Abstract
Background: Currently over 55% of end-stage renal disease (ESRD) patients are aged ≥60 years and patients >75 years represent the fastest growing segment of the dialysis population. We aimed to assess whether the Groningen frailty indicator (GFI) can be used to distinguish fit older ESRD patients, likely able to tolerate and benefit from dialysis, from frail older patients who need further evaluation with a geriatrician’s comprehensive assessment. Methods: All patients aged ≥65 years visiting the pre-dialysis unit at the Gelre hospital between 2007 and 2013 were included and underwent the GFI (n = 65). Patients with GFI ≥ 4 (frail) were referred for geriatric consultation (n = 13). Results of the GFI and nephrologists’ evaluation were compared with geriatrician’s assessment. Survival rates and outcomes after one year of follow up were recorded. Results: Twenty patients (32%) were identified as frail. Of the problems identified by the geriatrician in 13 patients, 55% were not reported in the nephrologists’ notes. The first year after inclusion, 30% of patients with a GFI ≥ 4 died, compared to 9% of fit patients (p = 0.04). Moreover, 90% of frail patients had been hospitalized one or more times, compared to 53% in the fit group (p = 0.005). Conclusion: Although the GFI can be a useful instrument to identify ESRD patients at risk, both the GFI and the nephrologists’ assessment failed to identify specific geriatric impairments. Further research is needed to develop a specific frailty indicator for ESRD patients and to determine the value and effect of a comprehensive geriatric assessment in ESRD patients.
Background
In the developed world, the number of elderly patients on dialysis is expanding rapidly. Currently >55% of end-stage renal disease (ESRD) patients are aged ≥60 years, and patients older than 75 years represent the fastest growing segment of the dialysis population.Citation1 This increase has been driven by a more liberal admission to renal replacement therapy, increased willingness to start dialysis therapy in the elderly and changing demographic factors.Citation2 A high degree of frailty, complex comorbid conditions, high mortality rate, and the presence of psychosocial and functional problems make the treatment of the elderly ESRD patient an ongoing challenge. Several studies found that a substantial part of older patients do not benefit from dialysis as they suffer from distressing symptoms like pain and depression.Citation3–5 Furthermore, dialysis is associated with a decline in functional status and a negative effect on quality of life.Citation6,Citation7 Specifically, for frail elderly patients dialysis may not confer a significant survival benefit.Citation8
Frailty is considered to be a state of decreased physiological reserves, arising from cumulative deficits in multiple organ systems and resulting in a diminished resistance to stressors.Citation9,Citation10 Frailty status can be assessed using a comprehensive geriatric assessment of the medical, psychosocial and functional abilities and limitations of an elderly person. Although this assessment has been proven of value in geriatric medicine in general and among older patients undergoing dialysis, it is a time-consuming procedure for the patient and there are not enough geriatricians available to assess all elderly ESRD patients.Citation11,Citation12 Therefore, it would be beneficial to identify a less-time consuming screening instrument in patients with ESRD that can detect potentially frail elderly, requiring further evaluation. The Groningen Frailty Indicator (GFI) has been proven to be a valuable and reliable instrument in predicting frailty among elderly.Citation13,Citation14 In geriatric oncology, several frailty screening tools are used to help identifying vulnerable patients,Citation15 including the GFI.Citation16,Citation17 As patients with advanced renal failure experience a symptom burden and impairment of quality of life similar to that of patients with terminal malignancy, such tools could perhaps also be used to screen for potentially frail elderly among geriatric ESRD patients.Citation18
In this study, we aimed to assess whether the GFI can be used to distinguish fit older ESRD patients, likely able to tolerate and benefit from dialysis, from frail older patients who need further evaluation with a geriatrician’s comprehensive assessment in a daily nephrology outpatient setting. Secondly, we compared the results of the GFI, respectively the nephrologists’ evaluation with the geriatrician’s assessment with regard to different geriatric domains.
Methods
Study design
This prospective cohort study was conducted between August 2007 and August 2013 at the pre-dialysis unit in the Gelre hospital in Apeldoorn, the Netherlands. In the pre-dialysis outpatient clinic, a multidisciplinary team consisting of nephrologists, specialized nurses, social workers and dieticians care for patients with ESRD, aiming to maintain renal function and to counsel patients regarding treatment options in case of progressive disease. All team members systematically report in an electronic patient record system that makes data collection over time fairly reliable. Patients are referred to the outpatient pre-dialysis clinic once the nephrologists expects need for dialysis in the near future, in general, if their estimated glomerular filtration rate (eGFR) falls <20 mL/min.
