such as cirrhosis, SIADH and heart failure.Citation17
AVP is also involved in cellular proliferation of breast, pulmonary and pancreatic cancers.Citation18 Renal cancer may synthesize and express the V2-receptor, resulting in a proliferative effect in CAKI-2 and A498 cell lines. Furthermore, this growth was completely avoided by the preventive addition of the V2-R antagonist (Satavaptan).Citation19 This study thus showed that aquaretic drugs might be used not only to prevent hyponatremia caused by chemotherapy or other drugs but also to inhibit proliferation of several cancers.
Hypernatremia
Hypernatremia, defined as serum Na >145 mEq/L, could be determined by an insufficient intake of water or renal insufficiency or diabetes insipidus. Hypernatremia causes cellular dehydration with aspecific symptoms including nausea, vomiting and fatigue; aggravation occurs with altered mental status, tremors, drowsiness, confusion and coma. Hypernatremia should not be corrected at rates of >1 mEq/h, to allow the cellular adaptation. Although hypernatremia is much less common than hyponatremia in hospitalized cancer patients, it was observed that it is associated with a high mortality rate.Citation20
Hypercalcemia
Hypercalcemia occurs when serum calcium exceeds the value of 10.4 mg/dL. It represents a common metabolic emergency in cancer patients, especially in the advanced stages of disease.Citation21 Hypercalcemia of malignancy is a paraneoplastic syndrome and a frequent complication of advanced breast and lung cancer, and multiple myeloma.Citation22
While some tumors produce parathyroid hormone (PTH)-like proteins, promoting the release of calcium from the bones, other tumors release cytokines that destroy the bone or produce vitamin D analogues, with more absorption of calcium.
The main clinical manifestations of hypercalcemia on kidney are nephrolithiasis and nephrocalcinosis, followed by progressive renal failure and, in severe cases, reduced ability to concentrate urine with polyuria and acute kidney damage.
Treatment of hypercalcemia consists in blocking the destruction of the bone through bisphosphonates (pamidronate, etidronate, clodronate, alendronate, ibandronate and zoledronic acid). Bisphosphonates are used for osteoporosis, but they are also useful for decreasing the risk of skeletal events and bone pain in case of metastases secondary to solid tumors or multiple myeloma. However, bisphosphonates can give side effects such as fever and nausea and osteonecrosis of the jaw and should be used with caution in patients with impaired renal function.Citation23,Citation24 Among bisphosphonates, pamidronate seems to have antiproliferative and apoptotic role in patients with metastatic prostatic cancer.Citation25
Other treatment options for hypercalcemia include calcitonin and mithramycin (or plicamycin). These drugs, characterized by liver, kidney and bone marrow toxicity, are reserved for patients who do not respond to previous therapies. Ten years ago, denosumab, a human monoclonal antibody, has been synthesized. It interacts with receptor activator of nuclear factor-kappa B (RANK)/RANK-ligand (L)/osteoprotegerin (OPG) pathway. Denosumab inactivates RANKL with high affinity and specificity, increasing bone mineral density and reducingbone resorption. It may cause osteonecrosis of the jaw, but to date, no case of renal impairment has been reported.Citation26
Finally, dialysis is necessary when hypercalcemia is associated with severe mental disorders, renal or cardiac insufficiency.
