Abstract
Purpose: Clinical use of cisplatin is limited by its nephrotoxicity. Cisplatin-induced nephrotoxicity is associated with an increase in oxidative stress, leading ultimately to kidney dysfunction. The aim of this study was to investigate the effect of pomegranate seed oil against nephrotoxicity induced by cisplatin in adult rats. Methods: Animals were divided into four groups. Group I received corn oil (1 mL/kg). Group II received cisplatin (8 mg/kg). Group III and IV received pomegranate seed oil (PSO) 0.4 mL/kg and 0.8 mL/kg one hour before cisplatin injection for 3 days, respectively. Blood samples were collected by cardiac puncture and used for measuring urea and creatinine concentration. Twenty-hour urine samples were collected to measure protein and glucose concentration. The right kidney fixed in formalin for histological examination and the left kidney was homogenized for measurement of malondialdehyde and total sulfhydryl groups. Results: A significant elevation of serum creatinine, urea, urinary glucose, protein concentrations, and non-significant decrease in total thiol content and increase in MDA level in kidney homogenates were observed in cisplatin-treated rats. Also cisplatin reduced animal’s body weight. Mild-to-moderate tubular cell necrosis, hyaline casts, and vascular congestion were observed in group II. PSO pre-treatment significantly decreased urinary protein, glucose, and serum creatinine concentration. PSO also caused a decrease in serum urea, renal MDA, and increase in thiol content, but the level of these parameters were not significant. Conclusion: The present results suggest that PSO is an effective agent for the prevention of cisplatin-induced renal dysfunction and oxidative damage in rat.
Introduction
Cisplatin (cis-diamminedichloroplatinum(II)) is an inorganic platinum-based antineoplastic drug. It is widely used for treatment of testicular, head and neck, ovarian and cervical carcinomas as well as non-small cell carcinoma of the lung.Citation1 However, it causes several toxicities particularly, nephrotoxicity, which is the main dose-limiting side effect of cisplatin.Citation2,Citation3 High concentration of cisplatin is reached in the S3 segment of the proximal tubule. Tubular injury is recognized as a major pathogenic factor in cisplatin-induced nephrotoxicity.Citation4 Copper transporter receptor1 (Ctr1) and organic cation receptor2(Oct2) are involved in cisplatin transport in tubular cells.Citation1,Citation4 Cisplatin causes impairment of kidney function and acute renal failure via several mechanisms including the inhibition of protein synthesis, generation of reactive oxygen species (ROS), mitochondrial dysfunction, DNA damage and apoptosis.Citation2,Citation5. Cisplatin induces oxidative stress in renal tubular cells through increasing ROS and decreasing antioxidant enzyme activity.Citation6 Many studies confirm oxidative stress as an important factor in tissues damage and antioxidant agents have been expected to either ameliorate or prevent the nephrotoxicity of cisplatin.Citation7–9 Pomegranate (Punnica granatum L.) from Punicaceae family is native to the region from northern India to Iran. It is also widely cultivated in parts of Southwest America, California, Arizona, and Africa.Citation10 Pomegranate has extensively been used in folk medicine for several purposes. Recently, studies have shown that pomegranate has several pharmacological activities, such as antimicrobial,Citation11,Citation12 antioxidant, anti-inflammatory, anticarcinogenic effects.Citation13 Pomegranate – derived products have shown beneficial effects on the treatment and prevention of various diseases, such as cancer, cardiovascular disease, neurological disorders diabetes, and other diseases.Citation14,Citation15 The aim of the present study was to investigate the protective effect of pomegranate seed oil (PSO) on cisplatin-induced nephrotoxicity in rat.
Materials and methods
Drugs and chemicals
Cisplatin was obtained from Mylan Company (France), TMP (tetramethoxypropane), Trizma base (Tris(hydroxymethyl) aminomethane), TBA (2-thiobarbituric acid), n-butanol, DTNB (2,2′-dinitro-5,5′-dithiodibenzoic acid), NaOH (sodium hydroxide), phosphoric acid, HCl (hydrochloric acid), Na2EDTA (ethylenediaminetetraacetic acid disodium salt), KCl (potassium chloride) and ether were purchased from Merck (Darmstadt, Germany). Pomegranate seed oil (d = 0.81 g/mL at 25 °C) was a kind gift from Urom Narin Co. (Uromeya, I. R. Iran).