All patients aged ≥65 years visiting the pre-dialysis unit in the Gelre hospital were included in this prospective cohort study and underwent the nurse-administered GFI at the date of inclusion (See Supplementary Table 1). This is a 15-item screening instrument to determine a person’s level of frailty.Citation19 The questions are subdivided into four geriatric domains: physical functioning [mobility, activities of daily living (ADL), vision, hearing and weight loss], cognitive functioning, social functioning (loneliness) and psychological functioning (anxiety, depression). Higher scores indicate higher levels of frailty. Patients with a GFI score of ≥4 were considered frail and were discussed with the geriatric nurse if referral for a geriatric consultation was likely to be beneficial. Additionally, patients could be referred for individual specific reasons to the geriatrician.
For included patients, the following data were collected from the patient’s medical file: age at inclusion, sex, marital status, level of education, dependence in ADL, smoking, number of prescription medications at inclusion, body mass index (BMI), presence of hypertension, cause of ESRD, median calculated creatinine clearance at inclusion, using the Cockroft–Gault formula. In addition, the Charlson Comorbidity Index was used as measure for comorbidity.Citation20 Medical records were inspected to determine if the multidisciplinary nephrology team paid attention to, and identified problems in the following eight domains: comorbidity, social situation, nutritional status, mobility, care dependency in ADL, mental health, cognition, vision and hearing. Caregiver burden could not be reliably retrieved and thus was not included. For all patients referred to the geriatrician, the geriatric files were consulted to determine how often a geriatrician identified problems in each of the geriatric domains. The problems observed by geriatricians were considered as the gold standard for the domain analyses.
Participants were followed until August 2013. Treatment for ESRD was determined at end of follow-up and categorized as “dialysis”, “conservative treatment only” or “planned for dialysis” (patients being prepared for dialysis but initiation was thus far not required). In addition, for the first year after inclusion, the following data were collected from patient’s medical files: number of hospital admissions and nights spent in the Gelre hospital, reason for admission (dialysis-related, kidney disease-related, not kidney disease-related) and dialysis-related complications. The date of death was collected from the Gelre hospital information system.
Ethics
The Medical Ethical Committee of the AMC declared that this study has been granted an exemption from requiring ethics approval. Informed consent for participation in the study was obtained from participants
Statistical analysis
Data were analyzed using SPSS software, version 20.0 (SPSS Inc., Chicago, IL). Patients were divided into two groups: GFI < 4 and GFI ≥ 4. For comparison between these two groups the χ2 test was used for nominal and ordinal variables, the t-test for continuous variables and the Mann–Whitney U-test for continuous variables not normally distributed. Survival analysis with Kaplan–Meier survival plots and logrank test were used to compare survival between the groups.
Results
Overall, 65 patients aged ≥65 years of age were assessed for frailty using the GFI at the pre-dialysis out-patient clinic of the Gelre hospital. Two patients were excluded because of reversible renal disease. The baseline characteristics of the remaining 63 patients are displayed in and results of the GFI in Supplementary Table 2.
Table 1. Patient characteristics at baseline (n = 63).
Median age of included patients was 75 years (range 66–92) and 35% were women; 65% of patients were married, 27% was widower and 8% was unmarried. Median Charlson comorbidity index was 1 (range 0–6) and patients used a median of eight types of prescription medications (range 1–18). Median eGFR at inclusion was 16 mL/min (range 5–34). Hypertensive nephropathy was the most common cause of ESRD (45%). Overall, twenty patients (32%) were classified as frail (GFI ≥ 4). There were no significant differences in age, sex distribution, medication use, Charlson Comorbidity Index score, GFR at inclusion or cause of ESRD between frail and non-frail patients. However, patients with GFI < 4 were significantly more ADL-independent than their counterparts with GFI ≥ 4 (91 versus 55%, p = 0.001).