Hypocalcemia
Hypocalcemia, defined as a level of serum calcium <8.5 mg/dL (2.2 mmol/L), represents a much more common event than hypercalcemia. It was a frequent complication of thyroidectomy, for accidental removal of the parathyroid glands. It can also be due to the use of bisphosphonates or denosumab, especially in patients with pre-existing deficiency of vitamin D. Hypocalcemia may be a complication of osteoblastic metastases (from breast and prostate cancer).Citation27,Citation28 Other causes may be a protein deficiency or a resistance to PTH, caused by the tumor itself or by treatments. Non-cancer causes are acute pancreatitis, alcoholism, sepsis, hepatic or renal impairment, therapy with certain antiepileptic drugs or with estrogen. An acute lowering of blood calcium may lead to syncope, heart failure and angina. Other detectable symptoms are muscle cramps, shortness of breath, numbness, or tingling in the extremities, cardiac arrhythmias, and tetanus. The chronic hypocalcaemia leads to the formation of cataracts, dry skin, brittle hair and nails, loss of teeth and itching. All patients treated for long time with bisphosphonates should receive, in advance, calcium and vitamin D by mouth. Patients with acute symptoms of hypocalcaemia should be treated with one or two 10-mL ampoules of 10% calcium gluconate diluted in 50–100 mL of 5% dextrose and infused slowly over 10 min until the re-establishment of normal values. It could be used for a continuous administration of calcium gluconate to prevent recurrence of hypocalcemia (ten 10-mL ampoules of 10% calcium gluconate in 1 L of 5% dextrose or 0.9% saline given at an initial infusion rate of 50 mL/h).Citation29
Anemia
Anemia in cancer patients can depend on the mechanisms triggered by chronic inflammatory disease or on the type of tumor. There may also be blood loss, hemolysis, bone marrow infiltration by the tumor cells and nutritional deficits. Anyway, anemia is mainly related to the toxic effects of radiotherapy and chemotherapy: >75% of patients treated with these therapeutic modalities suffered from mild to moderate anemia.Citation30
Erythropoietin is used in anemic patients with malignancies in order to reduce the need of blood transfusions, facilitate the oxygenation of the tumor and accentuate, through the effect of oxygen, the radio sensitivity improving the quality of life. Nevertheless, some reports have underlined adverse effects of erythropoietin in oncologic patients such as the reduction of survival, the progression of the neoplastic process and the increase of venous thrombosis incidence.Citation31
Recently, the Bennett’s study (SONAR) points out the indication to maintain hemoglobin levels between 10 and 12 g% in patients with chronic renal disease, and between 11 and 12 g% in patients on hemodialysis, because of the risks of myocardial infarction, stroke, venous thromboembolism and mortality of patients with Hb values >12 g/dL.Citation32
Acute tumor lysis syndrome
Acute Tumor Lysis Syndrome (TLS) arises from a sudden release of intracellular products coming from a massive lysis of tumoral cells into the blood circulation, spontaneous or secondary to radiotherapy or to chemotherapy. This complication is characterized by hyperuricemia, hypercalcemia, hyperphosphatemia, hyperazotemia (alone or in combination), and/or by AKI.Citation31 Hyperphosphatemia is common and associated with calcium phosphate precipitation, resulting in hypocalcemia. Moreover, primary renal failure could also be secondary to the precipitation of uric acid crystals into nephron ducts, in patients with hyperuricemia and hyperuricuria.
TLS occurs more frequently in acute or chronic leukemia, with high white blood cell count, and in malignant lymphoma, treated with radiotherapy or chemotherapy. TLS has been observed, although rarely, in patients with solid cancers or after treatment with non-cytotoxic drugs such as interferon alpha, tamoxifen and intrathecal MTX. TLS severity is favored by systemic diseases and by pre-existing kidney failure. Available prophylactic measures could decrease the incident of this syndrome, as well as the identification of patients at risk and the reduction or elimination of risk factors.
Urinary output should be valid to improve electrolytes excretions, including uric acid. An intensive hydration, which should be initiated 24–48 h before the beginning of the therapy and maintained during the entire treatment, and allopurinol administration, are standard prophylactic measures.Citation33
If the urinary flow is <4–5 L, then the use of diuretics could be appropriated.
Allopurinol, preventing the production of uric acid through xanthine oxidase inhibition, is usually administered at the dose of 300 mg, once or twice per day. The administration of allopurinol requires great caution because its interference with 6 mercaptopurine, azathioprine degradation, and marrow toxicity of cyclophosphamide. The recombinant urate oxidase (rasburicase), used in prophylactic treatment, represents a safe and effective alternative to allopurinol.Citation34
Interpretation of cancer biomarkers in renal patients
SheblCitation35 demonstrated a close relation between kidney disease and the presence of cancer, through a case–control study involving >1 million of cancer patients and almost 100,000 of controls patients with ESRD.
