Abstract
Aim: Increased arterial stiffness is strongly associated with cardiovascular diseases, while thrombotic events are more common than hemorrhagic events in hypertensive patients. Markers of a hypercoagulable state may also predict future cardiovascular events in hypertensive patients. Here, we speculated that increased arterial stiffness might lead to the development of a hypercoagulable state that can play a role in the thrombotic complications of hypertension. Soluble endothelial protein C receptor (sEPCR) is one such marker of hypercoagulation. The ambulatory arterial stiffness index (AASI) could be accepted as a non-invasive measure of arterial stiffness. The aim of this study was to investigate association of AASI with levels of sEPCR in newly diagnosed hypertensive patients. Materials and methods: The study included 263 newly diagnosed essential hypertensive patients and 55 healthy normotensive controls. All subjects underwent 24 h ambulatory blood pressure monitoring (ABPM); the AASI was derived from ABPM tracings. Plasma sEPCR was measured by ELISA. Results: Hypertensive patients (n = 263) had higher AASI, C-reactive protein (CRP) and sEPCR versus the normotensive healthy group (n = 55). Univariate analysis showed that AASI was positively associated with age (r = 0.212, p < 0.001) body mass index (r = 0.412, p < 0.001), pulse pressure (r = 0.350, p < 0.001), plasma sEPCR (r = 0.894, p < 0.001), 24-h heart rate (r = 0.176, p = 0.001) and inversely related to high-density lipoprotein (HDL) (r = −0.293, p < 0.001). Multivariate analyses revealed that sEPCR and HDL are independently correlated to AASI. Conclusion: We suggest that increased AASI is associated with elevated sEPCR. It might be responsible for subsequent thrombotic events in newly diagnosed hypertensive patients.
Introduction
Although aortic pulse wave velocity (PWV) is the gold-standard for evaluation arterial stiffness, the ambulatory arterial stiffness index (AASI) has been proposed as a surrogate measure of arterial stiffness. The AASI can be readily determined from ambulatory blood pressure recordings and independently has a predictive value for cardiovascular events, stroke and all-cause mortality in both hypertensive and normotensive subjects.Citation1–5 However, there is still controversy as to whether the AASI reflects arterial stiffness exactly because vascular resistance and heart rate—in addition to arterial stiffness—are the main factors that determine the AASI.Citation6,Citation7
The endothelial protein C receptor (EPCR) is a 46-kDa endothelial cell-specific type-I transmembrane protein, which plays a crucial role in regulating coagulation and inflammation. It is a ligand for Protein C and plays an important role in augmenting Protein C activation via the thrombin–thrombomodulin complexCitation8–10 A soluble form of EPCR (sEPCR) has recently been identified that can bind to protein C and activate protein C (APC) with the same affinity as transmembrane EPCR.Citation10,Citation11 Furthermore, sEPCR exhibits pro-inflammatory and pro-coagulant properties. An elevated sEPCR level appeared to be a risk factor for thrombosis,Citation10,Citation11 deep venous thrombosis,Citation12 strokeCitation13 and ST-segment elevation myocardial infarction.Citation14
Hypertension is related to a hypercoagulable state. Hypertensive patients have a tendency toward thrombotic rather than hemorrhagic events, although the underlying mechanism remains unclear.Citation15,Citation16 Some evidence suggests that there is a relationship between arterial stiffness and coagulation.Citation17,Citation18 However, the influence of AASI on the pro-thrombotic state, especially the level of sEPCR in newly diagnosed essential hypertension, remains unclear. The aim of this study was to evaluate the association of AASI with sEPCR in newly diagnosed essential hypertension patients.
Materials and methods
This cross-sectional study included 263 consecutive patients with newly diagnosed primary hypertension (Group 1) treated at our institution between June 2013 and March 2014. Individuals who had never had thrombosis or primary hypertension served as healthy controls (n = 55; Group 2). Physical examination findings, risk factors and medical history data were obtained for both the groups.
The exclusion criteria were diabetes mellitus, thromboembolic events, hypertriglyceridemia (>400 mg/dL), acute renal failure, renal replacement therapy, regular use of anti-inflammatory drugs, oral contraceptives or anticoagulants, a history of taking antihypertensive or lipid-lowering medication as well as myocardial infarction or cerebrovascular disease in the previous 6 months. The local ethics committee approved the protocol in accordance with the Helsinki Declaration (IRB number: 99950669/224). All of the participants provided written informed consent.