Of the geriatric domains, comorbidity (98%), social situation (97%) and nutritional status (89%) were most frequently addressed by the nephrology team among included patients. In addition, in 75% of patients data on mobility and care dependency in ADL were reported in the medical file of the nephrology team. However, much less attention was paid to mental health (52%) and cognition (41%) and almost none to impairments in vision (14%) and hearing (11%).
Geriatric consultation
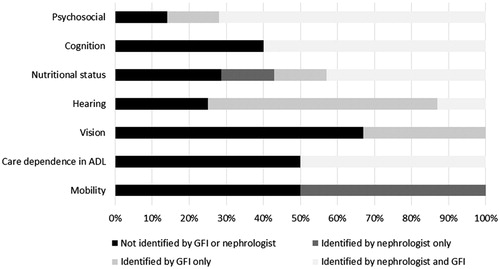
Two patients with a normal score on the GFI were referred to the geriatrician because the nephrologist suspected the presence of cognitive problems. Of the 20 patients with a GFI ≥ 4, eight were not referred: two refused referral to the geriatrician, but accepted follow up by geriatric nurse, three were considered not to be frail according to nephrologists’ evaluation and three were not referred for unclear reasons. Another patient was referred over 6 months after inclusion in this study; this patient was excluded from the following analyses (Supplementary Figure 1). Of the remaining thirteen patients seen by the geriatrician, eight suffered from hearing impairments—eleven with a GFI ≥ 4 and two with a normal GFI—seven were malnourished, seven had psychosocial impairments and six suffered from impaired mobility. In addition, five patients had cognitive impairments, four patients were care dependent in their ADL, and three were visually impaired. Of these issues diagnosed by the geriatrician, 55% were not reported in the nephrologists’ notes. These most frequently consisted of vision and hearing impairments (100 and 88% of issues not reported by nephrologist, respectively), followed by impaired mobility and care dependency in ADL (50% not reported), nutritional status (43% not reported), cognitive impairments (40% not reported) and psychosocial problems (29% not reported).
By comparison, the GFI was more sensitive to hearing impairments compared to nephrologists’ evaluation (), but failed to identify most impairment in mobility. In addition, the GFI identified many patients as having psychosocial issues, which were not confirmed in the geriatrician’s evaluation.
Follow-up
At the end of the follow-up, 45% of patients with a GFI ≥ 4 had received conservative treatment only, compared to 2% of fit patients (p < 0.001) (). Of the 53 patients opting for dialysis, fit patients were five times more likely to choose peritoneal dialysis than frail patients, of which 91% underwent hemodialysis.
Table 2. Treatment choices.
The first year after inclusion 10 patients died, 30% of patients with a GFI ≥ 4, compared to 9% of fit patients (p = 0.04, ). Moreover, 90% of frail patients had been hospitalized one or more times, compared to 53% in the fit group (p = 0.005). There was no difference in dialysis-related complications (p = 0.29) (). Survival was significantly higher among fit patients in the first year after inclusion (log rank p = 0.03) (). Among patients receiving conservative treatment 50% (5/10) died in the first year of follow up, compared to 9% (3/33) among patients on dialysis and 10% (2/20) among patients that were preparing for dialysis, but initiation of renal replacement therapy was thus far not required.
Figure 2. Kaplan–Meier survival curves, measured from the date of inclusion until 1 year of follow-up [n = 63, n (died) = 10].
![Figure 2. Kaplan–Meier survival curves, measured from the date of inclusion until 1 year of follow-up [n = 63, n (died) = 10].](/cms/asset/2c05c583-381a-40df-916c-3d66f061ff84/irnf_a_1077315_f0002_c.jpg)
Table 3. Outcomes after a year of follow-up, measured from the date of inclusion (date of GFI) (n = 63).
Discussion
This study explored whether the GFI can be used to identify frail patients who benefit from further geriatric evaluation in order to guide individual treatment decisions regarding dialysis therapy initiation. We found that geriatric impairments were prevalent with percentages ranging from 23% (visual impairment) to 62% (hearing impairment). One-third of patients were identified as frail according to the GFI, (GFI ≥ 4) and these patients more frequently required hospitalization. Both the GFI and the nephrologists’ assessment failed to identify relevant geriatric impairments that were detected after the geriatric consultation.