Animals
The study was performed on 32 adult male W/A rat, 190–220 g body weight (Animal House, School of Medicine, Mashhad, Iran). Animals were housed in a pathogen-free facility on a 12-h light/dark schedule and with ad libitum access to food and water. All animal procedures were approved by the University Ethics Committee and were in compliance with National Laws and with National Institutes of Health guidelines for the use and care of laboratory animals.
Experimental protocols
After acclimatization, animals were randomly divided into four groups (eight each). Group I (control) received 1 mL/kg of corn oil intraperitoneally (i.p.). In group II, a single dose of cisplatin (8 mg/kg) was administered (i.p). Groups III and IV were treated with PSO (0.4 and 0.8 mg/kg) 1 h before cisplatin (8 mg/kg) injection. All procedures were carried out between 10:00 and 12:00 am. The experiment lasted for 3 consecutive days. 24-h urine samples, for measuring glucose and protein concentration were collected, before scarifying animals. Animals were killed 24 h after the last injection, using ether anesthesia; blood samples were taken out by cardiac puncture for measuring the level of serum urea and creatinine. The right kidneys were removed and fixed in 10% neutral buffered formalin for histopathological examinations. The left kidneys were homogenized in cold KCl solution (1.5%, pH = 7) to give a 10% homogenate suspension and used for measuring malondialdehyde (MDA) and thiol content.
Histopathological examinations
Kidney tissues were collected from rats, washed by normal saline solution (0.9%), then fixed in 10% formalin solution, processed, and embedded in paraffin, and sectioned for histopathological studies. The sections were stained with hematoxylin and eosin and observed under light microscope.
Biochemical analysis
Measurement of serum urea and creatinine and urinary glucose and protein concentration
As indicators of kidney function, serum creatinine and urea levels were measured. Serum level of urea was measured colorimetrically using Autoanalyzer (Technicon RA-1000, London, England) and urea kit (Man Lab Company, Tehran, Iran). Serum creatinine concentration was measured by Jaffe’s method.Citation16 Urinary glucose concentration was determined by the enzymatic assay (glucose oxidase) and protein concentration was measured using the turbidimetric method.Citation17,Citation18
Determination of lipid peroxidation
Malondialdehyde level is identified as main product of lipid peroxidation that reacts with TBA to give a red color species [thiobarbituric acid reactive substance (TBARS) has peak absorbance at 532 nm].Citation19 The centrifuge tube containing 0.5 mL homogenate were mixed with 3 mL phosphoric acid (1%) and 1 mL TBA (0.6%). All tubes were placed in a boiling water bath for 45 min. Then the tubes were cooled and 4 mL n-butanol was added to the reaction mixture, vortexed for 1 min, and centrifuged at 20,000 rpm for 20 min. The absorbance of organic layer was measured at 532 nm. MDA content was expressed as nanomoles per gram of tissue.
MDA concentration of the kidney homogenates was calculated using the standard curve of MDA (concentration range of 0–40 μM).
Determination of sulfhydryl groups
The sulfhydryl (SH) group content in kidney homogenates was measured spectrophotometrically by using 2,2′-dinitro-5,5′-dithiodibenzoic acid (DTNB) as a coloring reagent. This reagent produces a measurable yellow-colored product when it reacts with sulfhydryl.
Fifty microliters homogenate was mixed with 1 mL Tris-EDTA buffer in test tube (pH = 8.6) and its absorbance was read at 412 nm (A1). To this was added to 20 μL of 10 mM DTNB. After 15 min at laboratory temperature, the absorption was measured again (A2). Blank (B) was the absorbance of DTNB reagent.Citation20
Total SH groups are calculated using the following equation:
Statistical analysis
Data were expressed as mean ± SEM. Statistical analysis was performed using Prism 6 software (La Jolla, CA). Data were analyzed using one-way analysis of variance (ANOVA) followed by the Tukey-Kramer post-hoc test for comparison between groups. The p-values less than 0.05 were considered to be statistically significant.
