Abstract
Background: In patients with IgA nephropathy (IgAN) lectin and alternative pathways of the complement can be activated. Our aim was to analyze the association of glomerular and extraglomerular C4d staining—the representative of lectin pathway—with demographic, clinical and histopathological findings in primary IgAN patients. Design: Seventy-three patients were enrolled and after re-evaluation 37 of them were included in this study. Biopsies were analyzed for staining with anti-C4d primary monoclonal antibody by immunohistochemistry. Patients were classified as positive and negative groups based on their glomerular C4d deposition. Groups were compared for their baseline clinical and histopathological findings. Results: Sixteen (43.2%) of 37 patients were C4d-positive. Glomerular C4d-staining was associated with more severe proteinuria (2906 mg/day vs. 1091 mg/day; p = 0.002), lower GFR (54.87 mL/min vs. 95 mL/min; p = 0.023), higher blood pressure (p = 0.022), more severe endocapillary hypercellularity (p < 0.001) and more severe tubular atrophy (p < 0.01). Mesangial IgM deposition was found to be associated with glomerular C4d staining and nephrotic range proteinuria. Conclusions: Glomerular C4d deposition was found to be associated with more unfavorable histopathological and clinical findings at the time of diagnosis. Association of mesangial IgM deposition with the activation of lectin pathway is a novel finding. Mesangial IgM deposition in our patients may reflect the genetic heterology of IgAN between diverse populations. However, since these data are about association, a cause-and-effect about IgM and IgAN cannot be proven solely with these findings.
Introduction
Being the most common glomerulonephritis world-wide, immunoglobulin A nephropathy (IgAN) is diagnosed according to the immunofluorescence findings of kidney biopsy. There is a wide spectrum of clinical findings both at the time of diagnosis and in follow-up.Citation1–5 Although IgAN used to be known as a benign condition, in a 20-year follow-up one-third of patients and in a 30-year follow-up almost half of the patients progress to end-stage renal disease (ESRD).Citation6,Citation7
It is important to distinguish patients who have unfavorable renal survival since they may benefit from immunosuppressive therapy. Lower GFR, higher degree of proteinuria and higher systolic blood pressures were associated with worse prognosis as higher histologic grade was.Citation8,Citation9 In the last decade after Roos et al. has shown that C4d, the representative of lectin and classic pathways of the complement system was associated with worse clinical findings at the time of diagnosis,Citation10 Espinosa et al. demonstrated the role of that marker as a new prognostic factor in IgAN.Citation11 Then, a multicenter retrospective cohort study showed that C4d is an independent risk factor for the development of ESRD;Citation12 this was recently supported by another single-center study.Citation13 Association of glomerular C4d with more unfavorable clinical and histopathological findings was shown in another preliminary study recently.Citation14 Lastly, several immunohistologic biomarkers were studied to assess their predictive values for disease progression.Citation15 Here, we wanted to evaluate patients with IgAN in our institution for C4d staining and analyze the associations between both clinical and histopathological findings with C4d staining.
Subjects and methods
Our study was approved by the Ethics Committee of our institution. Seventy-three patients who were diagnosed as IgAN between January 2003 and December 2013 were enrolled in this retrospective study. Patients who were younger than 18 years of age and who had a disease which may be respected to cause IgAN (lymphomas, Behçet’s disease, seronegative spondyloarthropathies, lymphomas, cirrhosis, solid-organ malignancies, inflammatory bowel diseases, celiac disease and infections with HIV, HBV, HCV, CMV and tuberculosis) were not included. Thereafter, 45 patients were identified as primary IgAN and kidney specimens of these were included in histopathological analysis. Renal biopsy specimens were evaluated with hematoxylin–eosin, Masson’s trichrome, periodic acid schiff and methenamine silver stained sections by light microscopy and staining with antibodies against immunoglobulin G (IgG), immunoglobulin A (IgA), immunoglobulin M (IgM), complement 3c, C1q, kappa and lambda for immunofluorescence. Presence of at least three non-sclerotic glomeruli was determined as a requirement to be able to assess glomerular C4d staining. So that 37 patients were established as to be eligible for the study. Medical records of patients were analyzed retrospectively.
Histopathological parameters
Mesangial hypercellularity, endocapillary hyperproliferation, segmental sclerosis and tubular atrophy (MEST) score was analyzed according to Oxford criteria. Crescent formation was classified as cellular and fibrotic. Results of immunohistochemical staining for IgG, IgA, IgM, C3c, C1q, kappa and lambda were classified as negative or positive.