The study reported an increased risk of cancer among individuals with ESRD. The end-stage renal disease was associated with a significantly elevated risk of stomach cancer, small intestine, colon, liver, biliary tract, lung and cervix cancer, as well as multiple myeloma and chronic myeloid leukemia. High risk was present for renal cancer and cancer of urinary tract. Among renal tumors, the most observed was the renal cell carcinoma. ESRD is associated with a low risk of prostate cancer, as observed in a collaborative study,Citation5 although in other studies, the prevalence of prostate cancer in ESRD patients was equal or higher compared with that of general population.Citation36,Citation37
ESRD patients are often affected by hepatitis due to HBV or HCV, or having HPV-related infections or were subjected to immunosuppressive therapy. The risk of developing cancer in these patients is increased not only for the renal disease but also for their immunodeficiency. ESRD patients are at high risk of hospitalization and death due to infection, especially related to cardiovascular disease, septicemia due to intravascular catheter-related infections, pneumonia and uremia; factors that might contribute to modify immune system in ESRD patients.
Also, in hemodialysis patients, the risk of cancer is increased as a recent study demonstrates,Citation38 with prevalence of cancer of male and female genital organs, renal cancer, gastrointestinal cancer, cancer of the urinary tract and skin cancer.
Cancer markers are a heterogeneous group of substances, above all glycoproteins, primarily produced by tumor that might come in handy for detection and follow up of many cancers. Unfortunately, these markers are often also associated with non-cancerous diseases, particular habits of life and diagnostic procedures. Moreover, in some circumstances, as just in case renal failure coexists, their clearance is altered and therefore their value becomes less reliable.Citation39 Furthermore, the quantity of cancer markers traceable in CKD patients might both increase because of a pro-inflammatory state linked with chronic renal disease or with smoking habit and decrease because of overt proteinuria. Some authors demonstrate that serum concentration of tumor markers may be affected not only by renal function but also by patients’ nutritional status.Citation40
Serum concentration of CA125 (in males), CYFRA 21-1, NSE neuron-specific enolase and SCC-Ag squamous cell carcinoma antigen, CEA, fPSA,Citation41 Chromogranin ACitation42 and beta-2-microglobulinCitation43 seems to be increased in CKD patients.Citation44,Citation45
As for as CA19-9 (Gastrointestinal antigen GICA), there are discordant data about its concentration in CKD patients. Some authors observed an increase of CA19-9 serum concentration in case of renal impairment,Citation44 while others identify no differences in CA19-9 serum concentration between patients with CRF compared to normal subjects.Citation45,Citation46
On the other hand, AFPCitation40 and CA125 (in female)Citation47 levels seem to be not modified by the presence of renal impairment, neither in ESDR nor in dialysis patients.Citation48
In hemodialysis patients, tumor marker serum concentration trend can be modified by using different type of hemodialysis membrane,Citation48 e.g. modifying the material of membrane or changing in pore diameter; cancer markers with a small molecular weight overpass filter, resulting in reduction of their serum concentrations. Some studies demonstrated that PSA level is not alternated in hemodialysis patients,Citation48–50 although other authors observed an increase in total PSA and in PSA index in this population.Citation51 Perhaps, the different results seen in the studies mentioned above are due to the different ages of the patients enrolled.
With regard to CA15-3, the majority of authors observed an increase in its serum concentration in hemodialysis patients unlike CKD patients,Citation46–53 whereas Xiaofang did not identify this kind of relationship.Citation44 It should be remembered that CA15-3 levels increase if concomitant hepatitis C infection co-exists.
AFP does not seem to be alternated in hemodialysis patients,Citation52–54 whereas CA19-9 serum concentration increases after hemodialysis.Citation46–52 As regards CA125, some authors observed that the increase concentration of this tumor marker after HD treatment might be gender specific (in male patients),Citation44 others hypothesized a relationship between elevated CA125 levels and HCV infection.Citation52 Further studies are needed to evaluate the diagnostic and prognostic value of tumor markers in order to avoid misdiagnosis and unnecessary diagnostic investigations in the presence of renal impairment.
