Abstract
Purpose: To evaluate the effectiveness of interventional therapy for complications of transplanted renal allografts. Materials and methods: Between January 2009 and March 2014, 14 patients underwent interventional therapy for complications of renal allografts. Complications included transplant renal artery stenosis (TRAS), TRAS combined with pseudoaneurysms, transplant renal venous kinking and ureteral obstruction (UO). Serum creatinine (S.Cr) levels were evaluated before and after procedure. The characteristics and procedure outcomes of these patients with vascular and nonvascular complications were also analyzed. Results: All primary procedures were successfully performed, which included percutaneous transluminal angioplasty (PTA) for TRAS (n = 4), stenting and coil embolization for TRAS combined with pseudoaneurysms (n = 1), stenting for renal vein kinking (n = 2), and percutaneous nephrostomy (PCN) for UO (n = 7) and secondary antegrade stent placement in six UO patients after 1 week of PCN. No major procedure related complications occurred. S.Cr level subsequently improved from 6.0 ± 3.6 to 2.6 ± 2.1 mg/dL (p < 0.001), as well as patients’ clinical features within 1 week after procedure. In our study, the onset time of vascular complications was earlier (<6 months) than nonvascular complications with significant difference (p < 0.001). During follow-up, the patient with TRAS and pseudoaneurysms suffered acute rejection 1 month after treatment and received transplant renal artery embolization. One patient with TRAS showed restenosis 4 months after procedure, and was retreated successfully with stenting. Thirteen cases reserved their transplanted renal allografts. Conclusion: Interventional therapy could be prior considered for transplanted renal allograft complications as its effectiveness and minimal invasiveness in saving the transplanted renal grafts.
Introduction
Renal transplantation has been the first treatment of choice for most patients with end stage renal disease. It provides higher long-term survival rates and improved quality of life compared with dialysis.Citation1 However, the imbalance of organ availability and demand is still a severe problem worldwide. This requires that each renal transplant should be optimized individually to achieve long-term survival of allograft and recipients.Citation2 Although the continuous advancement of surgical technical, immunosuppressive regimens and supportive therapy has led to improved outcomes and patient survival, a variety of vascular and nonvascular complications still frequently occur post-procedure, approximately accounting for 12–20% of all cases.Citation3 These complications may compromise allograft function and increase morbidity.
Surgery has been performed to salvage the kidney as the primary treatment for a long time. Nowadays, however, considering its invasiveness and substantial morbidity, interventional treatment has emerged as an eligible way to manage the complications of transplanted renal allografts,Citation4,Citation5 specially for arterial and venous kinking, transplant renal artery stenosis (TRAS) located at anastomosis or severe distal artery stenosis, and urinary obstruction (UO) occurs at middle and upper ureters.Citation3,Citation6,Citation7 To date, unfortunately, the experience of this minimal invasive treatment to preserve transplanted kidney allografts remains inclusive because of the limited cases.
Herein, we presented our experience with the minimally invasive salvage therapy for the complications post-transplantation period. Additionally, characteristics and procedure outcomes of vascular and nonvascular complications were also analyzed.
Materials and methods
Patients and study protocols
From January 2009 to March 2014, a total of 301 renal transplants were performed at our institute, of which 216 donors were living related and 85 were cadaveric. Fourteen cases had decreased renal function but unexplainable by rejection, drug toxicity, or dehydration. Meanwhile, these 14 patients were diagnosed of vascular disease or UO after kidney transplantation, and preferred to receive interventional treatment as a salvage therapy was involved in this study. Patients’ demographics and clinical data were retrospectively analyzed. The features of the vascular and nonvascular complications after interventional treatment were also compared.
This study has been performed in accordance with the Helsinki Declaration and its later amendments. Approval from our institutional review board was obtained for this study, and all patients provided informed consent.