The body mass index (BMI) was calculated by dividing the weight in kilograms by the height in square meters (kg/m2). Hypertension was diagnosed if the mean of three recordings in the clinic under basal conditions and separated in time revealed a systolic blood pressure (SBP) ≥ 140 and/or diastolic blood pressure (DBP) ≥ 90 mmHg. During each visit, at least two recordings were made. These were spaced more than one minute apart.Citation19 After diagnosis of hypertension, 24-h ambulatory blood pressure monitoring (ABPM) was performed during a day of standard activity with an adequate cuff for the size of the patient’s non-dominant arm. An ambulatory blood pressure monitor (Spacelabs 90217, Spacelabs Healthcare, Issaquah, Washington, DC) was programmed to obtain blood pressure measurements every 20 min while awake and every 30 min while resting; the patient reported the waking and sleeping hours. The ABPM was considered adequate if >80% of the total measurements were successfully obtained. From the hourly means of the ambulatory BP recordings, the daytime, nighttime and 24-h means of systolic, diastolic and mean blood pressure readings were calculated for each patient.
The AASI is defined as 1 minus the regression slope of the diastolic and systolic blood pressure values in individual subjects based on a non-invasive, 24-h ambulatory blood pressure recordings.Citation2 The slope was not forced through the origin.Citation2 The normal values of the AASI have been proposed to be <0.50 at 20 years and <0.70 at 80 years.Citation4,Citation5
One day after ABPM, laboratory data were obtained from venous blood samples drawn under standardized conditions after at least 12 h of fasting. The lipid profile, glucose, C-reactive protein (CRP) and creatinine levels were measured according to standard methods using an autoanalyzer (COBAS INTEGRA 800, Roche Diagnostics, Indianapolis, IN). An indirect measurement of low-density lipoprotein (LDL) was performed using the Friedewald equation [LDL = total cholesterol − high density lipoprotein − (triglycerides/5)]. The glomerular filtration rate (eGFR) was estimated from serum creatinine concentrations using the chronic kidney disease-epidemiology collaboration (CKD-EPI) equation.Citation20
The plasma sEPCR levels in the patient and healthy control groups were measured using a sandwich enzyme immunoassay (Catalog No: MBS703733; MyBioSource, Inc., San Diego, CA) one day after the ABPM. The sEPCR levels of 7.8–500 ng/mL were considered to be normal according to the laboratory’s standards. This assay has good sensitivity (lower limit of detection = 1.95 ng/mL) and intra- and inter-assay CVs of <8% and <10%, respectively.
The Kolmogorov–Smirnov test was used to determine whether the continuous variables were normally distributed. Normally distributed variables were given as the mean ± standard deviation, and categorical variables were given as percentages. The independent sample unpaired Student’s t-test was used for the continuous variables, and the categorical data were compared using the Chi-squared test. Multivariate linear regression analysis via the enter method was used to assess independent variables affecting AASI as the dependent variable. The variables (age, BMI, sEPCR, pulse pressure, HDL and/or 24-h rate) that were associated with AASI (p < 0.05) in univariate analysis were inserted into a multivariate regression model. A value of p < 0.05 was considered statistically significant. All of the statistical analyses were performed using SPSS version 20 (IBM, Armonk, NY).
Results
The demographic and clinical data of the two groups are summarized in . There were no significant differences between the groups in terms of age, sex distribution or smoking rates. However, the mean BMI was higher in Group 1 than in Group 2 (30.1 ± 2.7 vs. 24.6 ± 3.5; p < 0.01).
Table 1. Baseline characteristics and ambulatory blood pressure monitoring characteristics for the two groups.
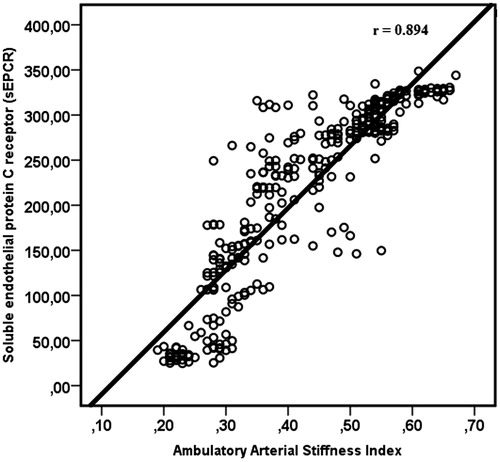
Group 1 had higher levels of plasma sEPCR compared to Group 2 (250.66 ± 68.75 ng/mL vs. 47.60 ± 21.93 ng/mL; p < 0.001) (). The AASI and CRP levels were significantly higher in Group 1 than in Group 2 (AASI, 0.462 ± 0.110 vs. 0.257 ± 0.040, p < 0.001 and CRP, 7.3 ± 1.9 vs. 3.1 ± 1.7, p = 0.028). Univariate analyses showed that AASI was positively related to age (r = 0.212, p < 0.001), BMI (r = 0.412 p < 0.001), pulse pressure (r = 0.350, p < 0.001), plasma sEPCR (r = 0.894, p < 0.001) () and 24-h heart rate (r = 0.176, p = 0.001); it was inversely related to HDL (r = −0.293, p < 0.001) in the entire group (). In contrast, there was no correlation of AASI with fasting blood glucose, eGFR, LDL, total cholesterol, triglyceride, uric acid or CRP levels ().