Results
Animal’s body weight
Both the initial and the final body weights of all rats was measured and presented in . A significant decrease in the final body weights was observed in rats treated with cisplatin in group I, II and III when compared to the control group.
Table 1. Initial and the final body weights in control group (group 1), cisplatin treated (group II) and PSO pre-treated rats (group III, IV).
Histopathological observations
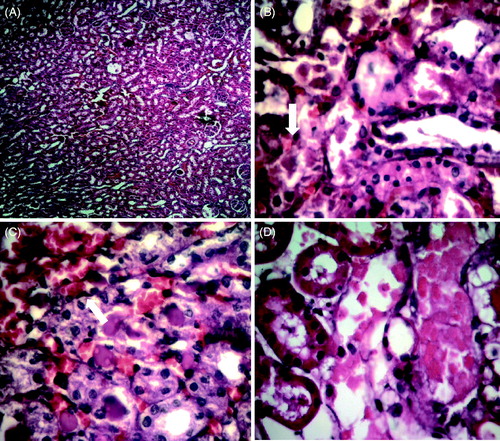
Histopathological changes in renal tissues in different treated groups are shown in . Rats treated with corn oil, showed normal kidney architecture and histology (glomeruli, tubules, interstitium, and blood vessels) (A). The renal tissues of rats treated with cisplatin showed several histological changes, such as mild-to-moderate tubular cell necrosis, hyaline casts, and vascular congestion (B, C). Most of the histopathological changes induced by cisplatin administration were prevented by pretreatment of PSO (0.4, 0.8 mL/kg) and minimal alterations (+/−) was observed in group III and IV (D).
Figure 1. Photomicrographs of histopathological alterations in renal tissues in response to cisplatin and PSO + cisplatin (H&E staining). Rat kidney sections from the control group showing normal histology (A, X100). Cisplatin-treated rat kidney showing tubular cell necrosis (B), hyaline casts and vascular congestion (C, X400). The arrows indicate tubular necrosis and hyaline casts. PSO + cisplatin-treated rats showed dramatic improvement in the histologic appearance. Minimal tubular necrosis was observed in group III and IV (D, X400).
Biochemical findings
Cisplatin caused a marked reduction in renal function, as characterized by significant increases in serum urea and creatinine levels with a concomitant increase in urinary glucose and protein concentration when compared with the control group (p < 0.05). Total thiol content was decreased after the administration of cisplatin but it was not significant (143.71 ± 1.12, p > 0.05). Cisplatin increased the levels of MDA but it was not significant (25.87 ± 1.80, p > 0.05; ).
Figure 2. Effect of pomegranate seed oil (0.4 and 0.8 mL/kg, i.p.) 1 h before injection of cisplatin (8 mg/kg) on concentration of urinary glucose (A), urinary protein (B), serum creatinine (C), serum urea (D), total thiol content (E) and renal MDA (F). Notes: Data were expressed as mean ± SEM (n = 8). *p < 0.05; ***p < 0.001 compared to cisplatin-treated group.
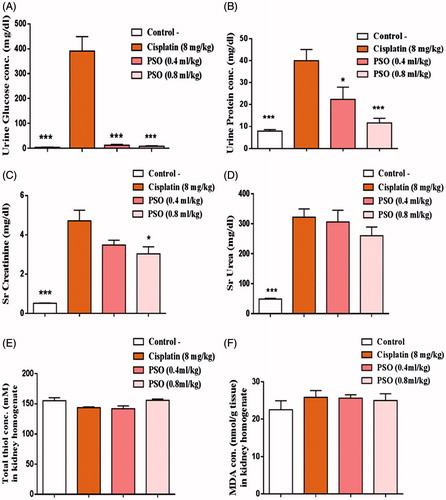
In groups treated with PSO, a significant decrease in protein and glucose excreted in urine (urine protein: 22.37 ± 5.62 in group III and 11.65 ± 2.13 in group IV; urine glucose: 12.43 ± 3.18 in group III and 8 ± 2.30 in group IV) as compared with cisplatin-treated group (p < 0.05). PSO pretreatment (0.8 mL/kg) caused a non-significant elevation in total thiol content (155.76 ± 2.23). Also, it decreased renal MDA but it was not significant (24.98 ± 1.82) when compared with the cisplatin-treated group (p > 0.05).