Biomarkers. NGAL: a biomarker between nephrology and oncology
Among all the potential biomarkers of kidney damage that are being studied in both plasma and urine, NGAL appears to emerge as a possible predictor not only of acute kidney impairmentCitation55 but also marker of progression of tumor disease. We have already spoken of its possible use as markers of CIN. NGAL is also a marker of CKD and its progression; in proteinuric patients with chronic renal disease, urinary and serum levels of NGAL are inversely related to indices of residual renal function.Citation56
Even in Oncology, NGAL find its place. NGAL is responsible for non-specific immune response; indeed the first and determinant recognized role of this protein relates to a direct participation in the mechanisms of innate non-specific immune response. It is also in charge of growth and cell differentiation, having an ant apoptotic effect. In effect, several proteins belonging to the lipocalin family seem to play a key role in the regulation of proliferation, differentiation and development of some human cancers.Citation57,Citation58
In a study, Bauer et al.Citation59 have confirmed the correlation between breast cancer and tissue expression of NGAL, especially in histological specimens of ductal carcinoma, and then also showed a correlation with the indexes of negative prognosis, as the high degree of dedifferentiation, presence of lymph node metastases and high proliferative index. The analysis also showed a significant negative association with survival and the absence of clinical recurrence, suggesting that the assessment of levels of NGAL may represent prognostic risk stratification and planning of anticancer therapies more intense. It seems that NGAL modulates positively the activity of metalloproteinase-9 (MMP9), enzyme protein involved in the resorption of the extracellular matrix and thus in tumor dissemination and metastasis. NGAL, by binding to MMP9, prevents its proteolytic degradation, with the exaltation of its enzymatic activity and therefore greater propensity to invasiveness and expansion of the tumor.Citation60
Conclusions
Cancer patient is a complex patient. Tumor disease is able to affect the body in its entirety, damage in several organs and systems, including the kidney. It is very important to know patients’ renal function, and closely monitor it, before and during treatment, using both MDRD formula and above all, specific biomarkers more early and sensitive than the increase of creatinine, like NGAL.
Physicians should identify cancer patients at risk of developing renal failure and should be able to set up a personalized therapy that takes into account the risks and benefits of treatment in order to look at the patient as a whole, avoiding sectorial approaches and emphasizing multidisciplinary support. Moreover, further studies are needed to identify molecules of acute renal damage that would allow you to monitor the patient during the natural history of the cancer disease and to take prompt action to prevent chronic kidney damage.
Declaration of interest
No external funding was secured for this study. The authors have no financial relationships relevant to this article to disclose. The authors have no conflicts of interest to disclose.
References
- Lameire NH, Flombaum CD, Moreau D, Ronco C. Acute renal failure in cancer patients. Ann Med. 2005;37(1):13–25
- Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–2831
- Wong G, Hayen A, Chapman JR, et al. Association of CKD and cancer risk in older people. J Am Soc Nephrol. 2009;20(6):1341–1350
- Arican A, Ozdemir N, Sezer S, et al. Tumor markers in hemodialysis patients. Transplant Proc. 1999;31(8):3367–3368
- Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet. 1999;354(9173):93–99
- Darmon M, Ciroldi M, Thiery G, Schlemmer B, Azoulay E. Clinical review: Specific aspects of acute renal failure in cancer patients. Crit Care. 2006;10(2):211. doi: 10.1186/cc4907
