Abstract
Background: The association between admission serum magnesium (Mg) levels and risk of developing septic shock in patients with systemic inflammatory response syndrome (SIRS) is limited. The aim of this study was to assess the risk of developing septic shock in hospitalized patients with SIRS with various admission Mg levels. Methods: This is a single-center retrospective study conducted at a tertiary referral hospital. All hospitalized adult patients with SIRS at admission who had admission Mg available from January 2009 to December 2013 were analyzed in this study. Admission Mg was categorized based on its distribution into six groups (<1.5, 1.5–1.7, 1.7–1.9, 1.9–2.1, 2.1–2.3, and >2.3 mg/dL). The primary outcome was septic shock occurring after hospital admission. Logistic regression analysis was performed to obtain the odds ratio (OR) of septic shock of various admission Mg levels using Mg with lowest incidence of shock, 2.1–2.3 mg/dL as the reference group. Results: Of 2589 patients with SIRS enrolled, septic shock occurred in 236 patients (9.1%). The lowest incidence of septic shock was when serum Mg was within 2.1–2.3 mg/dL. A reverse-checkmark curve emerged demonstrating higher incidences of septic shock associated with both hypoMg (<2.1) and hyperMg (>2.3). After adjusting for potential confounders, hypoMg (<1.5 mg/dL) was associated with an increased risk of developing septic shock with ORs of 1.86 (95% CI 1.07–3.27). Conclusion: Patients with SIRS and hypoMg (<1.5 mg/dL) at the time of admission had increased risk of developing septic shock during hospitalization.
Introduction
Septic shock is the most common cause of mortality in critically ill patients with a mortality rate reported to be as high as 50%.Citation1–3 Although multiple previous randomized controlled trials have attempted to identify effective interventions to prevent septic shock and improve the survival of these patients,Citation3–6 most were unsuccessful, and the mortality rate in patients with septic shock remains unacceptably high. Systemic inflammatory response syndrome (SIRS) is one of the risk factors for developing septic shock. Therefore, further studies are needed to identify patients with SIRS at high risk of developing septic shock during hospitalization.
Magnesium (Mg) gives a few functions in fields of energy generation, neurotransmitter release and also immune systems fighting against infection via inflammatory response and nitric oxide (NO) production.Citation7–19 Studies have shown that hypoMg is a potential risk factor of infections including acute bacterial infections including sepsis, bronchopneumonia and urinary tract infections.Citation13,Citation20 However, the effect of admission Mg levels on the risk of developing septic shock in patients with SIRS has not been examined. The objective of this study was to evaluate the risk of developing septic shock in hospitalized patients with SIRS across a spectrum of Mg levels.
Materials and methods
Study population
All research authorized adult (age 18 years or older) patients who presented with SIRS and admitted to Mayo Clinic Rochester—a tertiary referral hospital—from January 2009 to December 2013 with available admission serum Mg levels were enrolled. To increase the specificity of infected related SIRS, modified SIRS criteria were used and defined as at least 2 of 4 SIRS criteria within 24 h of presentation [body temperature less than 36 °C (96.8 °F) or greater than 38 °C (100.4 °F)], heart rate greater than 90 beats/min, tachypnea with greater than 20 breaths per minute, white blood cell (WBC) count less than 4000 cells/mm³ (4 × 109 cells/L) or greater than 12,000 cells/mm³ (12 × 109 cells/L), when it mandates that WBC or temperature be abnormal.Citation21 Exclusion criteria were patients who presented with septic shock at the time of admission. The local Institutional Review Board approved this study.
Data collection
Clinical characteristics, demographic information, and laboratory data were collected using manual and automated retrieval from the institutional electronic medical record system. The admission serum Mg level, defined as the first serum Mg level within 24 h of hospital admission was collected. eGFR was derived using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.Citation22 Chronic kidney disease was defined as a calculated eGFR less than 60 mL/min/1.73 mCitation2. The Charlson Comorbidity scoreCitation23 was computed for co-morbidities at the time of admission. Principal diagnoses were grouped based on ICD-9 codes at admission. The primary outcome was septic shock, defined as SIRS with refractory hypotension requiring vasopressors after the admission date.
Statistical analysis
Continuous variables are reported as mean ± SD for normally-distributed data and median (IQR) for non-normally distributed data. All categorical variables are reported as count with percentage. Baseline demographics and clinical characteristics were compared among admission Mg group, using ANOVA for continuous variables and the Chi-square test for categorical variables. We categorized admission Mg levels, based on six-quantile percentiles (10%, 25%, 50%, 75%, 90%): less than 1.5, 1.5–1.7, 1.7–1.9, 1.9–2.1, 2.1–2.3, and greater than 2.3 mg/dL. The Mg level of 2.1–2.3 mg/dL was selected as the reference group for outcome comparison since it was associated with the lowest incidence of septic shock (). We performed univariate analysis and then multivariate logistic regression analysis to evaluate the independent association between admission Mg levels and septic shock. Odds ratio (OR) with 95% confidence interval (CI) are reported. OR was adjusted for variables with statistically significant (p-value ≤ 0.05) differences between groups in univariate analysis. The adjusting variables were age, sex, baseline GFR, Charlson score, principal diagnosis, comorbidities, the presence of hypotension at hospital admission and the need for mechanical ventilator at hospital admission. Comorbidities were diabetes mellitus (DM) and congestive heart failure (CHF). A two-tailed p-value of less than 0.05 was considered statistically significant. All analyses were performed using JMP statistical software (version 10, SAS Institute, Cary, NC).
