Abstract
Background: In the present study, the protective and therapeutic effects of quercetin (QE) on renal injury induced by methotrexate (MTX) have been examined. Materials and methods: A total of 24 male rats were divided into the following three groups: control group, MTX group, and MTX + QE group. Rats in MTX group received 20 mg/kg of single dose of MTX, while those in MTX + QE group received 20 mg/kg of single dose MTX, in addition to 15 mg/kg of QE administered 30 min prior to MTX and in the following 5-day period as a single daily dose. At the end of the experimental period, renal tissues were removed for histopathological and biochemical assessments. Results: Light microscopic examination showed a disruption of the renal structure in rats in MTX group in the form of tubular degeneration and dilation, with shedding of the tubular epithelial cells into the lumen. QE treatment was associated with less marked degenerative changes, with a similar histological appearance to that of controls. Furthermore, QE treatment resulted in decreased the number of apoptotic cells. Biochemical assessments showed significantly higher malondialdehyde (MDA) levels in MTX group as compared to control and MTX + QE groups. superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) levels showed a significant decrease in MTX group as compared to controls. However, QE significantly suppressed MDA level, compensated deficits in the anti-oxidant defenses [reduced SOD, GSH-Px, and CAT levels] in kidney tissue resulted from MTX administration. Conclusions: In conclusion, renal toxic effects of MTX may be alleviated by QE.
Introduction
Methotrexate (MTX), a folic acid antagonist, is not only used as a chemotherapeutic agent for the treatment of leukemia, osteosarcoma, head and neck tumors, lung cancer, and certain other types of cancer, but also has anti-inflammatory, anti-proliferative, immunosuppressive, and anti-psoriatic effects, with appropriate clinical indications.Citation1
Despite this wide range of indications, MTX therapy is associated with a number of adverse reactions, particularly pneumonia, nephrotoxicity, and hepatotoxicity that may necessitate dose reductions or discontinuation of the treatment.Citation2,Citation3 In previous studies, oxidative injury mostly due to reactive oxygen species has been implicated in the majority of such adverse effects, particularly hepatotoxicity and nephrotoxicity.Citation3,Citation4
One of the most common effects observed after MTX use is the functional impairment in kidneys.Citation5 Administration of MTX to rats has been found to decrease glutathione (GSH) levels in kidneys and small intestines, and increase the levels of myeloperoxidase and malondialdehyde (MDA), which are indicators of the inflammatory response and lipid peroxidation, respectively.Citation3
Quercetin (QE) is an anti-oxidant compound and represents an important member of the flavonoids. QE has several biological functions such as anti-oxidant, anti-mutagenic, anti-cancer, anti-viral, anti-thrombotic, anti-ischemic, anti-inflammatory, and anti-allergic effects, as well as preventive effects on arteriosclerosis and coronary heart disease, and stimulation of cellular immunity.Citation6,Citation7 Studies at a cellular level found inhibitory effects of QE on colon, stomach, prostate, breast, cervix, ovarian, lung, and liver cancer cells. Also, it has been found to exert anti-aggregating effects on platelets and anti-hypertensive effects.Citation8 In a study by Ishikawa et al. involving renal glomerular cell cultures, QE showed anti-apoptotic effects as well.Citation9
Agents with potent anti-oxidant effects have been tested in terms of their efficacy to prevent MTX-related side effects, with significant success in some of these studies.Citation10,Citation11 However, the results of these studies with these agents have not been adequately reflected in the clinical studies due a number of factors such as the high cost of such chemicals or absence of licensed preparations.
Thus, we aimed to examine the anti-apoptotic and anti-oxidant effects of QE in MTX-induced nephrotoxicity in a rat model considering its low acquisition cost, wide availability, and proven in vivo and in vitro anti-oxidant effects.
Materials and methods
Animals
Twenty-four healthy Sprague Dawley male rats (8–10 weeks old) were utilized in this study. All animals received human care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health. Our study was approved by Namik Kemal University, Local Animal Ethics Committee and ethical rules were observed during our study (Permission number: NKU HADYEK 2014/04-3, 03.07.2014).
