Abstract
Background: The objective of this systematic review and meta-analysis was to evaluate the effectiveness and safety of rituximab as induction therapy in ABO-compatible, non-sensitized renal transplantation. Methods: A literature search for randomized controlled trials (RCTs) was performed from inception through February 2015. Studies that reported relative risks or hazard ratios comparing the risks of biopsy-proven acute rejection (BPAR), graft loss, leukopenia, infection or mortality in ABO-compatible, non-sensitized renal transplant recipients who received rituximab as induction therapy versus controls were included. Pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using a random-effect, generic inverse variance method. Results: Four RCTs with 480 patients were included in the meta-analysis. Pooled RR of BPAR in recipients with rituximab induction was 0.90 (95% CI 0.50–1.60). Compared to placebo, the risk of BPAR in rituximab group was 0.76 (95% CI 0.51–1.14, I2 = 0). The risk of leukopenia was increased in rituximab group with the pooled RR of 8.22 (95% CI 2.08–32.47). There were no statistical differences in the risks of infection, graft loss and mortality at 3–6 months after transplantation with pool RRs of 1.02 (95% CI 0.85–1.21), 0.55 (95% CI 0.21–1.48) and 0.58 (95% CI 0.17–1.99), respectively. Conclusion: This meta-analysis demonstrated insignificant reduced risks of BPAR, graft loss or mortality among in ABO-compatible, non-sensitized renal transplant recipients with rituximab induction. Although rituximab induction significantly increases risk of leukopenia, it appears to be safe with no significant risk of infection.
Introduction
A number of randomized controlled trials (RCTs) and meta-analyses indicate that induction therapy consisting of biologic antibodies and conventional immunosuppressive agent therapy is superior to conventional therapy alone in lowering renal allograft rejection and failure.Citation1,Citation2 Therefore, since 2009, the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline has recommended using a combination of immunosuppressive medications before, or at the time of renal transplantation.Citation3 Interleukin 2 receptor antagonists (IL2-RA) were recommended as the first-line induction therapy, while a lymphocyte-depleting agent was suggested for high immunologic risk transplantation. Despite current immunosuppressive protocols, acute rejection rates have still been reported as high as 10%.Citation4
Rituximab is a chimeric anti-CD20 monoclonal antibody that eliminates B lymphocytes.Citation5,Citation6 Rituximab has been used “off-label” in a variety of situations such as desensitization protocols for ABO-incompatible transplantation, human leukocyte antigen (HLA)-incompatible transplantation, treatments of post-transplant lymphoproliferative disease (PTLD), refractory cases with acute allograft rejection, chronic antibody-mediated rejection and recurrent glomerulonephritis following transplantation.Citation5–7 In addition to B-cell depleting effect, rituximab has been shown to provide direct inhibition of T-cell activation.Citation8 Thus, rituximab has been investigated for its use as induction therapy in ABO-compatible, non-sensitized renal transplantation.Citation9–12 Macklin et al.Citation13 recently performed a comprehensive review of the use of rituximab as induction therapy in renal transplantation, and concluded that available studies do not support the use of rituximab as induction therapy. However, comprehensive data regarding effect of acute rejection reduction and the risks of graft loss, leukopenia, infection and mortality in the use of rituximab induction therapy are limited.
The objectives of this systematic review and meta-analysis were to comprehensively accumulate all available data and pool the results in order to assess the effectiveness and safety of rituximab as induction therapy in ABO-compatible, non-sensitized renal transplantation.
Materials and methods
Search strategy
Two investigators (W.C. and C.T.) independently searched published studies and conference abstracts indexed in MEDLINE, EMBASE, the Cochrane database and ClinicalTrials.gov from inception through February using the search strategy described in Item S1 in online supplementary data. A manual search for additional relevant studies using references from retrieved articles was also performed.
Inclusion criteria
The inclusion criteria were as follows: (1) RCTs published as original studies or conference abstracts that evaluated the effectiveness and safety of rituximab as induction therapy versus controls in ABO-compatible, non-sensitized renal transplant recipients, (2) studies that provided data to calculate relative risks, hazard ratios, or standardized incidence ratios with 95% confidence intervals (CIs), and (3) a reference group composed of subjects with induction with other induction agents or placebo as control group.
Study eligibility was independently determined by the two investigators noted previously. Differing decisions were resolved by mutual consensus. The quality of each study was evaluated by using the Jadad quality-assessment scale.Citation14
Data extraction
A standardized data collection form was used to extract the following information: last name of first author, title of article, study design, year of study, country of origin, year of publication, sample size, definition of rituximab induction and control groups, baseline immunosuppression, infection prophylaxis regimen, and outcome assessment period.