Interventional techniques
For patients with vascular complications (TRAS, pseudoaneurysm, or drainage vein abnormality), either percutaneous transluminal angiography or venography was performed. During angiography, site, size, and number of lesions were documented. Percutaneous transluminal angioplasty (PTA) or PTA with stenting for vascular stenosis or superselective embolization for pseudoaneurysm was performed. Treatment procedure of TRAS was precisely documented as followed: A contralateral femoral artery access was approached regardless of end-to-end or end-to-side anastomosis by a Seldinger technique, then selective pelvic arteriography was performed to clarify stenotic lesions. A narrowing of luminal diameter over 50% was considered to be a hemodynamically significant stenosis.Citation8 PTA with balloon dilation was primarily performed for TRAS. For post-anastomotic stenosis, balloon-expandable stent (Palmaz, Cordis, FL) was preferred. Technical success of PTA was defined as a residual stenosis less than 30% and no flow-limiting intimal flap. For pseudoaneurysm, superselective catheterization followed by metallic coils embolization or covered stent placement was performed. Technical success was defined as no residual sac was indicated by followed angiography. As to iliac vein abnormality, lower limb venography was primarily performed to exclude thrombosis followed by venography of external iliac vein to exclude arteriovenous fistula. Then, common iliac vein angioplasty with bare stent was performed. Technical success was elucidated by a final venography that antegrade in-line flow from the femoral vein to the inferior vena cava was restored, and the kinking of the iliac vein was eliminated. For vascular cases, the protocol of antiplatelet or anticoagulation during perioperative period was performed as documented elsewhere in selected cases.Citation9,Citation10
As to UO patients (nonvascular), percutaneous nephrostomy (PCN) was primarily performed. After an initial nephrogram to diagnose the site and degree of ureteral obstruction (UO), an 8F nephrostomy catheter was used for decompression. Technical success of PCN was confirmed if the catheter drained urine spontaneously. Ureteral double-J stent was inserted after approximately 1 week when renal function improved. If the stent could not pass the stenotic area, dilatation with a balloon catheter was used before stent placement. Finally a PCN tube was placed through nephrostomy catheter, which was removed 48 h later when the patency of the stent was confirmed. Stent was usually removed in 6–12 weeks cystoscopically.
Potential post-procedure complications such as hemorrhage, hematoma, or infection were monitored by clinical manifestations and blood test as well as clinical outcomes. Follow-up was achieved by outpatient service and/or telephone interview periodically.
Statistical analysis
Statistical Package for the Social Sciences (SPSS 16.0, IBM, Armonk, NY) was used for all statistical analyses. The quantitative and qualitative variables were compared using the Student’s t-test and Fisher exact probability method, respectively. The statistical significance level was defined as p < 0.05.
Results
General demographics
Of these 14 patients, there were 10 males and 4 females, mean age 35.0 ± 8.3 years (age range: 18–49 years). Eight patients (57.1%) received living-related renal transplant, and the other six patients were cadaveric. The media time onset of complications was 4.5 months (interquartile ranged from 0.75 to 11.25 months). The types of anastomosis were end-to-end (n = 7) and end-to-side (n = 7). Clinical features included oliguria (n = 7), uncontrolled hypertension (n = 2, defined as primary hypertension being uncontrolled with present anti-hypertensive drugs or new onset of secondary hypertension), leg swelling (n = 2), decreased urine output (n = 1), and asymptomatic (n = 2). Seven patients were found vascular abnormalities, included TRAS (n = 4), TRAS combined with pseudoaneurysms (n = 1), and drainage vein abnormality (ipsilateral iliac vein kinking, n = 2). The rest seven patients were all with UOs. The data of patients’ characteristics are available in .
Table 1. Data of patients with complications after renal transplantation.
Interventional findings and treatment
Angiography showed five TRAS cases, two of them located in anastomosis, and three cases located in the distal part after anastomosis (one of them combined with 35 and 8 mm pseudoaneurysms near the anastomosis). Balloon dilation for two anastomosis stenosis () and balloon-expandable stent placement for the distal stenosis after anastomosis (n = 3, ) was performed successfully for all patients (residual stenosis range: 10–25%). For the case combined with distal part stenosis and pseudoaneurysms, fibered micro-coils were advanced into the sacs before stenting, and complete obliteration was elucidated by followed angiography. Decreased urine output (1500 mL/day) in the patient with pseudoaneurysms returned to baseline level (2000–3000 mL/day) 1 week later post-operatively. Uncontrolled hypertension in two TRAS patients showed remarkably improvement of blood pressure (From 160/110 and 172/108 mmHg to 140/90 and 130/80 mmHg, respectively) without taking antihypertensive drugs within 1 week. Iliac vein stenosis caused by severe kinking which was indicated by venography, one or two bare stents were delivered successively, respectively. No remarkable residual stenosis and kinking was left, and the pressure of distal iliac vein decreased impressively from 51 to 12 cm H2O, 44 to 10 cm H2O, respectively (). Left leg swelling in patients with iliac vein kinking was relieved gradually.