Group III, showed significant decrease in serum creatinine (3.03 ± 0.36, p < 0.05) and none significant decrease in serum urea (260 ± 29.24, p > 0.05) as compared with second group (serum creatinine: 4.72 ± 0.54, serum urea: 322.28 ± 27.34).
Discussion
The major limitation of cisplatin is nephrotoxicity. Nephrotoxicity occurs with high prevalence in the initial days of cisplatin chemotherapy.Citation9,Citation21 The underlying mechanism of cisplatin-induced nephrotoxicity is still not well known but many recent studies indicate that oxidative stress play an effective role in pathogenesis of kidney injury induced by cisplatin through increasing reactive oxygen species (ROS).Citation6,Citation22,Citation23 Therefore, antioxidants and free radical scavengers may influence the progression of renal oxidative damage and provide nephroprotection against irreversible cell damage induced by cisplatin.Citation24 Recently, much attention has been given to the possible role of natural antioxidants in protecting the kidneys against toxicity induced by cisplatin.Citation25,Citation26 Pomegranate seed oil, with a high content of conjugated fatty acids which punicic acid is the most common. Other components of pomegranate seed include γ-Tocopherol, ursolic acid, sterols (daucosterol, campesterol, stigmasterol, beta-sitosterol), hydroxybenzoic acids (gallic and ellagic), coniferyl 9-O-[beta-d-apiofuranosyl(1–6)]-O-beta-d-glucopyranoside, sinapyl 9-O-[beta-d-apiofuranosyl (1-6)]-O-beta-d-glucopyranoside,Citation24,Citation27 which showed antioxidant activity.Citation27 Moreover, it contains polyphenolic compounds, which were considered as antioxidant and anti-inflammatory compounds.Citation28,Citation29 Several studies reported anti-cancer and anti-inflammatory effects for these components, such as hydroxybenzoic acids, which cause inhibition of growth and induce apoptosis in human DU-145 prostate cancer cellsCitation30 and stroles inhibit pro-inflammatory cytokine production in mice.Citation31 PSO contains ellagic acid, an antioxidant compound that removes peroxy radical and prevents lipid peroxidation induced by Cu+2.Citation32 The present study showed that injection of cisplatin in rats resulted in deterioration of renal function and reduction in glomerular filtration rate as indicated by significant increase in serum urea and creatinine levels. Cisplatin also caused significant increases in urinary protein and glucose concentration. These biochemical parameters were correlated with the renal histological results. Histological analysis of the kidneys of cisplatin-treated rats revealed tubular cell necrosis, hyaline casts, and vascular congestion. This finding confirms kidney injury induced by cisplatin. These results are consistent with the previous studies on cisplatin-induced nephrotoxicity in experimental animals.Citation9,Citation23,Citation25 The reduction in body weight following cisplatin treatment in group II, III, and IV may possibly be due to cytotoxic effects on the gastrointestinal tract and by reduced ingestion of food.Citation33 In this study, cisplatin increased MDA (a secondary product of lipid peroxidation) and decreased thiol content in the kidney homogenates. Cisplatin can bind to the thiol (sulfhydryl) groups.Citation5,Citation8 Sulfhydryl (SH) groups are highly reactive constituents of non-protein and protein molecules and they play important role in several metabolic and biochemical processes, such as detoxification mechanisms, maintenance of protein systems, and enzymatic activation including antioxidant enzymes (superoxide dismutase, catalase, etc.).Citation29,Citation34 Depletion or inactivation of these molecules by cisplatin lead to the accumulation of endogenous ROS and oxidative stress.Citation35 Excessive production of ROS and LPO play a key role in renal damage induced by cisplatin.Citation2,Citation15 Oxidative