Abstract
Tubular epithelial-myofibroblast transition (TEMT) is an important process in renal tubulointerstitial fibrosis. Interleukin-1α (IL-1α) and transforming growth factor-β1 (TGF-β1) have been demonstrated to be key inducers of TEMT. In mouse embryonic fibroblast cells (NIH3T3), P311 protein induces phenotypic changes that are consistent to myofibroblast transformation. In the present study, we investigated the role of P311 gene and protein as well as potential mechanisms underlying TEMT in normal rat kidney tubular epithelial cells (NRK52E). Morphological and molecular changes were determined in NRK52E cells that were treated with IL-1α and/or P311 antibodies. The results showed that the NRK52E cells triggered by IL-1α became fibroblast-like cells, exhibiting hypertrophy of elongated and fusiform-shaped cells. IL-1α induced a time-dependent increase in P311 gene expression in NRK52E cells, with a peak time at 4 days. The expression levels of P311 gene were positively correlated with α-SMA and TGF-β1 gene expression levels. Anti-P311 antibody inhibited P311 and α-SMA expression in the presence of IL-1α. In contrast, anti-P311 antibody increased the expression of TGF-β1 gene in cells cultured with IL-1α. Therefore, P311 gene, together with α-SMA and TGF-β1 genes, was induced in the process of TEMT. P311 protein triggered by interleukin-1α may promote TEMT through a TGF-β1-independent pathway.
Introduction
Tubulointerstitial fibrosis is the final pathway to progressive kidney diseases and its histopathology features excess accumulation of extracellular matrix (ECM) in the renal interstitium. Tubulointerstitial fibrosis has been observed in various types of chronic kidney injury, eventually leading to end-stage renal disease (ESRD). Despite generic dilemma with α-smooth muscle actin (α-SMA) as a fibroblast maker, the monotonous process is characterized by de novo activation of α-SMA positive myofibroblasts, the principal effector cells with excess deposition of ECM in tubular interstitium.Citation1–11 Recent studies suggest that a large proportion of interstitial myofibroblasts is originated from tubular epithelial cells via tubular epithelial-myofibroblast transition (TEMT).Citation12 Numerous molecules associated with TEMT are not known, and the underlying mechanisms are now coming into focus. Transforming growth factor-β1 (TGF-β1) has been demonstrated to be the most potent inducer for initiating and completing the entire TEMT course.Citation13,Citation14 Interleukin-1 (IL-1) acts as a profibrogenic cytokine for inducing morphological and phenotypic transition in normal rat kidney tubular epithelial cells into myofibroblast-like cells in a TGF-β1-dependent mechanism.Citation15,Citation16
P311 gene (also referred to as pentylenetetrazol 17, PTZ17) is highly expressed in late-stage embryonic brain and adult cerebellum, hippocampus, and olfactory bulb in mice.Citation17 P311 protein is an 8-kDa intracytoplasmic protein of unknown biological functions.Citation18 In NIH3T3 fibroblast cells, P311 induces phenotypic changes that are consistent to myofibroblast transformation, independent of TGF-β1.Citation19 In myofibroblasts, P311 inhibits TGF-β1, TGF-β receptor 2, TGF-β1-activating matrix metalloproteinase-2 (MMP-2), and matrix metalloproteinase-9 (MMP-9) genes, with a significant decrease in collagen production.Citation20 P311 downregulates TGF-β1 gene expression partially by binding to TGF-β1 latency-associated protein (LAP), thereby decreases the TGF-β1 auto-induction.Citation20 However, the role of P311 protein in myofibroblast transformation in NRK52E cells has not been reported. Given this background, the present study aimed to investigate the levels of P311, α-SMA, and TGF-β1 genes and protein expression in the process of TEMT triggered by IL-1α in vitro. We sought to explore the potential mechanisms by which P311 acts on TEMT.