Experimental protocol
The rats were randomly allotted into one of three experimental groups: control, MTX, and MTX + QE; each group contains eight animals. Rats in the control group only received physiological saline through the intraperitoneal (i.p.) route. Rats in MTX group received a single 20 mg/kg i.p. dose of MTX (50 mg flakon®, Koçak Farma, Tekirdağ, Turkey) dissolved physiological saline. Those rats in MTX + QE group received a single dose of MTX on day 1 (20 mg/kg, in physiological saline, i.p.) in addition to a daily dose of QE (5 mg/kg, in physiological saline) administered 30 min prior to MTX and in the following 5-day period as a single daily dose.
At the end of the experiments, all rats were sacrificed by decapitation under ketamine-xylazine anesthesia. After bilateral nephrectomies were carried out, the right kidney was put into Bouin's solution for histopathological examination and left kidney was preserved at −80 °C until analysis of MDA, superoxide dismutase (SOD), catalase (CAT), and GSH-peroxidase (GSH-Px) levels was performed.
Dosage of MTX was determined from those described by relevant literatures.Citation11–13 The dose of QE was decided based on a recent study reported by Aktoz et al.Citation14
Histopathologic evaluation
Biopsies from the renal tissues of the rats were harvested and tissue fragments were fixed in Bouin's solution, embedded in paraffin, sectioned at 5 μm thickness and then stained with H&E. The preparations were evaluated by light microscope and photographed (Olympus CX41 microscope, Olympus, Tokyo, Japan).
Renal tissue injury index was performed quantitative analysis, the assessment was expressed as the sum of the individual score grades from 0 (no findings), 1 (mild), 2 (moderate), to 3 (severe) for each of the following parameters from kidney sections: tubular degeneration, tubular dilatation, tubular cell swelling, cellular vacuolization and glomerular congestion and degeneration.
TUNEL assay
Apoptosis was evaluated by the terminal dUTP nick end-labeling (TUNEL) assay. The TUNEL method, which detects fragmentation of DNA in the nucleus during apoptotic cell death in situ, was employed using an apoptosis detection kit (ApopTag® Peroxidase In Situ Apoptosis Detection Kit, Cat. No. S7100).
Three randomly selected slides, each of five different fields at high-power lens visual fields (×400), and the apoptotic cells were identified by the intense dark nuclear staining. Total cell population and TUNEL positive cells were counted. TUNEL positive cells were expressed as a percentage of total cells.Citation15
Lipid peroxidation and anti-oxidant enzyme analysis
The protein content of the supernatant was determined using the Lowry method.Citation16 MDA (nmol/mg protein), SOD (U/mg protein), CAT (U/mg protein), and GSH-Px (U/mg protein) levels were determined according to the methods of Buege and Aust,Citation17 Sun et al.,Citation18 Aebi,Citation19 and Paglia and Valentine,Citation20 respectively.
Statistical analysis
All statistical analyses were carried out using SPSS statistical software (S0064 Minitab Release 13, License number: WCP1331.00197). All data were presented in mean ± SD. Differences in measured parameters among the three groups were analyzed with a nonparametric test (Kruskal–Wallis). Dual comparisons between groups exhibiting significant values were evaluated with a Mann–Whitney U-test. These differences were considered significant when probability was less than 0.05.
Results
Histopathologic findings
The control group showed regular morphology with no evidence of histopathological changes (). MTX treatment showed prominent degenerative changes with tubular degeneration, tubular dilatation, tubular cell swelling, cellular vacuolization, glomerular congestion and degeneration in the renal cortical tissues (). On the contrary, QE cure was able to restore MTX-induced histopathological changes ().
Figure 1. Light microscopy of renal cortical tissues in different groups for H&E. (a) In control, normal renal tissue morphology was seen. (b) After MTX, severe tubuler and glomeruler damage was noted. (c) QE treatment prevented tubuler and glomeruler damage compared with alone MTX group. (d) Renal injury degree was significantly decreased in the MTX + QE group when compared to MTX group. Asterisk: glomerular congestion and degeneration, Arrowhead: tubular cell swelling, Arrow: tubular dilatation. (H&E, scale bar, 50 μm). ap < 0.001 compared to control group, bp < 0.01 compared to MTX group.
