Abstract
Objective: In this study, we aimed to investigate the effect of diclofenac sodium (DS) and melatonin (MEL) on kidney of the prenatally administered rats. Materials and methods: Pregnant rats were divided into the control, physiological saline, DS, and DS + MEL groups. All injections were given beginning from the 5th day after mating to the 15th day of the pregnancy. Physical dissector and Cavalieri principle were used to estimate the numerical density and total number of glomeruli and the volumetric parameters of kidney, respectively. Results: Our stereological results indicated that DS application during the pregnancy lead to decrease in the mean volume, numerical density, and total number of the glomeruli (p < 0.05). In addition, we determined that usage of the MEL with the DS caused increases in the mean volume, numerical density, and total number of the glomeruli (p < 0.05). So, there was no significant difference in terms of the any parameter between the CONT and DS + MEL groups (p > 0.05). Light microscopic investigation showed congestion in blood vessels and shrinkage of the Bowman's space in the DS group. Moreover, there was degeneration in nephrons including glomerulosclerosis and tubular defects, and an increase in the connective tissue in the kidneys of the DS-treated group. However, usage of the MEL with the DS caused preventing of these pathological alterations in the kidney. Discussion: We suggested that DS might lead to adverse effects in the kidneys of the rats that are prenatally subjected to this drug. Fortunately, these adverse effects can be prevented by the melatonin supplementation.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used for the treatment of various diseases, such as osteoarthritis, rheumatoid arthritis, ankylosing spondylitis, and acute muscle pain conditions. Diclofenac sodium (DS) is a drug, which belongs to the class of NSAIDs. NSAIDs generally prevent cyclooxygenase (COX) activity and so block prostaglandin production. Prostaglandins play a key role in inflammatory response by regulating immune function and in the control of cell proliferation.Citation1
Several reports indicated that the toxic doses of DS can cause nephrotoxicity in adult humans and experimental animals.Citation2 The effects of NSAIDs on the kidney of experimental animals have been extensively studied biochemically and histologically. But, there is a little knowledge about the effects of the DS on renal tissue of the developing kidney.Citation3
Gokcimen et al.Citation4 noticed that fetal neuronal apoptosis is significantly induced in DS-treated pregnant rats. Moreover, Chan et al.Citation5 showed that rodents treated with DS deliver fetuses with severe morphological abnormalities, such as defects of the palate, limbs, and ductus arteriosus. Recent studies report that trace concentrations of DS may induce toxic effects on different organisms as well as developmental, reproductive, and renal damage.Citation6,Citation7 In addition to these studies, Stepanova et al.Citation8 notice that the subchronic exposure of DS had effects on mortality and some parameters of oxidative stress (GST, GR, and TBARS). Occurring reactive oxygen species (ROS) and free radicals in tissues may lead to damage of the biologically important materials, such as DNA, protein, carbohydrates, and lipids.Citation9,Citation10 Melatonin (MEL) is a peptide hormone and it made by the pineal gland. It is a strong antioxidant and it is found in meats, grains, fruits, and some vegetables. In the literature, it was reported that MEL has neuroprotective effects on peripheral nerve that prenatally exposure to DS.Citation11,Citation12 But till now, there is no other study investigating possible protective effect of MEL on developing kidney prenatally subjected to DS.
Therefore, the present study was carried out to evaluate the effect of the DS and MEL on the kidney of adult male rats with the histological and stereological points of view.
Materials and methods
This study was approved by Ethic commission of animal researches in Ondokuz Mayıs University, Samsun-Turkey. Rats were obtained from the Animal Research Center of Ondokuz Mayıs University. During the study, rats were preserved on a 12:12-h day/night cycle in a temperature-controlled animal room (22 ± 1 °C) and access to food and water were supplied ad libitum. For this purpose, pregnant rats were divided into the five groups. Rats were followed during pregnancy by beginning from the 0th day of pregnancy and they were put in separate cages. At the 5th day of the mating, the DS, DS + MEL and PS injections were started.
The study groups used in this study are summarized as follows:
CONT Group: This group was not exposed to any special protocol.
PS Group: Between the 5th and15th days of pregnancy every day 1 mL/kg i.p. PS injection was made at 16:00 and17:00 pm.
DS Group: In this group, DS injections were intraperitoneally performed by beginning from 5th day to 15th day of pregnancy with a dose of 3.6 mg/kg/day (Voltaren 75 mg/3 mL, Novartis, İstanbul, Turkey) at 16:00–17:00 pm.