Figure 1. (a–c) An 18-year-old male with elevated serum creatinine level and uncontrolled hypertension 3 months post-operatively. (a) Angiography showed about 80% stenosis (arrowhead) at the anastomosis of transplant renal artery. (b) Balloon was dilated until no “waist-sign” (arrowhead) was left. (c) Subsequent angiography showed almost no residual stenosis (arrowhead).
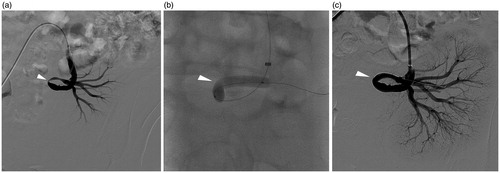
Figure 2. (a–d) A 33-year-old male with elevated serum creatinine level 6 days post-operatively. (a) Angiography showed about 80% stenosis (arrowhead) at the distal part of transplant renal artery near the renal hilum. (b) Balloon dilation was primary performed. Notice that the balloon diameter was selected in according with distal segmental artery (arrowhead). (c) Over 30% stenosis (arrowhead) was left after balloon dilation. (d) A balloon-expandable stent was deployed with almost no residual stenosis (arrowhead) left.
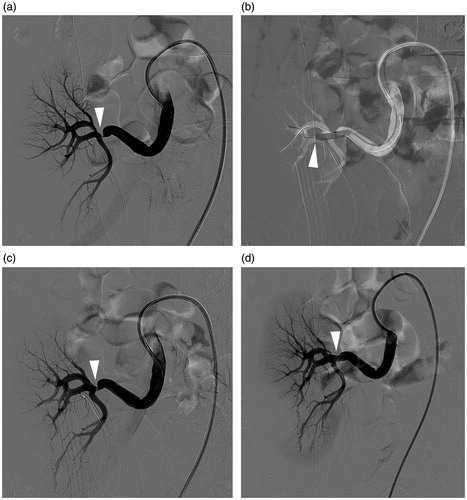
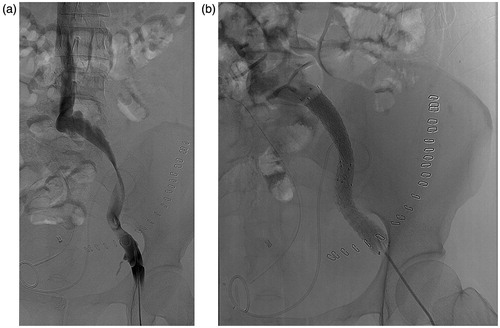
Figure 3. (a and b) A 44-year-old female with acute left leg swelling 14 days post-operatively. (a) Angiography showed extensive stenosis of the iliac vein caused by kinking. (b) Two bare stents in series were inserted and achieved perfect patency.
Antegrade pyelogram indicated obstruction located in either ureterocystic anastomosis (2/7) or distal ureter (5/7), PCN was subsequently performed successfully. Urine volume of seven UO patients was improved to 2879 mL on average (range 2200–4875 mL) on the seventh day after PCN. Then, six of the UO patients received interventional management with double-J stent, while one patient chose surgical treatment. Both pure interventional method and interventional followed surgical method acquired immediate patency.
In total, the primary interventional treatment achieve a 100% technical success, S.Cr level was improved from 6.0 ± 3.6 mg/dL before procedure to 2.6 ± 2.1 mg/dL within 1 week (p < 0.001). When compared vascular patients with nonvascular patients, we found that vascular complications occurred <6 months, while nonvascular occurred ≥6 months (p < 0.001), while there were no statistic significant differences in donor type (p = 0.592), S.Cr level before and after treatment (p = 0.559).
Follow-up and retreatment
During follow-up, the patient (case 4) with TRAS and pseudoaneurysms showed elevated S.Cr level and enlarged pseudoaneurysm (50 mm in diameter) by sonography 1 month after procedure. Acute rejection was indicated by renal biopsy. Positive management with immunosuppressive regimens failed to save the allograft, and this patient received endovascular renal graft artery embolization and subsequent dialysis. Another patient (case 3) with TRAS undergone PTA showed restenosis 4 months later by angiography (the anastomosis restenosis of 60%), and was retreated successfully with stenting. Thus, 13 cases (92.9%) reserved their transplanted renal allograft with stable renal function during the mean 17.0 months follow-up (1–45 months).
Discussion
In this study, we retrospectively analyzed 14 patients with four kinds of transplant renal complications. All complications were managed with a 100% technical success rate in the primary interventional treatment, and renal function improved significantly after procedure. Thirteen of 14 (92.9%) allografts were saved with this minimally invasive salvage therapy, which was invaluable considering the expensive cost of renal transplantation, as well as low quality of life with endless dialysis or second transplant.