Table 2. Univariate and multivariate regression analyses demonstrating factors showing correlation with ambulatory arterial stiffness index.
Univariate and multivariate linear regression analyses were performed to investigate the relationships between AASI and other variables. The multivariate linear regression analysis showed that sEPCR levels (β = 0.769, p < 0.001) and HDL levels (β = −0.197, p = 0.026) were independently related to AASI ().
Discussion
This study demonstrates that an increased AASI is associated with elevated levels of sEPCR. This finding is independent of potentially confounding factors that are positively related to AASI including age, BMI and pulse pressure. Newly diagnosed hypertensive patients have an increased AASI versus the healthy controls.
There has been controversy as to whether the AASI is a non-invasive indicator of arterial stiffness. The AASI is a composite index reflecting arterial stiffness, blood pressure variability and patients' diurnal cycles rather than just a measure of arterial stiffness.Citation21,Citation22 However, AASI is an independent predictor of cardiovascular and cerebrovascular outcomes in large population studies.Citation3–5,Citation23–25 Similar to other studies, we demonstrated higher AASI in the hypertensive patients versus controls.Citation26,Citation27 The mean AASI value of Group 1 in this study was 0.46 ± 0.11. This is very similar to the mean AASI values [0.47 ± 0.16;Citation26 0.51 ± 0.11Citation27] reported in other studies of hypertensive patients. Therefore, increased AASI values in newly diagnosed hypertensive patients suggest increased arterial stiffness. However, we cannot determine whether the increased AASI is a cause or a consequence of hypertension.
Thrombotic events are more common in hypertensive patients than hemorrhagic events, although the mechanism underlying this finding is unclear.Citation15–17 An increased sEPCR level is a pro-thrombotic marker, and its association with thrombotic events has been shown in various studies.Citation10,Citation11 This study is the first report of the association between sEPCR levels and AASI in newly diagnosed hypertensive patients. A strong, independent and positive relation between AASI and sEPCR was demonstrated in patients newly diagnosed with essential hypertension. sEPCR is generated at the endothelial surface through proteolytic cleavage via metalloprotease activity inducible by thrombin, IL-1, TNF-α or endotoxin.Citation8,Citation28 In addition, sEPCR is a very sensitive marker of endothelial dysfunction,Citation29 and elevated sEPCR levels could be responsible for thrombotic events.Citation8–10 The association of increased sEPCR levels and endothelial damage has been demonstrated in patients with preeclampsia.Citation30 An altered smooth muscle cell function, vascular inflammation, expression of modified vascular wall matrix proteins and endothelial dysfunction all play a role in the pathophysiology of arterial stiffness.Citation5,Citation7 Thus, this association can be explained by the common mechanism of endothelial dysfunction and inflammation. Although CRP levels were higher in hypertensive patients, there was no correlation between the AASI and CRP. The lack of a detectable correlation between the AASI and CRP could be due to the absence of serial CRP measurements and/or not measuring other inflammatory cytokines (e.g., IL-6 or TNF-α). Although the effect of inflammation cannot be completely excluded, the mechanism responsible for the association between AASI and sEPCR is more likely endothelial dysfunction. The reported association between increased sEPCR levels and endothelial dysfunction in patients with preeclampsia supports our speculation.Citation30 Other studies have demonstrated that a pro-thrombotic state can be induced by the activation of the renin–angiotensin system (RAS), which is associated with arterial stiffness in hypertensive patients.Citation31,Citation32
Remková et al. reported that hypertensive patients have a significant increase in sEPCR levels versus the healthy controls. The sEPCR level decreases in response to the treatment with an angiotensin II type 1 receptor blocker (ARB).Citation33 This finding suggests that the activation of the RAS can contribute to the increased sEPCR levels. In addition, activation of the RAS is associated with endothelial dysfunction and arterial stiffness.Citation34,Citation35 Our finding that the levels of sEPCR and AASI were increased in the hypertensive patients versus healthy controls is consistent with this report. In the light of these studies, RAS activation might be one of the mechanisms that is responsible for increased sEPCR and arterial stiffness. In sum, the results described above suggest that AASI might contribute to the thrombotic risk associated with increased sEPCR levels in newly diagnosed essential hypertension patients.