DS + MEL Group: In addition to the protocol of the DS group, from the 5th–15th days of the pregnancy 50 mg/kg i.p. melatonin (Lyophilized MEL Powder M5250 10 g (Sigma Aldrich Comp., Interlab A.S., Istanbul, Turkey) was daily given at 16:00–17:00 pm.
The kidneys were then removed from all animals and processed by graded alcohols (Sigma Chemical Comp., St. Louis, MO) and xylene (Sigma Chemical Comp., St. Louis, MO). Then, immersed in paraffin series and embedded in fresh paraffin (Merck, Darmstadt, Germany).
Sections were cut at 10-μm thicknesses from the blocked kidney tissues by using a rotary microtome (Leica RM 2135, Leica Instruments, Nussloch, Germany). Each slide was stained with hematoxylin–eosin dye (H–E) for light microscopic examination and stereological analysis (Leica RM 2135, Leica Instruments, Nussloch, Germany). For stereological analysis, a computer-assisted analysis system called Stereo Investigator software version 9.0 was used (Micro Bright Field Inc., Colchester, VT).
Stereology
Tissue sampling and stereological methods
Based on a pilot study, we obtained almost 240 sections from each kidney. We decided to select every 31st specimen and its adjacent sections as dissector pairs from the tissue sections using a systematic random sampling approximate. Because of this sampling strategy, nearly 15–20 section pairs for each kidney were used. The physical dissector-counting method was applicative to these section pairs to estimate the number of glomeruli in each kidney.
A 90-µm distance separated the first selected section and its adjacent section, called the dissector pair. A stereology analysis system (Stereoinvestigator 9.0, Microbrightfield, Colchester, VT) was used for the stereological estimation of the kidneys’ volume and number of glomeruli. The level of coefficient of errors (CE) for number of glomeruli and kidney volume estimation was determined to be in an admissible range.Citation13
Volume estimation with the cavalieri principle
Volumetric evaluation of a structure that has any shape and size can effectively obtained by the Cavalieri principle.Citation14 An important rule of this origin is as follows: in order to get a impartial estimate of the volume of an objective, the objective must be cut to serial and parallel planes separate by a fixed intervalCitation15,Citation16 and using a point-counting grid for the area estimate of section profiles. The point density of the grid was designed to obtain a proper CE for the serial paraffin sections of the study. The CE and coefficient of variation were estimated and the volumes of the kidneys were estimated with the following formulaCitation16:
where, V is the mean volume of the kidney, t is the mean section thickness, a/p is the inter-point area, and ΣP is the total number of points hitting whole serial sections of the kidney.
Estimation of glomerulus number
The boundary of a section that belongs to the dissector pair called the reference section was traced with Stereoinvestigator software and then it was used to determine the section’s cut surface area. The estimated reference section area of each kidney profile was divided into equal fields in the x- and y-axes of the microscope. Finally, the images of all fields in each step, as determined previously via motorized stages of the microscope, were taken using a charge-coupled device (CCD) camera. The same procedure was applied to the other section of the section pair, called the look-up section. Then, the adjacent fields were located on the PC screen and a suitable unbiased counting frame was manually placed on the kidney photographs with a fixed rule. Finally, the dissector-counting method was applied to these section pairs. Reference and look-up sections were reversed in order to double the number of dissector pairs without taking new sections.Citation13,Citation15
The mean numerical density of glomeruli (NVglo) was estimated using the following formula:
where,
is the total number of counted glomeruli seen in the reference sections but not in the look-up sections, t is the mean section thickness, and A is the area of the counting frame. Then, the total number of glomeruli was estimated by multiplying of the mean numerical density of glomeruli (NVglo) with mean volume of the cortex.
Statistical analysis
Data obtained from specimens were analyzed by using the one-way ANOVA and Bonferroni’s post-hoc test. Statistical analyses were performed using statistical package program SPSS 15.0 (IBM Corp., Armonk, NY) and statistical significance was determined when p values were less than 0.05.
Results
Stereological results
All stereological data are summarized in and submitted under subtitles with detail as given in the following sections.
Table 1. Morphometrical evaluations in the control and experimental groups with their ±SEM.