Materials and methods
Cell culture
The NRK52E cell line was obtained from American Type Culture Collection (Rockville, MD). NRK52E cells were cultured in Dulbecco’s modified Eagle’s medium with 4.5 g/L glucose (DMEM, Hyclone, Logan, UT) containing 1% fetal bovine serum (FBS, Hyclone, Logan, UT) at 37 °C in a 5% CO2 atmosphere in six-well plates (Corning, Tokyo, Japan). The optimal stimulus dose (10 ng/mL) of recombinant mouse IL-1α (Sigma Chemical Co, St Louis, MO) was obtained from our preliminary experiments.Citation16 To observe P311, α-SMA, TGF-β1 gene expression in TEMT, cells were cultured in the absence or presence of 10 ng/mL IL-1α for 1, 2, 3, 4 or 5 days. To examine effects of P311 on transition, anti-P311 antibodies (5 μg/mL) (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) were co-cultured for 4 days with IL-1α (10 ng/mL) in NRK52E culture.
Electron microscopy
The confluent cells on plastic cover slips were cultured for 4 days in the presence or absence of IL-1α. Cells were fixed in 2.5% glutaraldehyde in phosphate-buffered saline (PBS; PH 7.4) for 120 min, rinsed with PBS, and dehydrated through graded ethanol. Cells were then transferred to amyl acetate for 10 min, put on critical point drying, and then coated with gold. Cells were examined using a scanning electron microscope (Hitachi S-2300, Tokyo, Japan).
Immunohistochemistry
Confluent cells on plastic cover slips were fixed in 4% paraformaldehyde for 20 min at 4 °C and were then perforated in 0.3% Triton X-100 for 10 min on ice. Endogenous peroxidase was inactivated by incubation in 0.3% H2O2 for 20 min. The cells were preincubated with 1:20 normal sheep serum to block non-specific binding for 40 min at 37 °C. The cells were first incubated with anti-α-SMA antibodies (1:200, Santa Cruz, CA) or anti-P311 antibodies (1:200) for 2 h at 37 °C, and then incubated with goat anti-mouse IgG-HRP antibodies (1:200, Santa Cruz, CA) or goat anti-rabbit IgG-HRP antibodies (1:200, Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China) for 1 h at 37 °C. The cells were subsequently stained in diaminobenzidine (DAB) for 5 to 15 min to produce positive brown staining. Finally, the cells were stained with hematoxylin, dehydrated through graded ethanol (80, 95, 100%), cleared with dimethyl benzene, and cover-slipped in an aqueous mounting medium. The NRK52E cells stained with the anti-P311 and anti-α-SMA antibodies were determined by counting the number of positive-stained cells in a total of 1000 cells under high power (×400) microscope.
Real-time PCR
Total RNA was extracted from the cultured cells using TRIZOL Reagent (Invitrogen Biotechnology Co, Ltd., Shanghai, China) according to the manufacturer’s protocol. The cDNA were synthesized using 500 ng total RNA with the ExScript RT Reagent Kit (Perfect Real Time, Takara, Japan). The triplicate cDNA aliquots were amplified using SYBER green Real Time PCR Master Mix (Toyobo, Osaka, Japan). A PCR reaction of 20 μL volume (SYBER green Real Time PCR Master Mix 10 μL, forward primer 0.5 μL, reverse primer 0.5 μL, cDNA 1 μL, DEPC-H2O 8 μL) was amplified in Roche Light cycler (Roche, Basel, Switzerland). Quantitation was performed using Roche light cycler software 4.05 (Basel, Switzerland) and standardized to the housekeeping gene GAPDH. The primer pair sequences are listed in .
Table 1. Oligonucleotide primer sets for real-time PCR.
Western blot analysis
Cell lysates were prepared with 200 μL of cell protein extraction reagent (Kang Chen, Guangzhou, China) with protease inhibitor cocktail and phosphatase inhibitor cocktail (Kang Chen, Guangzhou, China). Protein concentration was measured with Brandford protein assay kit (GALEN, Beijing, China). Western blot analysis was performed as per manufacturer’s protocol with specific antibodies (P311 and α-SMA). Signal band intensity was quantitated using the NIH Image software (Bethesda, MD), and then normalized to β-actin. All experiments were repeated for three times.