- Ogawa T, Niho S, Nagai S, et al. Moderate renal dysfunction may not require a cisplatin dose reduction: A retrospective study of cancer patients with renal impairment. Int J Clin Oncol. 2013;18(6):977–982
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007
- Sahni V, Choudhury D, Ahmed Z. Chemotherapy-associated renal dysfunction. Nat Rev Nephrol. 2009;5(8):450–462
- Widemann BC, Balis FM, Kim A, et al. Glucarpidase, leucovorin, and thymidine for high-dose methotrexate-induced renal dysfunction: Clinical and pharmacologic factors affecting outcome. J Clin Oncol. 2010;28(25):3979–3986
- Perazella MA. Onco-nephrology: Renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;7(10):1713–1721
- McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4(Suppl 5):S3–S9
- Section 4: Contrast-induced AKI. Kidney Int Suppl. 2012;2(1):69–88
- Lacquaniti A, Buemi F, Lupica R, et al. Can neutrophil gelatinase-associated lipocalin help depict early contrast material-induced nephropathy? Radiology. 2013;267(1):86–93
- Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer. 2007;15(12):1341–1347
- Vantyghem MC, Balavoine AS, Wemeau JL, Douillard C. Hyponatremia and antidiuresis syndrome. Ann Endocrinol. 2011;72(6):500–512
- Bolignano D, Coppolino G, Criseo M, Campo S, Romeo A, Buemi M. Aquaretic agents: What’s beyond the treatment of hyponatremia? Curr Pharm Des. 2007;13(8):865–871
- North WG. Gene regulation of vasopressin and vasopressin receptors in cancer. Exp Physiol. 2000;85(Spec No):27S–40S
- Bolignano D, Medici MA, Coppolino G, et al. Aquaretic inhibits renal cancer proliferation: Role of vasopressin receptor-2 (V2-R). Urol Oncol. 2010;28(6):642–647
- Salahudeen AK, Doshi SM, Shah P. The frequency, cost, and clinical outcomes of hypernatremia in patients hospitalized to a comprehensive cancer center. Support Care Cancer. 2013;21(7):1871–1878
- Bergman PJ. Paraneoplastic hypercalcemia. Top Companion Anim Med. 2012;27(4):156–158
- Clines GA. Mechanisms and treatment of hypercalcemia of malignancy. Curr Opin Endocrinol Diab Obes. 2011;18(6):339–346
- Janovska Z. Bisphosphonate-related osteonecrosis of the jaws. A severe side effect of bisphosphonate therapy. Acta Med. 2012;55(3):111–115
- Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64(1):281–289
- Iguchi K, Tatsuda Y, Usui S, Hirano K. Pamidronate inhibits antiapoptotic bcl-2 expression through inhibition of the mevalonate pathway in prostate cancer PC-3 cells. Eur J Pharmacol. 2010;641(1):35–40
- Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet. 2011;377(9768):813–822
- Ikesue H, Tsuji T, Hata K, et al. Time course of calcium concentrations and risk factors for hypocalcemia in patients receiving denosumab for the treatment of bone metastases from cancer. Ann Pharmacother. 2014;48(9):1159–1165
- Szentirmai M, Constantinou C, Rainey JM, Loewenstein JE. Hypocalcemia due to avid calcium uptake by osteoblastic metastases of prostate cancer. West J Med. 1995;163(6):577–578
- Cooper MS, Gittoes NJ. Diagnosis and management of hypocalcaemia. BMJ. 2008;336(7656):1298–1302
- Barni S. Rosti G. Pronzato P. L'anemia nel paziente oncologico. In: Kluwer Health Italia, ed. L’Anemia in oncologia. Milano, MI: Springer; 2009:1–181
- Buemi M, Fazio MR, Bolignano D, et al. Renal complications in oncohematologic patients. J Investig Med. 2009;57(8):892–901
- Bennett CL, Spiegel DM, Macdougall IC, et al. A review of safety, efficacy, and utilization of erithropoietin, darbepoetin, and peginesatide for patients with cancer or chronic kidney disease: A report from the southern network on adverse reactions (SONAR). Semin Thromb Hemost. 2012;38(8):783–796
- Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844–1854
- Raghavendran M, Rastogi A, Dubey D, et al. Stones associated renal pelvic malignancies. Indian J Cancer. 2003;40(3):108–112
- Shebl FM, Warren JL, Eggers PW, Engels EA. Cancer risk among elderly persons with end-stage renal disease: A population-based case-control study. BMC Nephrol. 2012;13:65