Total volume of the proximal tubules
The total volume of the proximal tubules in the kidney of DS+MEL group was significantly higher than those of the CONT, PS and DS groups (p < 0.05). However, the total volume of the proximal tubules in the kidneys of the DS group was significantly higher than those of the CONT and PS groups (p < 0.05). There was no significant difference among to the CONT, PS and DS + MEL groups in terms of the total volume of the proximal tubules (p > 0.05).
Total volume of distal tubules
The total volume of the distal tubules in the kidney of the DS + MEL group was significantly higher than those of the CONT, PS and DS groups (p < 0.05). Furthermore, the total volume of the distal tubules was significantly increased in the DS group in comparison to those of the CONT and PS groups (p < 0.05).
Mean volume of the glomeruli
There was a significant difference between the CONT and DS groups (p < 0.05). In addition, volume of the glomeruli in the DS group was significantly decreased in comparison to those of the CONT, PS and DS + MEL groups (p < 0.05). There was no difference among to the CONT, PS and DS + MEL groups (p > 0.05).
Mean numerical density of the glomeruli
The mean numerical density of the glomeruli (glomeruli/mm3) in the DS group was significantly less than those of the CONT, PS and DS + MEL groups (p < 0.05). Additionally, there was no significant difference among the CONT, PS and DS + MEL groups (p > 0.05).
Total number of the glomeruli
The total number of glomeruli of the DS group was significantly decreased than those of the Cont, PS and DS + MEL groups (p < 0.05). However, the total number of glomeruli in the DS + MEL group was significantly more than that of the DS group (p < 0.05). Additionally, there was no significant difference among the CONT, PS, and DS + MEL groups (p > 0.05).
Histological results
The renal cortex of the control rats contained glomeruli, vessels, tubules, and interstitium. When evaluating these renal specimens by light microscopy on H-E stained sections, the overall cellularity of the glomeruli, the symmetry of the glomerulus and the thickness of the capillary walls were normal. Renal tubules (the long and winding neck) formed as the proximal tubule, the loop of Henle and the distal tubule.
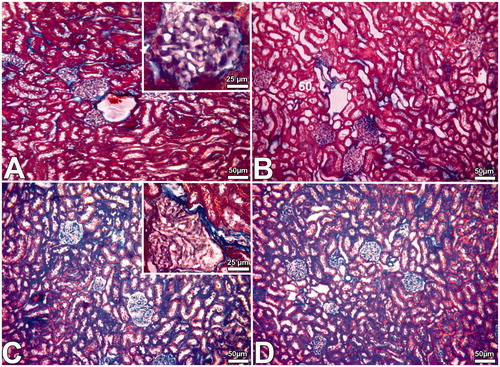
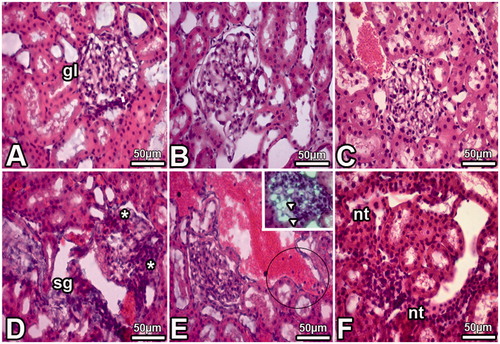
Our results indicate that DS application leads to pathological alterations in the kidney. Bowman spaces were smaller and irregular shaped glomeruli were seen according to that of the control sections ( and ). Light microscopic investigation showed congestion in blood vessels, degeneration in nephrons, including glomerulosclerosis and tubular defects, and an increase in the connective tissue in the kidneys of the DS treated group (). In addition, there were many mononuclear leukocytes in the sections of the DS group (). However, MEL treatment groups have seen normally tissue structures ( and ). There were normally shaped glomeruli, Bowman spaces, and tubules.
Figure 1. Images obtained from the kidney of the DS (D, E, F), Cont (A), PS (B) and DS + MEL (C) groups. Notes: Tubular necrosis (nt), sclerosis (sg), dilated glomerular capillaries (arrowheads), many mononuclear cells (in circled area) and segmental necrosis (*) of the glomeruli were detected in kidney samples of the DS group.
Discussion
DS is commonly used for certain medical purposes by women in the different periods, such as pregnant and lactating, although it has certain harmful effects on body organs.Citation17 The present investigation was constructed to study the effect of DS, which administered during pregnancy, on the morphometric and histological alterations of the kidney in adult male rats. The results of the present work clearly illustrated that the application of 3.6 mg/kg body weight of DS to pregnant rat during gestation (5–15 days) had induced noticeable results in the histological features of the kidney of such treated animals.