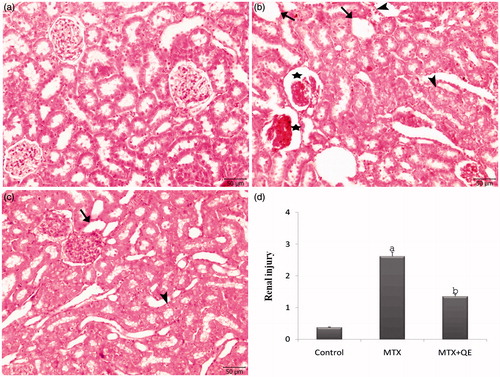
In addition, renal tissue injury index was higher in MTX group comparing to MTX + QE group significantly, and it was higher in MTX + QE group comparing to control group significantly ().
TUNEL findings
TUNEL method was used in order to determine apoptosis in renal tissues of all groups. In control group, only a few TUNEL positive cells were observed (). After the MTX treatment a great number of TUNEL positive apoptotic cells were observed on the wall of tubules and glomeruli (). QE therapy was seen to decrease the number of apoptotic cells ().
Figure 2. TUNEL staining of renal cortical tissues in different groups. (a) In control group, a few TUNEL positive cells were observed in the renal cortical tissues. (b) The TUNEL positive cells were significantly higher in the renal cortical tissues of the MTX treated group. (c) QE treatment markedly decreased the number of TUNEL positive cells. (d) The apoptotic index was significantly decreased in the MTX+QE group when compared to MTX group. (Arrow: TUNEL positive cells), (TUNEL, scale bar, 50 μm). ap < 0.001 compared to control group, bp < 0.01 compared to MTX group.
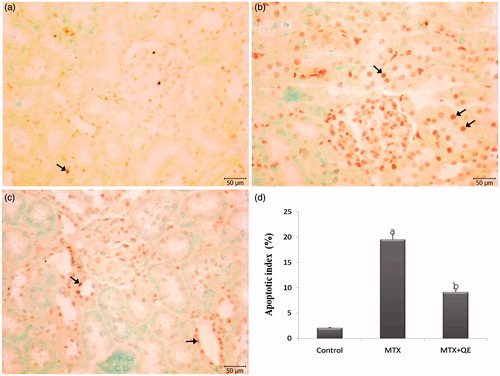
TUNEL positive cells were counted and apoptotic index values were calculated for all groups. The percent of TUNEL positive cells increased in the renal tissue after MTX administration as compared with control group and it was found statistically to decrease significantly in MTX + QE group as compared with the values of MTX group ().
Lipid peroxidation and anti-oxidant enzyme assay
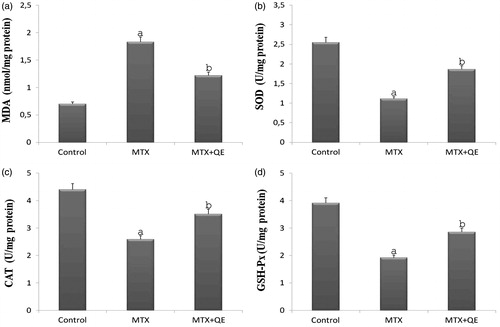
In our study performed on rats, SOD, CAT and GSH-Px levels from anti-oxidant defense systems were determined spectrophotometrically. However, MDA levels occurring as a result of lipid peroxidation and used as an important parameter in introducing oxidative damage were measured by the same method. MDA levels of renal tissue were significantly increased in the renal tissue of rats treated with MTX. However, this elevation was significantly suppressed when QE treatment followed MTX administration (). Renal tissue of rats showed significantly reduction of SOD, CAT and GSH-Px levels after MTX treatment. These anti-oxidant enzymes level was higher in MTX + QE group comparing to MTX group significantly ().