stress, including ROS can activate death receptor-mediated apoptotic pathways in cisplatin-induced renal tubular cell death. Specifically, ROS can act as second messengers during receptor-mediated apoptosis. Thus, oxidative stress and death receptor-mediated apoptotic cascades are considered to interact synergistically.Citation36,Citation37 Non-significant alterations of renal MDA and thiol content may be due to low dose of cisplatin. PSO administration 1 h before cisplatin treatment significantly reduced the development of cisplatin-induced acute renal failure as evidenced by functional and histopathological findings. PSO significantly reduced urinary glucose and protein concentration. This process shows that PSO restores glucose uptake and protein and prevents their excretion. Although significant difference in renal MDA and thiol content between groups was not observed, but according to other parameters, such as serum creatinine, urinary protein, and glucose and histological changes, PSO in 0.8 mL/kg act as a nephroprotective agent against cisplatin-induced toxicity. Boroushaki et al. previously showed that PSO was effective in preventing renal damage induced by nephrotoxins (e.g. gentamicin, mercuric chloride) and significantly attenuated the increase of MDA concentration in kidney tissue.Citation20,Citation37 This is probably due to free radicals scavenging and antioxidant properties. These protective effects of PSO may be due to the presence of variety of biologically active compounds. Antioxidant and free radicals scavenging properties of PSO may be partially responsible for its nephroprotective activity.
Conclusions
The present results suggest that PSO can be an effective agent against the toxic effects of cisplatin both at the biochemical and histological levels and shows beneficial effects in cisplatin-induced kidney dysfunction and organ damage, but explanation and mechanism of this protection need further studies.
Declaration of interest
The authors declare that there are no conflicts of interest.
This investigation was financially supported by Pharmacological Research Center of Medicinal Plants, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
References
- Al-Kharusi N, Babiker HA, Al-Salam S, et al. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: A dose-dependent study. Eur Rev Med Pharmacol Sci. 2013;17:299–310
- Santos NAG, Catao CS, Martins NM, et al. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504
- Kilic U, Kilic E, Tuzcu Z, et al. Melatonin suppresses cisplatin-induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutrition Metab. 2013;10:7–15
- Pabla N, Murphy RF, Liu K, Dong Z. The copper transporter Ctr1 contributes to cisplatin uptake by renal tubular cells during cisplatin nephrotoxicity. Am J Physiol Renal Physiol. 2009;296:F505–F511
- Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: Molecular mechanisms. Cancer Ther. 2003;1:47–61
- Iseri S, Ercan F, Gedik N, Yuksel M, Alican I. Simvastatin attenuates cisplatin-induced kidney and liver damage in rats. Toxicology. 2007;230:256–264
- Srinivasan KK, Mathew JE, Joseph K, Vachala SD, Malini S. Effect of ethanol extract of Graptophyllum pictum (L.) Griff. on cisplatin induced nephrotoxicity in rats. Herba Polonica. 2011;57:51–65
- Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: A review of some recent research. Food Chem Toxicol. 2006;44:1173–1183
- Palipoch S, Punsawad C. Biochemical and histological study of rat liver and kidney injury induced by cisplatin. J Toxicol Pathol. 2013;26:293–299
- Jurenka J. Therapeutic applications of pomegranate (Punica granatum L.): A review. J Altern Med Rev. 2008;13:128–144
- El-Sherbini GM, Ibrahim KM, El Sherbiny ET, Abdel-Had NM, Morsy TA. Efficacy of Punica granatum extract on in-vitro and in-vivo control of Trichomonas vaginalis. J Egypt Soc Parasitol. 2010;40:229–244