During the recent years, an increasing number of studies and researches have been published concerning the nephrotoxic effects of several drugs, including NSAIDs, in developing fetus. Sabry Sahar et al.Citation3 indicated that treatment of pregnant subjects with NSAIDs during pregnancy could cause renal dysgenesis in neonates. The light microscopic investigation and morphometric data approved that DS has negative effects on the kidney and could disrupt the normal renal morphology and structure.
Siu et al.Citation18 reported that DS crosses the human placenta easily during the first trimester. Therefore, possible toxic effects of DS were among the main causes of the histological changes. It was observed in present study that toxic effect was in the kidney because of diffusion and excretion of DS through the renal tissue. Also, Triebskorn et al.Citation19 and Hussain et al.Citation20 observed histopathological changes in liver and kidney of some animals, which are treated with DS. Additionally, DS increases the rate of pregnancy termination and it may have potential teratogenic effects.Citation21 It was been reported by Chan et al.Citation22 that exposure to high levels of DS also results in embryo toxicity in rat whole embryos. They also demonstrated in another study that DS-treated rat embryos develop pronounced malformations in the caudal neural tube and hindlimb.Citation22
DS at high dose causes alterations in hematological, biochemical parameters and histopathological changes in liver and kidney of male rats. These adverse effects may contribute to oxidative stress induced by the drug.Citation2 Our study examined the toxic effects in tissues that may have occurred by oxidative stress.
The synthesis by pineal gland melatonin is the strong antioxidant hormone.Citation23,Citation24 Due to the lipophilic nature, MEL can easily pass the blood–brain barrier. MEL is an electron-rich molecule and it has direct antioxidant properties because of its both oil and water-soluble character. Thus, it easily enters to the cytosol of the each cell and the other structures in the cells and therefore, it is more effective than the vitamins and the minerals.Citation25
Yurt et al.’sCitation26 study on the obese rats suggested that after the melatonin treatment, kidney structures and glomerular morphology was returned to normal, healthy structure. Our DS-MEL treatment group had same property. In the melatonin-treated group, that is not any decrease in the number of glomeruli, mean volume of glomeruli, mean volume of proximal and distal tubules. These results are consistent with the strong antioxidant effects of melatonin. Additionally, that can detoxify the harmful effects of drugs.
We suggested that DS usage might lead to renal deformities because of histopathological changes, such as vascular congestion, tubular defects, and connective tissue enlargement of the kidney in rats that are prenatally subjected to this drug. These drugs are widely used for therapeutic purposes in humans. These negative effects may get prevented by the exogenous melatonin application. Therefore, further investigations are needed to confirm these findings.
Declaration of interest
The authors report no conflicts of interest.
References
- Chae JP, Park MS, Hwang YS, et al. Evaluation of developmental toxicity and teratogenicity of diclofenac using Xenopus embryos. Chemosphere. 2015;120:52–58
- El-Maddawy zkh, El-Ashmawy IM. Hepato-renal and hematological effects of diclofenac sodium in rats. Global J Pharmacol. 2013;7:123–132
- Sabry Sahar A, Samia M, Sakr Shahin MA. Histological and ultrastructural studies on the effect of diclofenac sodium on the renal cortex of fetuses of albino mice. Global J Pharmacol. 2014;8:369–377
- Gokcimen A, Ragbetli MC, Bas O, et al. Effect of prenatal exposure to an anti-inflammatory drug on neuron number in cornu ammonis and dentate gyrus of the rat hippocampus: A stereological study. Brain Res. 2007;1127:185–192
- Chan LYS, Chiu PY, Siu NSS, Wang CC, Lau TK. Diclofenac-induced embryo toxicity is associated with increased embryonic 8-isoprostaglandin F2 alpha level in rat whole embryo culture. Reprod Toxicol. 2002;16:841–844