Enzyme-linked immunosorbent assay
The supernatant was collected. TGF-β1 protein concentration was measured using an enzyme-linked immunosorbent assay kit (R&D, Minneapolis, MN) according to the method recommended by the manufacturer.
Statistical analysis
Results are expressed as the mean ± SEM. Statistical comparisons were performed by using SPSS 13.0 software (Chicago, IL). Significance was determined by one-way ANOVA. The R represents the correlation coefficient for spearman’s test. For comparisons between two groups, unpaired Student’s t-test was used, and p value of less than 0.05 was taken as significant.
Results
IL-1α induced morphological and phenotypic transition of NRK52E cells
Effects of anti-P311 antibodies on the IL-1α-induced gene expression of P311, α-SMA and TGF-β1
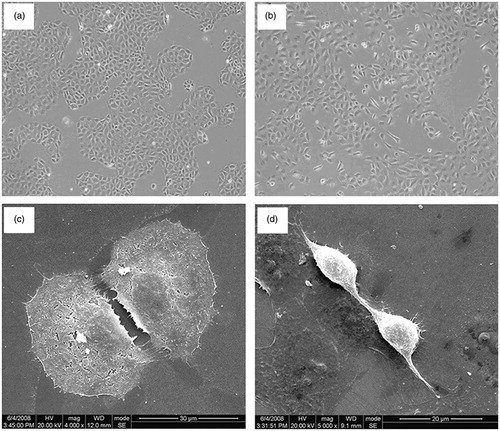
Cultured NRK52E cells showed a confluent monolayer of cells with classic epithelial cobblestone morphological characteristics evident by phase-contrast microscopy (). Ultrastructural analysis showed that these cells had a cubic or round shape, apical-basal polarity, and numerous microvilli on the surface (). Confluent NRK52E cells co-cultured with IL-1α (10 ng/mL) for 4 days showed clear morphological changes, with many cells becoming fibroblast-like in appearance. The transformed cells showed marked hypertrophy, became elongated, and lost their cobblestone growth pattern (). Scanning electron microscopy showed a loss of apical-basal polarity and surface microvillus on the transformed cells. They were elongated, disassociated from neighboring cells, and highly invasive, with many cytoplasmic projections. Some transformed cells developed a new front-end, back-end, fibroblast-like polarity, with many prolonged cytoplasmic projections present at the new front end (). The results were consistent with our previous report.Citation16
Figure 1. IL-1α-induced fibroblast-like morphological changes in cultured NRK52E tubular epithelial cells. (a) Confluent cells cultured in medium alone for 4 days to maintain the cobblestone epithelial morphological state and growth pattern. (b) Cells co-cultured with IL-1α showed marked hypertrophy, became elongated, and developed an invasive growth pattern. (c) Cells grown in medium alone displayed a normal aggregated epithelial growth pattern with apical-basal polarity and numerous microvillus on the surface. (d) Cells co-cultured with IL-1α showed fibroblast-like morphological transition.
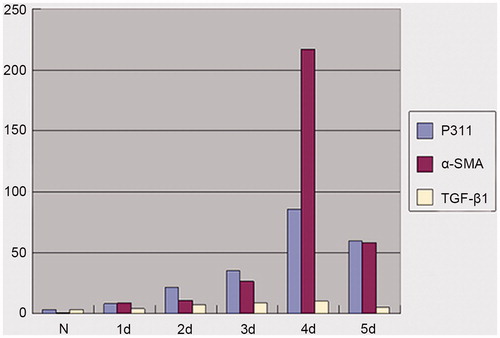
Accelerated stimulation time with IL-1α (10 ng/mL) induced time-dependent increases in P311, α-SMA, and TGF-β1 gene expression in NRK52E cells (). Peak levels of expression for all genes were at 4 days.