Results
A total of 3667 patients with SIRS with available Mg levels within 24 h were identified. After excluding 1078 patients with septic shock at presentation, 2589 unique patients with SIRS were enrolled.
Baseline characteristics
Of 2589 patients, 2362 (91%) patients were Caucasian and 1491 (58%) were male (). Mean age was 61 ± 18 years. Patient age was positively correlated with admission Mg levels, when eGFR was inversely correlated with admission Mg levels. Patient comorbidities included HTN (52%), DM (21%), CAD (18%), and CHF (7%).
Table 1. baseline clinical characteristics.
Admission Mg and the incidence of septic shock
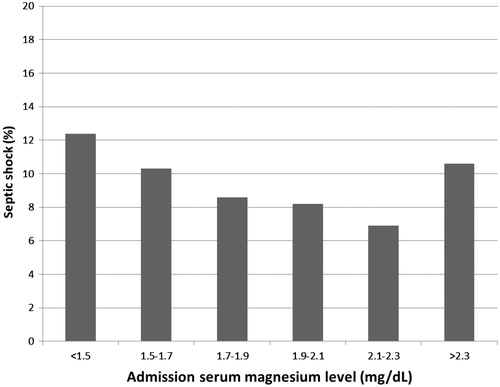
Of 2589 patients with SIRS enrolled, septic shock occurred in 236 patients (9.1%). The lowest incidence of septic shock (6.9%) was when serum Mg within 2.1–2.3 mg/dL (). A reverse-checkmark curve emerged demonstrating higher incidences of septic shock associated with both hypoMg (<2.1) and hyperMg (>2.3). When the incidences of septic shock in patients with serum Mg <1.5, 1.5–1.7, 1.7–1.9, 1.9–2.1 and >2.3 mg/dL were 12.4%, 10.3%, 8.6%, 8.2% and 10.6%, respectively.
Admission Mg and risk of septic shock
To assess whether admission Mg levels contributed to the septic shock development, logistic regression models were built, using 2.1–2.3 mg/dL as a reference range. Unadjusted admission Mg level of less than 1.5 mg/dL was associated with an increased risk of septic shock with ORs of 1.90 (95% CI 1.11–3.25) (). When adjusted for all variables including age, sex, eGFR, Charlson Comorbidity Score, history of DM, CHF, principal diagnosis, mechanical ventilator use and the presence of hypotension at hospital admission, the association remained statistically significant in Mg less than 1.5 mg/dL. Admission hypoMg (<1.5 mg/dL) was associated with an increased risk of septic shock (OR 1.86; 1.07–3.27). However, admission HyperMg (>2.3 mg/dL) was not significantly associated with the risk of developing septic shock (OR 1.40; 0.82–2.43) ().
Table 2. ORs for the association between admission serum Mg levels and the need for vasopressor use in hospital.
Discussion
In this study, we showed that admission Mg level in patients with SIRS was correlated with the incidence of septic shock during hospitalization. The lowest incidence of septic shock was when serum Mg within 2.1–2.3 mg/dL and there was a reverse-checkmark curve demonstrating higher incidences of septic shock associated with both hypoMg (<2.1) and hyperMg (>2.3). Patients with SIRS and hypoMg (<1.5 mg/dL) at the time of admission had 1.86-fold increased risk of developing septic shock during hospitalization.
There are several plausible explanations for the increased risk of septic shock in patients with SIRS and hypoMg at admission. First, Mg has been known to play an important role in the regulation of immune systems and inflammatory response.Citation8–12 In animal models, increased levels of proinflammatory cytokines (IL-6, TNF-alpha) have been reported under Mg deprivation.Citation8–10 HypoMg can result in an increase in inflammation by the activation of macrophages, neutrophils and endothelial cells.Citation11,Citation12 Moreover, apoptosis of thymus gland was shown in Mg-deficient rats.Citation24 Second, Mg also plays an important role in the release of NOs from the cell and one of NO functions is preventing infections in the body cavities, such as sinusitis, pneumonia and infection of mucosa in many organs.Citation13 HypoMg prevents cells from releasing their NOs and blood vessels cannot dilate properly, resulting in increased risk of recurrent infection.Citation14–16 Third, the presence of Mg is mandatory for the formation of thiamine pyrophosphate from thiamine (vitamin B1).Citation25 The lack of performance of thiamine can result in low levels of gastric acid and lead to increased risk of gastrointestinal infections. Cojocaru et al.Citation20 demonstrated that hypoMg was significantly associated with acute bacterial infections including sepsis, bronchopneumonia, urinary tract infections. Altogether, our study is the first to demonstrate that hypoMg at time of admission in patients with SIRS is an important predictor of developing septic shock.