To our knowledge, this is the first study that investigated whether a frailty index is able to discriminate fit older ESRD patients, from frail older patients with high risk of complications from renal replacement therapy. However, some aspects of our study warrant comment. First, the limited number of patients may have hampered our ability to detect differences in outcomes between both groups. Secondly, the limited duration of follow-up makes it difficult to determine whether the associations found are also predictive of long-time prognosis. Another weakness is that only patients with GFI ≥ 4 were further evaluated with a geriatric assessment, whereas patients with GFI < 4 were not. Thus, we do not know the prevalence of geriatric impairments among the patients with a low GFI, and therefore cannot determine the sensitivity of this screening tool. Finally, not all patients with a GFI ≥ 4 were referred to a geriatrician, potentially introducing some selection bias.
The challenge in today’s pre-dialysis practice is to identify which patients will benefit from dialysis and which will be better off with conservative treatment. The lack of large prospective studies and heterogeneity in the elderly population makes it difficult to differentiate between these two groups of patients. Previous studies have set out to identify prognostic factors for frailty to predict to whom dialysis would be of benefit and to whom not, and found a range of potentially relevant factors, such as comorbid conditions, non-ambulatory status, age, low body weight, unplanned dialysis and severe behavioral disorder.Citation21,Citation22 A third study found that the so called “surprise question” (“Would I be surprised if this patient died within the next 6 months?”) proved to be of use in identifying dialysis patients with a high risk for early mortality.Citation23 Our study demonstrates that the GFI can also be useful in identifying patients at risk for poor outcome irrespective of treatment.
Currently, nephrologists base their treatment decisions on clinical experience, general guidelines which are not age-specific, and the doctors’ and patients’ preferences. Our study shows that nephrologists do not focus on impairments in specific geriatric domains. Previous studies have also found that elderly dialysis patients frequently suffer from multiple geriatric impairments, not all of which are identified by the nephrology team.Citation24 This appears to be due in large part to the fact that these issues are not specifically looked for. In our study, we found that visual and hearing impairments, cognitive dysfunction and psychosocial issues were not reported most often. It is unlikely that neurosensory impairments will influence the outcome of dialysis and so it can be debated where the fact that these are not reported is relevant. However, psychological and cognitive problems are not only highly prevalent, they also have a significant impact on the patient’s quality of life as well as the outcome of dialysis. Dementia among ESRD patients was associated with an increased risk of death (RR 1.48, 95% CI 1.32–1.66) and dialysis withdrawal (RR 2.01, 95% CI 1.57–2.57), and better mood and satisfaction in daily life were associated with improved survival in patients on hemodialysis.Citation25,Citation26
Recently, the American society of nephrology recommends involvement of a geriatrician in the multidisciplinary care team to aid in decision-making for elderly ESRD patients and for a thorough and objective appraisal of the health status of elderly people.Citation27–29 However, there are no published models that describe how interactions between dialysis teams and geriatricians might be facilitated. We also observed in our study that patients may be reluctant to be investigated by a geriatrician, perhaps based on prejudices. In addition, we do not yet know to which extent problems on different geriatric domains influence outcomes like mortality, complications, independency and quality of life. Therefore, more research is needed to optimize care and decision-making for the growing elderly ESRD population.
Conclusions
Our results show that a significant part of the elderly ESRD-patients are frail. The nephrologist is frequently faced with the difficult task of deciding whether dialysis would be of benefit to the individual older patient. In order to make a good decision regarding treatment, it is helpful to distinguish fit older ESRD patients from frail ones, considering the strong relation between frailty and adverse outcomes. Our study shows that the nephrologist and the GFI are not always able to identify relevant geriatric impairments and a comprehensive geriatric assessment has complementary value. Through assessing the medical, psychosocial and functional capabilities and limitations of an elderly person systematically, a geriatrician can aid in determining what will be the best treatment for maximizing quality of life years. The GFI could be of value to limit the time-consuming comprehensive geriatric assessment to the patients that will benefit most from renal replacement therapy. Given the complexity and importance of decisions regarding treatment in older ESRD patients and starting dialysis, we strongly recommend the involvement of a geriatrician in decision making. We are a long way from being able to stratify patients as suitable or unsuitable for dialysis based on a frailty indicator or geriatric assessment. Therefore, the value and effect of a comprehensive geriatric assessment, the development of a specific ESRD frailty indicator and the identification of the right target group among ESRD patients need to be further evaluated in large multicentre cohort studies.