We further analyzed the relationship of expression levels of P311, α-SMA, and TGF-β1 genes. The expression levels of P311 gene were strongly associated with expression levels of both α-SMA and TGF-β1 genes. Spearman’s correlation assay between P311 gene expression and α-SMA gene expression showed remarkable significant (r = 0.999, p < 0.01). P311 expression was also significantly correlated with the expression of TGF-β1 gene (r = 0.829, p < 0.05).
To examine effects and explore the mechanism of P311 on transition, the cells were cultured for 4 days with IL-1α (10 ng/mL) or IL-1α (10 ng/mL) plus anti-p311 antibodies (5 μg/mL). The effects of anti-P311 antibodies on P311, α-SMA, and TGF-β1 gene expression were analyzed by RT-PCR and shown in . Compared to the control cells, there was a significant increase in P311, α-SMA, and TGF-β1 gene expression levels in the cells stimulated with IL-1α (p < 0.05). The cells treated with IL-1α plus anti-p311 antibodies expressed less α-SMA, but more P311 and TGF-β1 mRNAs compared with IL-1α stimulating cells (p < 0.05).
Table 2. Expression of P311, α-SMA, and TGF-β1 in all groups.
Effect of anti-P311 on protein levels of P311, α-SMA, and TGF-β1
Immunohistochemistry staining was used to evaluate the morphology and number of the NRK52E cells expressing P311 and α-SMA protein. The cultured cells showed a normal epithelial morphology and cobblestone growth pattern, with only occasional cells staining positive for P311 and α-SMA (). The NRK52E cells initiating transition by IL-1α (10 ng/mL, 4 days) exhibited hypertrophy, an elongated morphology, with strong P311 staining (p < 0.05) compared with unstimulated cells (). The NRK52E cells cultured in the presence of IL-1α and anti-P311 antibodies expressed less P311 protein (p < 0.05) compared with IL-1α-stimulating cells ().
Figure 3. P311 protein and α-SMA protein in tubular epithelial-myofibroblast transition (TEMT). NRK52E cells were grown for four days on glass slides in the presence or absence of IL-1α or a neutralizing anti-P311 antibody. Cells expressing P311 protein or α-SMA protein were detected by immunohistochemistry. (a) Cells cultured in medium alone showed a normal epithelial morphology state. Only rare cells stained positive for P311. (b) Cells co-cultured with IL-1α caused marked TEMT. (c) The addition of neutralizing anti-P311 antibody produced much inhibition of P311 protein expression. (d) Cells cultured in medium alone showed a normal epithelial morphology and rare cells were stained positive for α-SMA. (e) Cells co-cultured with IL-1α caused marked TEMT. Strong α-SMA staining was demonstrated in many transformed cells. (f) The addition of neutralizing anti-P311 antibody obviously decreased the expression of α-SMA protein. This is one of three experiments that produced similar results.
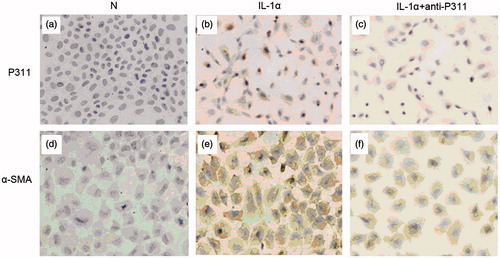
Addition of IL-1α (10 ng/mL) to confluent cells resulted in the strong cytoplasmic staining for α-SMA in about 50% of the cells (p < 0.05) compared with untreated cells (). Many α-SMA+ showed marked hypertrophy, and elongated shape, indicating transition into myofibroblasts. Less accumulation of α-SMA+ cells were detected in IL-1α plus anti-P311 group () comparing to IL-1α group (p < 0.05).