AASI has been previously shown to be a marker of vascular aging.Citation2–5 In this study, only a weak positive correlation was found between AASI and age. This might be due to the narrow age range (1–54 years) of the patients in this study and the exclusion of older patients (>65 years). A positive correlation was found between AASI and BMI similar to other studies.Citation26,Citation27
Some evidence has suggested that HDL-C is inversely and independently associated with arterial stiffness in the general populationCitation36 as well as in hypertensive patients.Citation37 The results of our study are compatible with other reports in the literature, which show that while HDL is inversely correlated with AASI, it is an independent predictor of AASI.
This study has several limitations. First, this was a cross-sectional study in a human population—it cannot establish a causal link between sEPCR levels and AASI. Second, heart rate and vascular resistance are factors that influence AASI; hence, AASI is not an ideal indicator of arterial stiffness. Third, the sEPCR levels were measured only once rather than serially, which would have been more appropriate. Fourth, we did not use both an invasive and noninvasive method to assess endothelial dysfunction.
In conclusion, higher AASI values were associated with increased levels of sEPCR in newly diagnosed essential hypertension. We suggest that newly diagnosed hypertensive patients who have high AASI values could have a higher risk of thrombotic events due to the elevated levels of sEPCR. If our results are validated by longitudinal studies, increased sEPCR levels could be one of the mechanisms responsible for the thrombotic events in newly diagnosed hypertensive patients together with increased AASI.
Declaration of interest
The authors have no conflict of interest to declare.
References
- Li Y, Wang JG, Dolan E, et al. Ambulatory arterial stiffness index derived from 24-hour ambulatory blood pressure monitoring. Hypertension. 2006;47(3):359–364
- Dolan E, Li Y, Thijs L, et al. Ambulatory arterial stiffness index: Rationale and methodology. Blood Press Monit. 2006;11:103–105
- Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34(7):647–651
- Aznaouridis K, Vlachopoulos C, Protogerou A, et al. Ambulatory systolic–diastolic pressure regression index as a predictor of clinical events: A meta-analysis of longitudinal studies. Stroke. 2012;43(3):733–739
- Kollias A, Stergiou GS, Dolan E, O'Brien E. Ambulatory arterial stiffness index: A systematic review and meta-analysis. Atherosclerosis. 2012;224(2):291–301
- Kips JG, Vermeersch SJ, Reymond P, et al. Ambulatory arterial stiffness index does not accurately assess arterial stiffness. J Hypertens. 2012;30(3):574–580
- Chirinos JA. Arterial stiffness: Basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5(3):243–255
- Laszik Z, Mitro A, Taylor FB Jr, et al. Human protein C receptor is present primarily on endothelium of large blood vessels: Implications for the control of the protein C pathway. Circulation. 1997;96(10):3633–3640
- Van de Wouwer M, Collen D, Conway EM. Thrombomodulin–protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–1383
- Esmon CT. The endothelial protein C receptor. Curr Opin Hematol. 2006;13:382–385
- Liaw PC, Neuenschwander PF, Smirnov MD, et al. Mechanisms by which soluble endothelial cell protein C receptor modulates protein C and activated protein C function. J Biol Chem. 2000;275:5447–5452
- Uitte de Willige S, Van Marion V, Rosendaal FR, et al. Haplotypes of the EPCR gene, plasma sEPCR levels and the risk of deep venous thrombosis. J Thromb Haemost. 2004;2:1305–1310
- Ulu A, Gunal D, Tiras S, et al. EPCR gene A3 haplotype and elevated soluble endothelial protein C receptor (sEPCR) levels in Turkish pediatric stroke patients. Thromb Res. 2007;120(1):47–52
- Tanalp AC, Oduncu V, Erkol A, et al. Soluble endothelial protein C receptor levels and protein C activity in patients with acute ST-segment elevation myocardial infarction. Coron Artery Dis. 2013;24(3):209–216
- Varughese GI, Lip GY. Is hypertension a prothrombotic state? Curr Hypertens Rep. 2005;7(3):168–173