Statistical analysis
Review Manager 5.2 software (The Cochrane Collaboration, Oxford, UK) was used for data analysis. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird.Citation15 Given the high likelihood of between study variances, a random-effect model was used rather than a fixed-effect model. Statistical heterogeneity was assessed using Cochran’s Q test. This statistic was complemented with the I2 statistic, which quantifies the proportion of the total variation across studies that is due to heterogeneity rather than chance. An I2 of 0–25% represents insignificant heterogeneity, 26–50% low heterogeneity, 51–75% moderate heterogeneity and >75% high heterogeneity.Citation16 The presence of publication bias was assessed by funnel plots of the logarithm of odds ratios versus their standard errors.Citation17
Results
The search strategy yielded 690 potentially relevant articles: 608 were excluded based on the title and abstract indicating that they clearly did not fulfill inclusion criteria on the basis of article type, study design, population, or outcome of interest (Item S2 available online at http://informahealthcare.com/doi/suppl/[doinumber]). The remaining 82 articles underwent full-length review, with 78 excluded because they were not RCTs (n = 9), studied the outcomes of patients with ABO-incompatible or highly sensitized patients (n = 33) or did not report outcomes of interest (n = 36). Four RCTsCitation9–12 with 480 patients met our inclusion criteria and were included in the meta-analysis. contains detailed characteristics and quality assessment of all included studies.
Table 1. Main characteristics of the studies included in this meta-analysis.
The risks of acute rejection and allograft loss in patients with rituximab induction
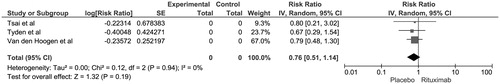
The pooled risk ratio (RR) of biopsy-proven acute rejection (BPAR) in recipients with rituximab induction was 0.90 (95% CI 0.50–1.60, I2 = 34). shows the forest plot of the included studies. We also performed a sensitivity analysis excluding the study by Clatworthy et al.Citation11 as it was the only study comparing rituximab to daclizumab. Compared to placebo, the risk of BPAR in rituximab group excluding Clatworthy et al. was 0.76 (95% CI 0.51–1.14, I2 = 0) (). A majority of rejection episodes were acute cellular rejections. Studies by Clatworthy et al.Citation11 and Tyden et al.Citation18 reported no antibody-mediated rejection episodes in 3 and 6 months, respectively. The pooled RR of allograft loss at 6 months in patients receiving rituximab induction was 0.55 (95% CI 0.21–1.48, I2 = 0%).
Figure 1. Forest plot of included RCTs comparing risk of biopsy-proven acute rejection in recipients with rituximab induction versus control; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error.
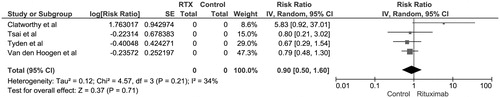
Figure 2. Forest plot of included RCTs comparing risk of biopsy-proven acute rejection in recipients with rituximab induction versus placebo; square data markers, RRs; horizontal lines, 95% CIs, with marker size reflecting statistical weight of study using random-effects meta-analysis. Diamond data markers, overall RRs and 95% CIs for outcomes of interest. IV, inverse variance; SE, standard error.
The safety profiles of rituximab induction
The risk of leukopenia (<2 to 3 × 109 cells/L) was increased in rituximab group with the pooled RR of 8.22 (95% CI 2.08–32.47) (Figure S1). There was no statistical difference in the risk of infection or mortality between recipients with rituximab induction versus controls with pool RRs of 1.02 (95% CI 0.85–1.21) and 0.58 (95% CI 0.17–1.99), respectively as shown in Figures S2 and S3. Van den Hoogen et al.Citation9 reported the risk of malignancy at 24 months of 1.03 (95% CI 0.40–2.66).
Evaluation for publication bias
Overall, assessments of publication bias were limited due to small numbers of included studies. Funnel plots to evaluate publication bias with RCTs regarding the risk of BPAR in recipients with rituximab induction are summarized in Figures S4 and S5. Overall, the publication bias was insignificant.
Discussion
This current meta-analysis revealed no significant reduction in acute rejection risk in the use of rituximab as induction therapy. The quality of evidence is supported by the low heterogeneity of the included studies. Although the risk of leukopenia is 8.22-fold increased in rituximab therapy, there is no significant increase in risk of infection. In addition, induction with rituximab alone does not reduce the rates of graft loss or mortality among in ABO-compatible, non-sensitized renal transplant recipients.
There are several plausible explanations for insignificant reduced acute rejection risk in recipients who received rituximab as induction therapy alone. First, although induction with rituximab leads to B cell depletion that lasts for over 15 months, a reduction in B cells in the peripheral blood occurs within 1–3 days after the administration.Citation19 Most of included studies used rituximab within 1 day prior to or after surgery.Citation9,Citation10,Citation12 Second, the inactivation of T-cell by rituximab was transient and restored after 3 months after the infusion. Third, Clatworthy et al.Citation11 described the elevation of cytokine or “cytokine storm” after rituximab induction and proposed that these mediators may facilitate antigen presentation, resulting in acute cellular rejection. The study by Clatworthy et al.Citation11 compared the effectiveness of rituximab versus daclizumab, an IL2-RA, and found higher incidence of acute rejection in rituximab group and the study was prematurely halted. Induction therapy with rituximab alone therefore should not be recommended as induction therapy.