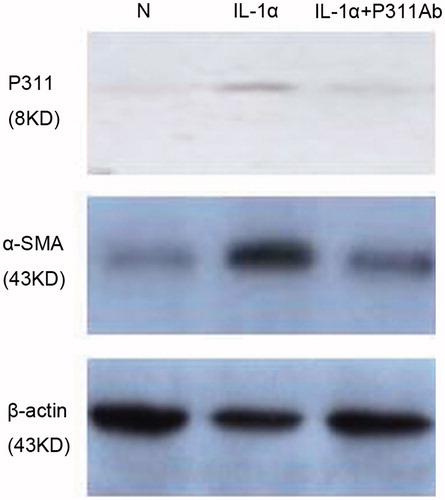
Consistent with immunohistochemistry staining, western blot analysis of cell lysates also showed that IL-1α induced evident increases in the P311 and α-SMA protein expression, which was inhibited by the addition of anti-P311 antibodies (, ). In addition, the supernatant was collected and assayed using an ELISA to measure total TGF-β1 protein. As shown in , there was a significant increase in the cells stimulated with IL-1α and IL-1α plus anti-P311 antibodies (p < 0.05).
Discussion
The present study demonstrated that IL-1α-induced significant increases in P311 gene and protein expression, thus causing transition of normal rat tubular epithelial cells (NRK52E) into myofibroblasts. As the extent of fibrosis is an important predictor of outcome in patients with renal disease, much work has been done to analyze the mechanisms that promote TEMT and lead to interstitial fibrosis. To date, P311 protein has not been investigated and reported in renal cells. We, for the first time, linked P311 protein with TEMT in NRK52E cells.
Alpha-SMA is the marker of myofibroblasts, a major source of extracellular matrix proteins in fibrotic lesions in the kidney and other tissues.Citation2–5,Citation21 TGF-β1, a multifunctional cytokine with profibrogenic activity, has been involved in mediating renal fibrosis in experimental and human glomerulonephritis.Citation22 TGF-β1 up-regulates protein synthesis of ECM components, increasing the accumulation of ECM proteins and promoting TEMT.Citation14,Citation23,Citation24 With that as background, the present study focuses on the correlation between P311 with α-SMA and TGF-β1. The results demonstrated that the gene expression level of P311 was positively correlated to that of α-SMA and TGF-β1, suggesting that P311 may be involved in TEMT of NRK52E cells.
In the process of TEMT in NRK52E cells, the mechanisms of P311 protein were also explored. Anti-P311 antibodies induced an increase of P311 gene expression in the presence of IL-1α, indicating a possible positive feedback of P311 protein itself. In previous studies, it has been reported that P311 gene expression induces phenotypic changes of myofibroblast transformation.Citation19 In this study, anti-P311 antibodies repressed α-SMA gene expression, providing further supportive evidence for the role of P311 protein in TEMT.
In NIH3T3 cells, P311 protein inhibits TGF-β receptor 2, TGF-β1-activating MMP-2 and MMP-9 and induces TGF-β1-independent myofibroblast transformation.Citation19 P311 induces myofibroblast ameboid migration, depending on RalA activation that could be abolished by TGF-β1.Citation19 P311 protein has also been shown to down-regulate TGF-β1 gene expression by binding to the latency associated protein.Citation20 Consistently, expression of TGF-β1 gene in the cells cultured with IL-1α plus anti-P311 bodies was increased in this study. Therefore, P311, induced by IL-1α, may be involved in the transition of NRK52E cells into myofibroblasts in a TGF-β1-independent way.
In summary, in the process of TEMT induced by IL-1α, P311 was involved, independent of TGF-β1. P311 protein may play a key role in TEMT and cellular activation, and has the potential to be identified as a marker of tubulointerstitial fibrosis in chronic renal injury. Combined with conventional antagonists, P311 protein as a component of signaling pathway leading to TEMT may offer a new strategy for therapeutic target of kidney diseases with renal tubulointerstitial fibrosis.
Acknowledgements
The authors are grateful to Prof. Xian-ming Mo and Wen-tong Meng of Sichuan University, Huaxi Hospital Stem Cell Lab for technical advice.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by Basic Research Program of Sichuan Scientific committee (No. 0040205301280) and National Basic Research Program of China (No. 2007CB947802).