Our relatively lower vascular complication rate (2.3%, 7/301) compared with previous reported rate of 3–15%Citation3 may be based on the improved surgical technique. Similarly, TRAS was the most frequent vascular complication as reported in other studies. The treatment strategy is still controversial for the early-happened stenosis located in the anastomotic arteries. In our series, anastomotic stenosis was primarily treated with PTA in the present study, because it was usually caused by trauma during surgeryCitation11 and might be rebounded easily. While post-anastomotic stenosis may happen due to rejection, turbulent flow caused by kidney malposition, or artery twisting, or compression.Citation12 Unlike anastomotic stenosis, post-anastomotic stenosis was usually insensitive to PTA due to intractable etiology characters (hyperplasia caused by rejection or artery kinking) and procedure insufficiency (insufficient dilation by PTA: because the balloon diameter was usually selected in according with distal segmental artery, which was usually smaller than proximal part as shown in ). Herein, for post-anastomic stenosis, we used stenting primarily and decreased overall restenosis rate (20%) compared with balloon or balloon and stent (62% and 30%, respectively) reported by Voiculescu et al.Citation13
Iliac vein stenosis was rarely reported and may lead to thrombosis and graft dysfunction.Citation14 It is usually caused by the insertion of femoral dialysis catheters prior to transplantation, or donor-recipient size discrepancy, thrombosis, inflammatory reaction secondary to fluid collections, or compression caused by hematoma intra- or post-transplantation.Citation14–17 Iliac vein kinking affected the anastomosis site after renal transplantation was even rarer. In our study, both cases occurred within 1 month (14 and 21 days, respectively) post-operatively, this early extensive kinking may be caused by inflammatory reaction secondary to insufficient drainage and inconspicuous blood residual. In our experience, PTA alone showed little improvement because of the elastic recoil. Herein, bare stent insertion was preferred to provide enough radial force for dilation, and impressive patency was achieved.
In our study, UO accounted for 2.3% (7/301) patients, which was in the range of previously reported (2–9% of renal transplants).Citation4 The course is generally classified as early (<3 months) and late (>3 months).Citation18 Early stenosis typically occurs at the distal ureter near the ureterovesical junction and tends to be a result of mechanical causes and responds favorably to percutaneous therapy, whereas late stenosis often occurs more proximal provoking by generalized or focal fibrosis resulting from ischemia or rejection, which has a dull result post minimally invasive treatment.Citation19–21 Interestingly, all the courses of UO were ≥6 months, possibly because of the routine using of double-J stent for 1 month post-transplantation. For these patients, pure balloon dilation or stent immediately after PCN in one session could not achieve high successful rate.Citation20,Citation22 In our experience, PCN was undisputed the first step as decompression could improve renal function quickly, as well as decrease incidence of infection and morbidity compared with the stent insertion in one session. As to the second step after renal function recovery, stent placement was done justified by mini-invasive character. As in our results, the step-by-step manipulation and relatively long stent reservation guaranteed the technical success.
The patient who lost transplanted allograft was a complicated case with TRAS concurrent with extrarenal pseudoaneurysms. The latter complication is extremely rare (<1%) and usually affected by the arterial anastomosis owing to surgical technique and infection.Citation3 Unlike intrarenal pseudoaneurysm, which is often asymptomatic and can be treated conservatively.Citation23 Extrarenal pseudoaneurysm can be devastating for their potential of rupture.Citation3 However, management choices for extrarenal pseudoaneurysms are still controversial. In our case, acute angled main renal branch made it difficult for covered stent placement, and superselective embolization with detachable coils was performed as its relatively narrow neck. Embolization was performed before PTA of the distal artery stenosis because of the possibility that balloon dilation of the distal stenosis could increase blood pressure of the proximal part and subsequently caused pseudoaneurysms rupture. However, we anticipated the renal parenchymal perfusion could be improved after solving the hemodynamic disturbance caused by “steal phenomenon”.Citation4 Unfortunately, this patient lost his allograft for irreversible acute rejection with enlarged sac again 1 month later. Therefore, except the morphological improvement with interventional management, sufficient immunosuppressive protocols were still in need.
Some limitations should be mentioned. First, this was a retrospective study with limited cases. Second, S.Cr level was chosen as the criteria to evaluate transplant renal function because all patients in our study had decreased renal function, which made it possible to combine the complications and to evaluate the efficacy of interventional treatment. However, creatinine level may be influenced by age, gender, race and body weight, and different kinds of complications. Therefore, the quantificational accuracy of this index or a combination of other predictors requires further consideration.