Although rituximab induction seems to be safe and there was no significant increased risk of bacterial or opportunistic infection at 6 months, data on long-term effects are limited. Despite no significant increased or reduced mortality risk in rituximab induction therapy at 6 months, Tyden et al.Citation18 reported a statistically significant increase in mortality in the rituximab group at 3-year follow-up and 75% of deaths in rituximab treated recipients were from cardiovascular causes. This raises the concern of adverse cardiovascular effects from rituximab since B-lymphocytes, particularly B1a-lymphocytes, were recently found to provide an are atheroprotective effect.Citation20
There are several limitations of the present analysis. First, rituximab was given as induction therapy at the day of surgery in most included studies.Citation9,Citation10,Citation12 Therefore, we cannot assess the effects of rituximab administration 1–2 weeks prior to renal transplantation as its use for desensitization.Citation7 Second, there are no currently published studies assessing the effects of rituximab plus a standard induction regimen. An ongoing RCT, ReMIND (RituxiMab INDuction in renal transplantation; ClinicalTrials.gov identifier – NCT01095172 will likely elucidate if rituximab provides any benefit or risk when it is combined with basiliximab, an IL2-RA. Lastly, all included studies assessed most clinical outcomes at 3 to 6 months after transplantation. However, the effects of rituximab especially a reduction in B cells may last for over 15 months after the administration.Citation19 A future study is ultimately required to address these long-term outcomes of rituximab induction in renal transplantation.
In summary, this meta-analysis shows no significant reduced risk of BPAR, graft loss or mortality among in ABO-compatible, non-sensitized renal transplant recipients with rituximab induction. Although rituximab induction significantly increases risk of leukopenia, it appears to be safe with no significant risk of infection. Future studies on effects of rituximab induction in addition to current standard induction regimen may be indicated.
Supplementary material available online
Supplementary Item S1 and S2 and Figures S1-S5.
Supplemental File
Download MS Word (266.7 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bakr MA, Nagib AM, Donia AF. Induction immunosuppressive therapy in kidney transplantation. Exp Clin Transplant. 2014;12(Suppl. 1):60–69
- Webster AC, Playford EG, Higgins G, Chapman JR, Craig JC. Interleukin 2 receptor antagonists for renal transplant recipients: A meta-analysis of randomized trials. Transplantation. 2004;77:166–176
- Kasiske BL, Zeier MG, Chapman JR, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: A summary. Kidney Int. 2009;77:299–311
- Matas AJ, Smith JM, Skeans MA, et al. Optn/srtr 2012 annual data report: Kidney. Am J Transplant. 2014;14(Suppl. 1):11–44
- Ramanath V, Nistala R, Chaudhary K. Update on the role of rituximab in kidney diseases and transplant. Expert Opin Biol Ther. 2012;12:223–233
- Barnett AN, Hadjianastassiou VG, Mamode N. Rituximab in renal transplantation. Transpl Int. 2013;26:563–575
- Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for desensitization in renal transplantation. Transplantation. 2014;98:794–805
- Stroopinsky D, Katz T, Rowe JM, Melamed D, Avivi I. Rituximab-induced direct inhibition of t-cell activation. Cancer Immunol Immunother. 2012;61:1233–1241
- van den Hoogen MW, Kamburova EG, Baas MC, et al. Rituximab as induction therapy after renal transplantation: A randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. 2015;15:407–416
- Tyden G, Genberg H, Tollemar J, et al. A randomized, doubleblind, placebo-controlled, study of single-dose rituximab as induction in renal transplantation. Transplantation. 2009;87:1325–1329
- Clatworthy MR, Watson CJ, Plotnek G, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685
- Tsai MK, Lee CY, Yang CY, Yeh CC, Hu RH, Lee PH. Rituximab induction therapy provided additional immunosuppressive effect and functional benefit to non-sensitized renal transplant recipients. Am J Transplant. 2012;12:319
- Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab as induction therapy in renal transplantation. Transplant Rev (Orlando). 2015;29:103–108
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872
- Tyden G, Ekberg H, Tufveson G, Mjornstedt L. A randomized, double-blind, placebo-controlled study of single dose rituximab as induction in renal transplantation: A 3-year follow-up. Transplantation. 2012;94:e21–e22
- Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6:2418–2428
- Kyaw T, Tay C, Krishnamurthi S, et al. B1a b lymphocytes are atheroprotective by secreting natural igm that increases igm deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–840