Figure 3. Renal tissue MDA, SOD, CAT, and GSH-Px levels in control, MTX, and MTX + QE groups. Tissue MDA levels were significantly increased, SOD, CAT, and GSH-Px levels were significantly decreased in MTX rats in comparison to control rats. Treatment of QE significantly decreased the elevated tissue MDA levels and increased of reduced SOD, CAT, and GSH-Px levels in the renal tissues. ap < 0.001 compared to control group, bp < 0.01 compared to MTX group.
Discussion
MTX is an effective chemotherapeutic agent in cancer treatment, as well as its use in other indications such as rheumatoid arthritis, psoriasis, Crohn's disease, and ulcerative colitis.Citation21–23 Despite its widespread use, it is also associated with a number of serious side effects, particularly nephrotoxicity.Citation24
In previous experimental studies, MTX has been administered to rats at varying doses and routes of administration. In our study, MTX was given with a single i.p. dose of 20 mg/kg as previously described in certain tissue toxicity studies.Citation11,Citation25
Although the exact mechanisms of MTX-associated nephrotoxicity have not been clearly understood, recent studies suggest that it may be due to oxidative stress.Citation26 QE is a very potent anti-oxidant providing protection against oxidative injury through prevention of the formation of hydroxyl radicals via Fe and Cu. Previous experimental work on nephrotoxicity confirmed the QE's efficacy on reducing the oxidative injury.Citation27,Citation28 Similarly, we also preferred QE for our study based on its proven anti-oxidant and anti-apoptotic efficacy.
Reduction in GSH levels due to MTX treatment results in a weakening of the anti-oxidant defense system protecting cells against the effects of reactive oxygen species such as superoxide anion, hydroxyl radicals, hydrogen peroxide, and hydrochloride.Citation29 Also, MTX has been found to lead to tissue injury through lipid peroxidation.Citation3 Again, MTX was found to increase MDA levels, and certain anti-oxidant compounds could reduce MDA levels.Citation3,Citation5,Citation29 In a 2011 study by Asvadi et al.Citation12 rats had increased levels of MDA upon administration of MTX, while SOD and GSH-Px were found to decrease. Similarly other studies found increased levels of MDA and decreased levels of anti-oxidant enzymes SOD, CAT, and GSH-Px with administration of MTX. Anti-oxidant molecules in the same studies resulted in a reduction of oxidative injury when coadministered with MTX.Citation13,Citation30 Cafeic acid phenylester, a potential free radical scavenger, has been found to exert protective effects through the regulation of oxidation/anti-oxidation balance.Citation31 Similar findings have been observed with the use of taurine, which corrected the oxidative tissue injury caused by MTX as detected by biochemical assays.Citation32 Consistent with previous studies, MTX significantly increased the renal MDA level, while it in caused a decline in SOD, CAT, and GSH-Px. However, the anti-oxidant molecule utilized in our study, i.e., QE decreased MDA levels, which were increased by MTX, and in increased the concentrations of anti-oxidants such as SOD, CAT, and GSH-Px.
High dose intake of MTX is known to result in the development of acute renal failure.Citation24 Although the mechanism underlying the renotoxic effects of MTX has not been fully elucidated, its precipitation in renal tubules, or direct toxic effect of the original molecule or its metabolite has been implicated.Citation33 Also, high dose MTX may precipitate in renal tubules, causing acute renal failure.Citation34 Significant pathological changes in the renal tubules and glomeruli have been reported to occur in response to MTX use.Citation4,Citation5 Vardi et al.Citation13 in their study showed significant structural damage including glomerulosclerosis and apoptosis upon MTX administration. Kose et al.Citation35 reported MTX-related effects such as tubular desquamation and brush-border injury in the proximal tubules. Other studies showed degeneration and dilation in proximal and distal tubular epithelial cells in addition to glomerular degenerative changes.Citation25,Citation36 In the present study, in line with these previous reports, QE was able to alleviate the histopathological injury caused by MTX in renal tissues.