Supplemental Files
Download Zip (17.1 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- ERA-EDTA Registry. ERA-EDTA Registry Annual Report 2011. Academic Medical Center Department of Medical Informatics (ed). Amsterdam, The Netherlands: Academic Medical Center; 2013
- Kramer A, Stel V, Zoccali C, et al. An update on renal replacement therapy in Europe: ERA-EDTA Registry data from 1997 to 2006. Nephrol Dial Transplant. 2009;24:3557–3566
- Kurella M. Incidence, management, and outcomes of end-stage renal disease in the elderly. Curr Opin Nephrol Hypertens. 2009;18:252–257
- Davison SN. Pain in hemodialysis patients: Prevalence, cause, severity, and management. Am J Kidney Dis. 2003;42:1239–1247
- Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–2494
- Kurella TM, Covinsky KE, Chertow GM, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547
- Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: A systematic review. Adv Chronic Kidney Dis. 2007;14:82–99
- Murtagh FE, Marsh JE, Donohoe P, et al. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22:1955–1962
- Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: A consensus report. J Am Geriatr Soc. 2004;52:625–634
- Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27:17–26
- Ellis G, Whitehead MA, O’Neill D, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211
- Aucella F, Stoico L, Cicchella A, et al. Comprehensive geriatric assessment in the hemodialysis elderly population. J Nephrol. 2012;25(Suppl. 19):S85–S89
- Peters LL, Boter H, Burgerhof JG, et al. Construct validity of the Groningen Frailty Indicator established in a large sample of home-dwelling elderly persons: Evidence of stability across age and gender. Exp Gerontol. 2015;69:129–141
- Peters LL, Boter H, Buskens E, Slaets JP. Measurement properties of the Groningen Frailty Indicator in home-dwelling and institutionalized elderly people. J Am Med Dir Assoc. 2012;13:546–551
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: A systematic review. Lancet Oncol. 2012;13:e437–e444
- Baitar A, Van FF, Vandebroek A, et al. Evaluation of the Groningen Frailty Indicator and the G8 questionnaire as screening tools for frailty in older patients with cancer. J Geriatr Oncol. 2013;4:32–38
- Tegels JJ, De Maat MF, Hulsewe KW, et al. Value of geriatric frailty and nutritional status assessment in predicting postoperative mortality in gastric cancer surgery. J Gastrointest Surg. 2014;18:439–445
- Saini T, Murtagh FE, Dupont PJ, et al. Comparative pilot study of symptoms and quality of life in cancer patients and patients with end stage renal disease. Palliat Med. 2006;20:631–636
- Steverink N, Slaets JPJ, Schuurmans H, van Lis M. Measuring frailty: Developing and testing the GFI (Groningen Frailty Indicator). Gerontologist 2001;41:236–237
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383
- Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146:177–183
- Couchoud C, Labeeuw M, Moranne O, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24:1553–1561
- Moss AH, Ganjoo J, Sharma S, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol. 2008;3:1379–1384
- Parlevliet JL, Buurman BM, Pannekeet MM, et al. Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: A cross-sectional comparative and feasibility study. BMC Nephrol. 2012;13:30
- Kanamori H, Nagai K, Matsubara T, et al. Comparison of the psychosocial quality of life in hemodialysis patients between the elderly and non-elderly using a visual analogue scale: The importance of appetite and depressive mood. Geriatr Gerontol Int. 2012;12:65–71
- Kurella M, Mapes DL, Port FK, Chertow GM. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2006;21:2543–2548
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol. 2005;55:241–252
- Wiggins J, Bitzer M. Geriatric assessment for the nephrologist. Semin Dial. 2012;25:623–627
- Gambert SR, American Society of Nephrology. Geriatric Nephrology Curriculum, Chapter 26: Comprehensive Geriatric Assessment: A Multidimensional Process Designed to Assess an Elderly Person's Functional Ability, Physical Health, Cognitive and Mental Health, and Socio-Environmental Situation [online]. Available at: https://www.asn-online.org/education/distancelearning/curricula/geriatrics/Chapter26.pdf. Accessed August 12, 2015