This study has several limitations. First, this is a single-center, retrospective study. Second, the patient population in this study is relatively homogeneous (predominantly Caucasian). Further studies with more heterogeneous population are desirable to ascertain the clinical effects of admission Mg on septic shock in a broad patient population. In addition, the malnutrition has been suggested to be a cause of hypoMg and important factor for worse outcome.Citation26,Citation27 However, the data regarding albumin were limited since they were not commonly measured in hospitalized patients. Lastly, the effects of normalizing Mg levels and the risk of septic shock were not the focus of our present study. Future studies assessing the effects of Mg replacement in hypoMg patients with SIRS and at the time of admission are required.
In summary, this study demonstrates that patients with SIRS and hypoMg (<1.5 mg/dL) at the time of admission may carry higher risk of developing septic shock during hospitalization.
Declaration of interest
We do not have any financial or non-financial potential conflicts of interest..
Notes
* Institution: This work was performed at Mayo Clinic in Rochester, MN.
References
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174
- Brun-Buisson C, Meshaka P, Pinton P, Vallet B. Episepsis: A reappraisal of the epidemiology and outcome of severe sepsis in french intensive care units. Intensive Care Med. 2004;30:580–588
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316
- Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein c for severe sepsis. N Engl J Med. 2001;344:699–709
- Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377
- Annane D, Sebille V, Charpentier C, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288:862–871
- Altura BM. Basic biochemistry and physiology of magnesium: A brief review. Magnes Trace Elem. 1991;10:167–171
- Tam M, Gomez S, Gonzalez-Gross M, Marcos A. Possible roles of magnesium on the immune system. Eur J Clin Nutr. 2003;57:1193–1197
- Weglicki WB, Phillips TM, Freedman AM, Cassidy MM, Dickens BF. Magnesium-deficiency elevates circulating levels of inflammatory cytokines and endothelin. Mol Cell Biochem. 1992;110:169–173
- Weglicki WB, Phillips TM. Pathobiology of magnesium deficiency: A cytokine/neurogenic inflammation hypothesis. Am J Physiol. 1992;263:R734–R737
- Mak IT, Dickens BF, Komarov AM, Wagner TL, Phillips TM, Weglicki WB. Activation of the neutrophil and loss of plasma glutathione during Mg-deficiency – Modulation by nitric oxide synthase inhibition. Mol Cell Biochem. 1997;176:35–39
- Malpuech-Brugere C, Nowacki W, Daveau M, et al. Inflammatory response following acute magnesium deficiency in the rat. Biochim Biophys Acta. 2000;1501:91–98
- Unal M, Tamer L, Pata YS, et al. Serum levels of antioxidant vitamins, copper, zinc and magnesium in children with chronic rhinosinusitis. J Trace Elem Med Biol. 2004;18:189–192
- Reinhart RA. Clinical correlates of the molecular and cellular actions of magnesium on the cardiovascular system. Am Heart J. 1991;121:1513–1521
- Pearson PJ, Evora PR, Seccombe JF, Schaff HV. Hypomagnesemia inhibits nitric oxide release from coronary endothelium: Protective role of magnesium infusion after cardiac operations. Ann Thorac Surg. 1998;65:967–972
- Johnson S. The multifaceted and widespread pathology of magnesium deficiency. Med Hypotheses. 2001;56:163–170
- Cheungpasitporn W, Thongprayoon C, Kittanamongkolchai W, et al. Proton pump inhibitors linked to hypomagnesemia: A systematic review and meta-analysis of observational studies. Ren Fail. 2015. [Epub ahead of print]
- Cheungpasitporn W, Thongprayoon C, Erickson SB. Admission hypomagnesemia and hypermagnesemia increase the risk of acute kidney injury. Ren Fail. 2015. [Epub ahead of print]
- Cheungpasitporn W, Thongprayoon C, Mao MA, et al. Hypomagnesaemia linked to depression: A systematic review and meta-analysis. Intern Med J. 2015;45:436–440
- Cojocaru IM, Cojocaru M, Tanasescu R, Iacob SA, Iliescu I. Changes of magnesium serum levels in patients with acute ischemic stroke and acute infections. Rom J Intern Med. 2009;47:169–171
- Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the advance trial): A randomised controlled trial. Lancet. 2007;370:829–840
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251
- Malpuech-Brugere C, Nowacki W, Gueux E, et al. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br J Nutr. 1999;81:405–411
- Dyckner T, Ek B, Nyhlin H, Wester PO. Aggravation of thiamine deficiency by magnesium depletion. A case report. Acta Med Scand. 1985;218:129–131
- Wada T, Hirayama T, Hibino Y, Fukuhara Y, Kanno Y. Malnutrition as cause of hypomagnesemia. Kidney Int. 2014;86:856
- Karakelleoglu C, Orbak Z, Ozturk F, Kosan C. Hypomagnesaemia as a mortality risk factor in protein-energy malnutrition. J Health Popul Nutr. 2011;29:181–182