- Port FK, Ragheb NE, Schwartz AG, Hawthorne VM. Neoplasms in dialysis patients: A population-based study. Am J Kidney Dis. 1989;14:119–123
- Kamata T, Fushimi K. Prevalence of prostate cancer in end-stage renal disease patients. Urol Int. 2008;80:419–424
- Marquardt P, Krause R, Schaller M, Bach D, Von Gersdorff G. Vitamin D and cancer in incident hemodialysis patients. Anticancer Res. 2015;35:1181–1188
- Coppolino G, Bolignano D, Rivoli L, Mazza G, Presta P, Fuiano G. Tumour markers and kidney function: A systematic review. BioMed Res Int. 2014;2014:647541
- Tong HL, Dong ZN, Wen XY, Gao J, Wang B, Tian YP. Impact of chronic kidney disease on serum tumor markers concentrations. Chin Med J (Engl). 2013;126(2):274–279
- Bruun L, Savage C, Cronin AM, Hugosson J, Lilja H, Christensson A. Increase in percent free prostate-specific antigen in men with chronic kidney disease. Nephrol Dial Transplant. 2009;24(4):1238–1241
- Hsiao RJ, Mezger MS, O’Connor DT. Chromogranin A in uremia: Progressive retention of immunoreactive fragments. Kidney Int. 1990;37(3):955–964
- Drüeke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009;22(4):378–380
- Xiaofang Y1, Yue Z, Xialian X, Zhibin Y. Serum tumour markers in patients with chronic kidney disease. Scand J Clin Lab Invest. 2007;67(6):661–667
- Filella X, Cases A, Molina R, et al. Tumor markers in patients with chronic renal failure. Int J Biol Mark. 1990;5(2):85–88
- Zeferos N, Digenis GE, Christophoraki M, Alexopoulos I, Kostakis Gyftaki AH, Moulopoulos S. Tumor markers in patients undergoing hemodialysis or kidney transplantation. Nephron. 1991;59(4):618–620
- Menzin AW, Kobrin S, Pollak E, Goodman DBP, Rubin SC. The effect of renal function on serum levels of CA 125. Gynecol Oncol. 1995;58(3):375–377
- Lye WC, Tambyah P, Leong SO, Lee EJ. Serum tumor markers in patients on dialysis and kidney transplantation. Adv Perit Dial. 1994;10:109–111
- Arik N, Adam B, Akpolat T, Hasil K, Tabak S. Serum tumour markers in renal failure. Int Urol Nephrol. 1996;28(4):601–604
- Maoujoud O1, El Machtani S, Asseraji M, et al. Serum tumor markers in hemodialysis patients. Int J Artif Organs. 2014;37(2):126–132
- García-Sánchez C1, Corchuelo-Maillo C, Congregado-Ruiz CB, et al. PSA levels in patients on hemodialysis treatment. Arch Esp Urol. 2013;66(10):939–944
- Tzitzikos G, Saridi M, Filippopoulou T, et al. Measurement of tumor markers in chronic hemodialysis patients. Saudi J Kidney Dis Transplant. 2010;21(1):50–53
- Estakhri R, Ghahramanzade A, Vahedi A, Nourazarian A. Serum levels of CA15-3, AFP, CA19-9 and CEA tumor markers in cancer care and treatment of patients with impaired renal function on hemodialysis. Asian Pac J Cancer Prev. 2013;14(3):1597–1599
- Bertolini L, Meschi M, Detrenis S, Maggiore U, Savazzi G. Serum concentration of some tumor markers in renal failure. Recenti Prog Med. 2005;96(5):221–225
- Ronco C. Biomarkers for acute kidney injury: Is NGAL ready for clinical use? Crit Care. 2014;18(6):680. doi: 10.1186/s13054-014-0680-0
- Bolignano D, Coppolino G, Lacquaniti A, Nicocia G, Buemi M. Pathological and prognostic value of urinary neutrophil gelatinase-associated lipocalin in macroproteinuric patients with worsening renal function. Kidney Blood Press Res. 2008;31(4):274–279
- Perrin C, Patard JJ, Jouan F, et al. The neutrophil gelatinase-associated lipocalin, or LCN 2, marker of aggressiveness in clear cell renal cell carcinoma. Clin Neuropathol. 2010;29(5):317–322
- Bratt T. Lipocalins and cancer. Biochim Biophys Acta. 2000;1482(1–2):318–326
- Bauer M, Eickhoff JC, Gould MN, Mundhenke C, Maass N, Friedl A. Neutrophil gelatinase-associated lipocalin (NGAL) is a predictor of poor prognosis in human primary breast cancer. Breast Cancer Res Treat. 2008;108(3):389–397
- Fernàndez CA, Yan L, Louis G, Yang J, Kutok JL, Moses MA. The matrix metallo-proteinase-9/neutrophil gelatinase associated lipocalin complex plays a role in breast tumor growth and is present in the urine of breast cancer patients. Clin Cancer Res. 2005;11(15):5390–5395