- Braga LC, Shupp JW, Cummings C. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339
- Lansky P, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206
- Hartman RE, Shah A, Fagan AM, et al. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol Dis. 2006;24:506–515
- Mena P, Girones-Vilaplana A, Moreno DA, Girones-Vilaplana C. Pomegranate fruit for health promotion: Myths and realities. Func Plant Sci Biotech. 2011;5:33–42
- Masson P, Ohlsson P, Bjorkhem I. Combined enzymatic – Jaffe’s method for determination of creatinine in serum. Clin Chem. 1981;27:18–21
- Lott JA, Turner K. Evaluation of Trinder’s glucose oxidase method for measuring glucose in serum and urine. Clin Chem. 1975;21:1754–1760
- Mc Elderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein compared. Clin Chem. 1982;28:356–360
- Hosseinzadeh H, Sadeghnia HR, Ziaee T. Safranal, a constituent of Crocus sativus (saffron), attenuated cerebral ischemia induced oxidative damage in rat hippocampus. J Pharm Pharmaceut Sci. 2005;8:394–399
- Boroushaki MT, Asadpour E, Sadeghnia HR, Dolati K. Effect of pomegranate seed oil against gentamicin-induced nephrotoxicity in rat. J Food Sci Technol. 2014;51:3510–3514
- Rubera I, Duranton C, Melis N, Cougnon M, Mograbi B, Tauc M. Role of CFTR in oxidative stress and suicidal death of renal cells during cisplatin-induced nephrotoxicity. Cell Death Dis. 2013;4:355–365
- Borrego A, Zamora ZB, Gonza R, et al. Protection by ozone preconditioning is mediated by the antioxidant system in cisplatin-induced nephrotoxicity in rats. Mediators Inflamm. 2004;13(1):13–19
- Nematbakhsh M, Zahra Pezeshki Z, Eshraghi-Jazi F, et al. Vitamin E, vitamin C, or losartan is not nephroprotectant against cisplatin-induced nephrotoxicity in presence of estrogen in ovariectomized rat model. Int J Nephrol. 2012;2012:284896. doi: 10.1155/2012/284896
- Lansky P, Newman RA. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J Ethnopharmacol. 2007;109:177–206
- Behling EB, Sendao MC, Francescato HDC, Antunes LMG, Costa RS, Bianchi Mde L. Comparative study of multiple dosage of quercetin against cisplatin-induced nephrotoxicity and oxidative stress in rat kidneys. Pharmacol Rep. 2006;58(4):526–532
- Antunes LMG, Darin JDC, Bianchi MLP. Effects of the antioxidants curcumin or selenium on cisplatin-induced nephrotoxicity and lipid peroxidation in rats. Pharmacol Res. 2001;43:145–150
- Wang RF, Xie WD, Zhang Z, et al. Bioactive compounds from the seeds of Punica granatum (pomegranate). J Nat Prod. 2004;67:2096–2098
- Yamasaki M, Kitagawa T, Koyanagi N, et al. Dietary effect of pomegranate seed oil on immune function and lipid metabolism in mice. Nutrition. 2006;22:54–59
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat kidneys. Ren Fail. 2010;32:612–617
- Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453
- Ndhlala AR, Moyo M, Staden JV. Natural antioxidants: Fascinating or mythical biomolecules? Molecules. 2010;15:6905–6930
- Ramanathan L, Das NP. Inhibitory effects of some natural products on metal-induced lipid oxidation in cooked fish. Biol Trace Elem Res. 1992;34:35–44
- Mora LdeO, Antunes LM, Francescato HD, Bianchi Mde L. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517–522
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007
- Sanchez-Gonzalez PD, Lopez-Hernandez FJ, Perez-Barriocanal F, Morales AI, Lopez-Novoa JM. Quercetin reduces cisplatin nephrotoxicity in rats without compromising its anti-tumour activity. Nephrol Dial Transplant. 2011;1–12. [Epub ahead of print]. doi: 10.1093/ndt/gfr195
- Tsuruya K, Tokumoto M, Ninomiya T, et al. Antioxidant ameliorates cisplatin-induced renal tubular cell death through inhibition of death receptor-mediated pathways. Am J Physiol Renal Physiol. 2003;285:F208–F218
- Boroushaki MT, Mollazadeh H, Rajabian A, et al. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren Fail. 2014;36(10):1581–1586