Conclusion
In conclusion, interventional therapy is safe and effective in treating the complications after kidney transplantation. It could be preferentially considered technique for saving transplanted renal allograft because of its effectiveness and minimal invasiveness.
Declaration of interest
All authors declare no conflicts of interest.
References
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–1730
- Chen GD, Shiu-Chung Ko D, Wang CX, et al. Kidney transplantation from donors after cardiac death: An initial report of 71 cases from China. Am J Transplant. 2013;13(5):1323–1326
- Kobayashi K, Censullo ML, Rossman LL, et al. Interventional radiologic management of renal transplant dysfunction: Indications, limitations, and technical considerations. Radiographics. 2007;27(4):1109–1130
- Hedegard W, Saad WEA, Davies MG. Management of vascular and nonvascular complications after renal transplantation. Tech Vasc Interv Radiol. 2009;12(4):240–262
- Šurlan M, Popovič P. The role of interventional radiology in management of patients with end-stage renal disease. Eur J Radiol. 2003;46(2):96–114
- Aktas S, Boyvat F, Sevmis S, Moray G, Karakayali H, Haberal M. Analysis of vascular complications after renal transplantation. Transplant P. 2011;43(2):557–561
- Rajiah P, Lim YY, Taylor P. Renal transplant imaging and complications. Abdom Imaging. 2006;31(6):735–746
- Patel NH, Jindal RM, Wilkin T, et al. Renal arterial stenosis in renal allografts: Retrospective study of predisposing factors and outcome after percutaneous transluminal angioplasty. Radiology. 2001;219(3):663–667
- Sobieszczyk P, Eisenhauer A. Management of patients after endovascular interventions for peripheral artery disease. Circulation. 2013;128(7):749–757
- Titus JM, Moise MA, Bena J, Lyden SP, Clair DG. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53(3):706–712
- Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol. 2004;15(1):134–141
- Akbar SA, Jafri SZH, Amendola MA, Madrazo BL, Salem R, Bis KG. Complications of renal transplantation. Radiographics. 2005;25(5):1335–1356
- Voiculescu A, Schmitz M, Hollenbeck M, et al. Management of arterial stenosis affecting kidney graft perfusion: A single-centre study in 53 patients. Am J Transplant. 2005;5(7):1731–1738
- Jones G, Tibballs J, Al-Akraa M, Sweny P. Iliac vein stenosis as a reversible cause of renal transplant dysfunction. Nephrol Dial Transpl. 2004;19(9):2415–2416
- Fava M, Loyola S, Flores P, Del Campo F. External iliac vein thrombosis after renal transplantation: Treatment by thrombolysis and stent placement: A case report. Transplantation. 1997;64:928–930
- Koster-Kamphuis L, Die CE, Der Vliet JA, Monnens L. Early transient leg swelling at the side of renal transplant in two children. Pediatr Transplant, 2006;10(1):112–113
- Campsen J, Bang TJ, Kam I, Gupta R. May-Thurner syndrome complicating left-sided renal transplant. Transplantation. 2010;89(7):904–906
- Leonardou P, Gioldasi S, Pappas P. Percutaneous management of ureteral stenosis of transplanted kidney: Technical and clinical aspects. Urol Int. 2011;87(4):375–379
- Kaskarelis I, Koukoulaki M, Georgantas T, et al. Ureteral complications in renal transplant recipients successfully treated with interventional radiology. Transplant P. 2008;40(9):3170–3172
- Bhagat VJ, Gordon RL, Osorio RW, et al. Ureteral obstructions and leaks after transplantation: Outcome of percutaneous antegrade ureteral stent placement in 44 patients. Radiology. 1998;209:159–167
- Fontaine AB, Nijjar A, Rangaraj R. Update on the use of percutaneous nephrostomy/balloon dilation for the treatment of renal transplant leak/obstruction. J Vasc Interv Radiol. 1997;8:649–653
- Bachar GN, Mor E, Bartal G, Atar E, Goldberg N, Belenky A. Percutaneous balloon dilatation for the treatment of early and late ureteral strictures after renal transplantation: Long-term follow-up. Cardiovasc Inter Rad. 2004;27(4):335–338
- Ladinsky GA, Goral S. Macroscopic hematuria in a kidney transplant recipient: A rare cause. Am J Kidney Dis. 2006;47(1):e3–e7