References
- Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7(12):2495–2508
- Badid C, Mounier N, Costa AM, Desmoulière A. Role of Myofibroblasts during normal tissue repair and excessive scarring: Interest of their assessment in nephropathies. Histol Histopathol. 2000;15(1):269–280
- Tang WW, Van GY, Qi M. Myofibroblast and α1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997;51(3):926–931
- Högemann B, Gillessen A, Böcker W, Rauterberg J, Domschke W. Myofibroblasts-like cells produce mRNA for type I and III procollagens in chronic active hepatitis. Scand J Gastroenterol. 1993;28(7):591–594
- Zhang HY, Gharaee-Kermani M, Zhang K, Karmiol S, Phan SH. Lung fibroblast alpha-smooth muscle actin expression and contractile phenotype in bleomycin-induced pulmonary fibrosis. Am J Pathol. 1996;148(2):527–537
- Alpers CE, Hudkins KL, Floege J, Johnson RJ. Human renal cortical interstitial cells with some features of smooth muscle cells participate in tubulointerstitial and crescentic glomerular injury. J Am Soc Nephrol. 1994;5(2):201–209
- Goumenos DS, Brown CB, Shortland J, el Nahas AM. Myofibroblasts, predictors of progression of mesangial IgA nephropathy? Nephrol Dial Transplant. 1994;9(10):1418–1425
- Roberts IS, Burrows C, Shanks JH, Venning M, McWilliam LJ. Interstitial myofibroblasts: Predictors of progression in membranous nephropathy. J Clin Pathol. 1997;50(2):123–127
- Badid C, Desmoulière A, McGregor B, et al. Interstitial alpha-smooth muscle actin: A prognostic marker in membranous nephropathy. Clin Nephrol. 1999;52(4):210–217
- Essawy M, Soylemezoglu O, Muchaneta-Kubara EC, Shortland J, Brown CB, el Nahas AM. Myofibroblasts and the progression of diabetic nephropathy. Nephrol Dial Transplant. 1997;12(1):43–50
- Zhang G, Moorhead PJ, el Nahas AM. Myofibroblasts and the progression of experimental glomerulonephritis. Exp Nephrol. 1995;3(5):308–318
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: Pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15(1):1–12
- Fan JM, Ng YY, Hill PA, et al. Transforming growth factor-beta regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56(4):1455–1467
- Dinarello CA, Wolff SM. The role of interleukin-1 in disease. New Engl J Med. 1993;328:106–113
- Fan JM, Huang XR, Ng YY, et al. Interleukin-1 induces tubular epithelial-myofibroblast transdifferentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J Transplant. 2001;37(4):820–831
- Studler JM, Glowinski J, Lévi-Strauss M. An abundant mRNA of the embryonic brain persists at a high level in cerebellum, hippocampus and olfactory bulb during adulthood. Eur J Neurosci. 1993;5(6):614–623
- Mariani L, McDonough WS, Hoelzinger DB, et al. Identification and validation of P311 as a glioblastoma invasion gene using laser capture microdissection. Cancer Res. 2001;61(10):4190–4196
- Pan D, Zhe X, Jakkaraju S, Taylor GA, Schuger L. P311 induces a TGF-beta1-independent nonfibrogenic myofibroblast phenotype. J Clin Invest. 2002;110(9):1349–1358
- Paliwal S, Shi J, Dhru U, Zhou Y, Schuger L. P311 binds to the latency associated protein and down regulates the expression of TGF-β1 and TGF-β2. Biochem Biophys Res Commun. 2004;315(4):1104–1109
- Jinde K, Endoh M, Sakai H, Kurokawa K, Atkins RC, Lan HY. Epithelial-myofibroblast transdifferentiation (EMT) in glomerular crescent progression and tubulointerstitial (TI) fibrosis in human glomerulonephritis. J Am Soc Nephrol. 1999;10:550 A
- Iwano M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. Curr Opin Nephrol Hypertens. 2004;13:279–284
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112(10):1486–1494
- Ponda M, Siconolfi-Baez L, Kopp JB, Angeletti RH, Hostetter TH, Bitzer M. NGAL and extracellular matrix proteins in TGF-beta induced chronic renal fibrosis. Am J Kidney Dis. 2007;49(4):B66