MTX causes the formation of DNA defects leading to apoptosis through the inhibition of purine and pyrimidine.Citation37 Also, activation of caspase represents another mechanism through which MTX may be causing apoptosis.Citation38 In studies by Vardi et al.Citation13 and Kocaman and ColakogluCitation39 an increased number of apoptotic cells have been detected with MTX administration. However, Asvadi et al.Citation12 observed a decrease in the number of apoptotic cells after an increase caused by apoptosis by the use of pentoxifylline, a molecule with anti-oxidant properties. Also, QE was found to possess apoptosis-reducing properties in heavy metal renal injury due to lead.Citation40 Again, Ozyurt et al.Citation41 were able to reduce the radiation-induced apoptotic changes by QE. Similar to previous publications, we have been able to show that MTX was associated with increased apoptosis in renal tissues, and that QE reduced apoptotic changes induced by MTX.
Conclusions
Our results demonstrate that oxidative stress plays an essential role in MTX-induced renal toxicity in rats. The findings of this study show that QE is capable of reducing MTX-induced renal oxidative injury through its anti-oxidant and anti-apoptotic effects, which were evaluated both histologically and biochemically. However, further research will be necessary to understand the mechanisms by which QE is able to prevent renal damage.
Declaration of interest
The authors report no conflicts of interest.
References
- Feagan BG, Alfadhli A. Methotrexate in inflammatory bowel disease. Gastroenterol Clin North Am. 2004;33:407–420
- Abraham P, Kolli VK, Rabi S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct. 2010;28(5):426–433
- Jahovic N, Cevik H, Sehirli AO, Yegen BC, Sener G. Melatonin prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pineal Res. 2003;34(4):282–287
- Kolli VK, Abraham P, Isaac B, Selvakumar D. Neutrophil infiltration and oxidative stress may play a critical role in methotrexate-induced renal damage. Chemotherapy. 2009;55(2):83–90
- Sener G, Ekşioğlu-Demiralp E, Çetiner M, et al. l-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol. 2006;22:47–60
- Elik M, Serdaroglu G, Ozkan R. Mirisetin ve Kuersetin Bilesiklerinin Antioksidan Etkinliklerinin DFT Yontemiyle İncelenmesi. Fen Bilimleri Dergisi. 2007;28(2):53–65
- Kahraman A, Erkasap N, Koken T, Serteser M, Aktepe F, Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology. 2003;183:133–142
- Abdel-Raheem IT, Abdel-Ghany AA, Mohamed A. Protective effect of quercetin against gentamicin-induced nephrotoxicty. Biol Pharm Bull. 2009;32(1):61–67
- Ishikawa Y, Kitamura M. Bioflavonoid quercetin inhibits mitosis and apoptosis of glomerular cells in vitro and in vivo. Biochem Biophys Res Commun. 2000;279:629–634
- Acipayam C, Bayram I, Daglioglu K, et al. The protective effect of hesperidin on methotrexate-induced intestinal epithelial damage in rats: An experimental study. Med Princ Pract. 2014;23(1):45–52
- Bozkurt M, Em S, Oktayoglu P, et al. Carvacrol prevents methotrexate-induced renal oxidative injury and renal damage in rats. Clin Invest Med. 2014;37(1):19–25
- Asvadi I, Hajipour B, Asvadi A, Asl NA, Roshangar L, Khodadadi A. Protective effect of pentoxyfilline in renal toxicity after methotrexate administration. Eur Rev Med Pharmacol Sci. 2011;15(9):1003–1009
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A. The protective effects of Prunus armeniaca L (apricot) against methotrexate-induced oxidative damage and apoptosis in rat kidney. J Physiol Biochem. 2013;69:371–381
- Aktoz T, Kanter M, Uz YH, Aktas C, Erboga M, Atakan IH. Protective effect of quercetin against renal toxicity induced by cadmium in rats. Balkan Med J. 2012;29:56–61
- Jawan B, Goto S, Pan TL, et al. The protective mechanism of magnolol, a Chinese herb drug, against warm ischemia–reperfusion injury of rat liver. J Surg Res. 2003;110:378–382