- Kakar P, Lip GY. Hypertension: Endothelial dysfunction, the prothrombotic state and antithrombotic therapy. Expert Rev Cardiovasc Ther. 2007;5(3):441–450
- Regnault V, Perret-Guillaume C, Kearney-Schwartz A, et al. Tissue factor pathway inhibitor: A new link among arterial stiffness, pulse pressure, and coagulation in postmenopausal women. Arterioscler Thromb Vasc Biol. 2011;31(5):1226–1232
- Wykretowicz J, Guzik P, Krauze T, et al. Fibrinogen and D-dimer in contrasting relation with measures of wave reflection and arterial stiffness. Scand J Clin Lab Invest. 2012;72(8):629–634
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289:2560–2572
- Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612
- Schillaci G, Parati G. Ambulatory arterial stiffness index: Merits and limitations of a simple surrogate measure of arterial compliance. J Hypertens. 2008;26:182–185
- Laugesen E, Erlandsen M, Knudsen ST, et al. Ambulatory arterial stiffness index: A composite index reflecting arterial stiffness, blood pressure variability and patients’ diurnal cycle. J Hypertens. 2011;29:2278–2279
- Hansen TW, Staessen JA, Torp-Pedersen C, et al. Ambulatory arterial stiffness index predicts stroke in a general population. J Hypertens. 2006;24:2247–2253
- Leoncini G, Ratto E, Viazzi F, et al. Increased ambulatory arterial stiffness index is associated with target organ damage in primary hypertension. Hypertension. 2006;48:397–403
- Ben-Dov IZ, Gavish B, Kark JD, et al. A modified ambulatory arterial stiffness index is independently associated with all-cause mortality. J Hum Hypertens. 2008;22:761–766
- Catena C, Bernardi S, Sabato N, et al. Ambulatory arterial stiffness indices and non-alcoholic fatty liver disease in essential hypertension. Nutr Metab Cardiovasc Dis. 2013;23(4):389–393
- Chen H, Hua Q, Hou H. Association of hemoglobin with ambulatory arterial stiffness index in untreated essential hypertensive patients without anemia. Intern Med. 2011;50(22):2759–2765
- Gu JM, Katsuura Y, Ferrell GL, et al. Endotoxin and thrombin elevate rodent endothelial cell protein C receptor mRNA levels and increase receptor shedding in vivo. Blood. 2000;95:1687–1693
- Kurosawa S, Stearns-Kurosawa DJ, Carson CW, et al. Plasma levels of endothelial cell protein C receptor are elevated in patients with sepsis and systemic lupus erythematosus: Lack of correlation with thrombomodulin suggests involvement of different pathological processes. Blood. 1998;91:725–727
- Saposnik B, Peynaud-Debayle E, Stepanian A, et al. Elevated soluble endothelial cell protein C receptor (sEPCR) levels in women with preeclampsia: A marker of endothelial activation/damage? Thromb Res. 2012;129(2):152–157
- Lip GYH, Blann AD, Edmunds E, et al. Baseline abnormalities of endothelial function and thrombogenesis in relation to prognosis in essential hypertension. Blood Coagul Fibrinolysis. 2002;13:35–41
- Kotliar C, Kempny P, Gonzalez S, et al. Lack of RAAS inhibition by high-salt intake is associated with arterial stiffness in hypertensive patients. J Renin Angiotensin Aldosterone Syst. 2014;15(4):498–504
- Remková A, Kratochvíl'ová H, Durina J. Impact of the therapy by renin–angiotensin system targeting antihypertensive agents perindopril versus telmisartan on prothrombotic state in essential hypertension. J Hum Hypertens. 2008;22(5):338–345
- Aroor AR, Demarco VG, Jia G, et al. The role of tissue renin–angiotensin–aldosterone system in the development of endothelial dysfunction and arterial stiffness. Front Endocrinol (Lausanne). 2013;4:161
- Mahmud A, Feely J. Arterial stiffness and the renin–angiotensin–aldosterone system. J Renin Angiotensin Aldosterone Syst. 2004;5(3):102–108
- Wang X, Du Y, Fan L, et al. Relationships between HDL-C, hs-CRP, with central arterial stiffness in apparently healthy people undergoing a general health examination. PLoS One. 2013;8(12):e81778
- Miao DM, Ye P, Xiao WK, et al. Influence of low high-density lipoprotein cholesterol on arterial stiffening and left ventricular diastolic dysfunction in essential hypertension. J Clin Hypertens (Greenwich). 2011;13(10):710–715