- Brun L, Bernier M, Losier R, Doe K, Jackman P, Lee H. Pharmaceutically active compounds in Atlantic Canadian sewage treatment plant effluents and receiving waters and potential for environmental effects as measured by acute and chronic aquatic toxicity. Environ Toxicol Chem. 2006;26:2163–2176
- Saravanan M, Karthika S, Malarvizhi A, Ramesh, M. Ecotoxicological impacts of clofibricacid and diclofenac in common carp (Cyprinuscarpio) finger lings: Hematological, biochemical, ionorregulatory and enzymological response. J Hazardous Mater. 2011;195:188–194
- Stepanova S, Praskova E, Chromcova L, et al. The effects of diclofenac on early life stages of common carp (Cyprinus carpio). Environ Toxicol Pharmacol. 2013;35:454–460
- Islas-Flores H, Gómez-Oliván LM, Galar-Martínez M, Colín-Cruz A, Neri-Cruz N, García-Medina S. Diclofenac-induced oxidative stress in brain, liver, gill and blood of common carp (Cyprinus carpio). Ecotoxicol Environ Saf. 2013;92:32–38
- Aygün D, Kaplan S, Odaci E, Onger ME, Altunkaynak ME. Toxicity of non-steroidal anti-inflammatory drugs: A review of melatonin and diclofenac sodium association. Histol Histopathol. 2012;27:417–436
- Kaplan S, Esrefoglu M, Aktas A, et al. The effect of prenatal exposure of a non-steroidal anti-inflammatory drug on the optic nerve of female rats: A stereological, histological, and electron microscopic study. J Matern Fetal Neonatal Med. 2013;26:1860–1864
- Keskin I, Kaplan S, Kalkan S, Sutcu M, Ulkay MB, Esener OB. Evaluation of neuroprotection by melatonin against adverse effects of prenatal exposure to a nonsteroidal anti-inflammatory drug during peripheral nerve development. Int J Dev Neurosci. 2014;41:1–7
- Altunkaynak BZ, Altunkaynak ME. Relationship of body weight and volume of liver. A morphometrical and stereological study. Saudi Med J. 2007;28:891–895
- Altunkaynak BZ, Ozbek E, Aydin N, et al. Effects of haloperidol on striatal neurons: Relation to neuronal loss (a stereological study). Fol Neuropathol. 2011;49:21–27
- Altunkaynak BZ, Önger ME, Altunkaynak ME, Ayrancı E, Canan S. Brief introduction to stereology and sampling strategies: Basic concepts of stereology. Neuro Quantol. 2012;1:31–43
- Altunkaynak BZ, Unal D, Altunkaynak ME, et al. Effects of diabetes and ovariectomy on rat hippocampus (a biochemical and stereological study). Gynecol Endocrinol. 2012;28:228–233
- Bloor M, Paech M. Non-steroidal antiinflammatory drugs during pregnancy and the initiation of lactation. Anesth Analg. 2013;116:1063–1075
- Siu SSN, Yeung JHK, Lau TK. A study on placental transfer of diclofenac in first trimester of human pregnancy. Hum Reprod. 2000;15:2423–2425
- Triebskorn R, Casper H, Heyd AR, Köhler HR, Schwaiger J. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part II: Cytological effects in liver, kidney, gills and intestine of rainbow trout (Oncorhynchusmykiss). Aquatic Toxicol. 2004;68:151–166
- Hussain IM, Khan Z, Khan A, Javed I, Saleemi MK. Toxicological effects of diclofenac in four avian species. Avian Pathol. 2008;37:315–321
- Cassina M, De Santis M, Cesari E, et al. First trimester diclofenac exposure and pregnancy outcome. Reprod Toxicol. 2010;30:401–404
- Chan LY, Chiu PY, Siu SSN, Lau TK. A study of diclofenac-induced teratogenicity during organogenesis using a whole rat embryo culture model. Hum Reprod. 2001;16:2390–2393
- Kavaklı A, Acet A, Parlakpınar H, Akpolat N, Şahna E. Ratlarda beyin iskemi – reperfüzyonu sonucu oluşan morfolojik değişikliklere melatonin ve pinealektomi’nin etkisi. FÜ Sağ Bil Derg. 2007;21:63–66
- Doğan A. Sirkadiyen ritmin izofluran uygulanan yeni doğan ratlarda nörotoksisite üzerine etkisinin araştırılması. İzmir, Uzmanlık Tezi: Dokuz Eylül Üniversitesi Tıp Fakültesi Anesteziyoloji ve Reanimasyon Anabilim Dalı; 2008
- Manda K, Reiter RJ. Melatonin maintains adult hippocampal neurogenesis and cognitive functions after irradiation. Prog Neurobiol. 2010;90:60–68
- Yurt KK, Kayhan E, Altunkaynak BZ, Tümentemur G, Kaplan S. Effects of the melatonin on the kidney of high fat diet fed obese rats: A stereological and histological approach. J Exp Clin Med. 2013;30:153–158