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275
- Buege AJ, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310
- Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500
- Aebi H. Catalase. In: Bergmeyer HU, ed. Methods of Enzymatic Analysis. New York and London: Academic Press; 1974: 673–677
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterisation of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169
- Tilling L, Townsend S, David J. Methotrexate and hepatic toxicity in rheumatoid arthritis and psoriatic arthritis. Clin Drug Invest. 2006;26:55–62
- Sendur N, Karaman G, Savk H. Akut Metotreksat Toksisitesinin Erken Belirtisi; Deri Ülserleri. T Klin Tıp Bilimleri. 2002;22:593–596
- Al-Motagabani MA. Histological and histochemical studies on the effects of methotrexate on the liver of adult male albino rat. Int J Morphol. 2006;24(3):417–422
- Caglar Y, Ozgur H, Matur I, et al. Ultrastructural evaluation of the effect of N-acetylcysteine on methotrexate nephrotoxicity in rats. Histol Histopathol. 2013;28:865–874
- Cakır T, Ozkan E, Dulundu E, et al. Caffeic acid phenethyl ester (CAPE) prevents methotrexate-induced hepatorenal oxidative injury in rats. J Pharm Pharmacol. 2011;63(12):1566–1571
- Pinheiro FV, Pimentel VC, De Bona KS, et al. Decrease of adenosine deaminase activity and increase of the lipid peroxidation after acute methotrexate treatment in young rats: Protective effects of grape seed extract. Cell Biochem Funct. 2010;28(1):89–94
- Chaudhary S, Ganjoo P, Raiusddin S, Parvez S. Nephroprotective activities of quercetin with potential relevance to oxidative stress induced by valproic acid. Protoplasma. 2015;252(1):209–217
- Hou Y, Zeng Y, Li S, et al. Effect of quercetin against dichlorvos induced nephrotoxicity in rats. Exp Toxicol Pathol. 2014;66(4):211–218
- Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:274–278
- Abdel-Raheem IT, Khedr NF. Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexate-induced kidney damage in rats. Naunyn-Schmiedeberg's Arch Pharmacol. 2014;387:341–353
- Uzar E, Sahin O, Koyuncuoglu HR, et al. The activity of adenosine deaminaz and the level of nitric oxide in spinal cord of methotrexate administered rats: Protective effect of caffeic acid phenethyl ester. Toxicology. 2006;218(2–3):125–133
- Cetiner M, Sener G, Sehirli AO, et al. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death. Toxicol Appl Pharmacol. 2005;209(1):39–50
- Lankelma J, van der Klein E, Ramaekers F. The role of 7-hydroxymethotrexate during methotrexate anti-cancer therapy. Cancer Lett. 1980;9:133–142
- Oktem F, Yilmaz HR, Ozguner F, et al. Methotrexate-induced renal oxidative stress in rats the role of a novel antioxidant caffeic acid phenethyl ester. Toxicol Ind Health. 2006;22:241–247
- Kose E, Beytur A, Vardı N, Turkoz Y, Ekinci N, Ekincioglu Z. Metotreksat'ın neden olduğu akut böbrek hasarına karşı montelukast'ın etkileri. İnönü Üniv Tıp Fak Derg. 2011;18:73–77
- Morsy MA, Ibrahim SA, Amin EF, Kamel MY, Rifaai RA, Hassan MK. Curcumin ameliorates methotrexate-induced nephrotoxicity in rats. Adv Pharmacol Sci. 2013;2013:387071
- Uraz S, Tahan V, Aygun C, et al. Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci. 2008;53:1071–1077
- Papaconstantinou HT, Xie C, Zhang W, et al. The role of caspases on methotrexate induced gastrointestinal toxicity. Surgery. 2001;130:859–865
- Kocaman N, Colakoglu N. Tekrarlayan Dozlarda Metotreksat Uygulamasının Sıçan Böbrek Dokusu Üzerine Etkileri. Firat Med J. 2013;18(4):198–202
- Liu CM, Ma JQ, Sun YZ. Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ Toxicol Pharmacol. 2010;30(3):264–271
- Ozyurt H, Cevik O, Ozgen Z, et al. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic Res. 2014;48(10):1247–1255