C4d staining
Three-micrometer sections of formaldehyde-fixed renal tissue were stained in Benchmarck Ultra machine using ultra view universal Dab detection kit. Antigen retrieval was performed in ultra CCl solution for 64 min in 95 °C. Primary monoclonal anti-C4d antibody (Cell Marque®, SP91 clone, Rocklin, CA) was applied and incubated for 32 min.
Biopsies were categorized as positive or negative based on glomerular C4d immunohistochemical staining. Patients were classified as “positive” if >25% of the glomeruli were positive for C4d. Staining was defined as “global” when >50% of mesangial area was affected and as “segmental” otherwise. Assessment for tubular epithelium and interlobular capillaries was also performed.
After immunohistochemical staining patients were classified as positive and negative groups based on their glomerular C4d staining-status. Positive and negative groups were compared for their demographic (patient age and sex), clinical (degree proteinuria, serum creatinine level, creatinine clearance, presence of macroscopic hematuria, presence of hypertension or use of anti-hypertensive medicine, use of immunosuppressive, need for renal replacement therapy) and histopathological (C4d staining in glomerular and extraglomerular sites of kidney; Oxford classification system criteria; mesangial immunoglobulin depositions; presence of crescent formation) findings. We calculated the estimated GFR (eGFR) according to modification of diet in renal disease (MDRD) study equation.
Statistical analysis
Chi-square and Fisher’s exact tests were used to compare qualitative values. Results are expressed as mean ± standard deviation. A p-value of <0.05 was considered to be statistically significant. For numerical data analysis, Chi-square and Fisher's exact test were used; for variant analysis, the Mann–Whitney U test and correlation analysis were used. Calculations were performed using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL) 16.0 version.
Results
Baseline characteristics of patients are presented in . After histopathological analysis of kidney biopsy specimens of 45 patients who were diagnosed as primary IgAN, eight were excluded due to insufficient non-sclerotic glomeruli for C4d staining. Of remaining 37 patients, 11 were females. Sixteen patients were C4d positive (43.2%). Four of women patients and 12 of men were in glomerular C4d-positive group. Glomerular C4d staining was global in four patients. In addition to glomerular C4d staining in 16 patients, tubular C4d staining was observed in four patients, while interlobular arterial C4d staining was shown in eight patients (). Peritubular capillary involvement for C4d staining was not observed in any patient.
Figure 1. C4d staining in different sites of kidney biopsy specimens of patients with primary IgAN. (A) C4d staining in a segmentally sclerotic mesangial area. (B) C4d staining in another mesangial area with no sclerosis. (C) C4d staining in renal tubular epithelium. (D) Interlobular arterial C4d staining (stained with anti-C4d monoclonal antibody; magnification ×20).
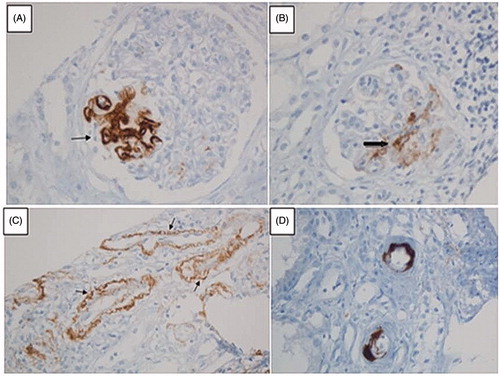
Table 1. Basic characteristics of all included patients at the time of diagnosis.
Other histopathological analyses
When patients were evaluated based on Oxford classification, 28 (75.7%) were M1, 17 (45.9%) were E1, 27 (73%) were S1, 15 (40.5%) were T0 and 12 (32.4%) were T1. Crescent formation was observed in 12 (32.4%) patients, while 7 of them were cellular in type.
Association between glomerular C4d staining and other baseline histopathological analyses
Glomerular C4d staining was associated with endocapillary hypercellularity (p < 0.001), interstitial fibrosis/tubular atrophy (p < 0.001) and mesangial IgM deposition (p = 0.006). Glomerular depositions of immunoglobulins and complements in glomerular C4d positive and negative groups are as shown in . Association of glomerular C4d with mesangial hypercellularity score, segmental sclerosis score and crescent formation were not statistically significant. Statistical significance was not found when analysis was done for cellular and fibrotic crescent types, separately ().
Table 2. Glomerular depositions of immunoglobulins and complement molecules in glomerular C4d positive and negative groups.
Table 3. Association between glomerular C4d staining and baseline histopathological analyses.
Association between glomerular C4d staining and baseline clinical findings
Statistically significant association of positive glomerular C4d was observed in the presence of hypertension, higher eGFR and more severe proteinuria at the time of diagnosis ().
Table 4. Association between glomerular C4d staining and baseline clinical findings.
C4d staining in extraglomerular sites
In four patients tubular epithelial staining with C4d was observed. This was not statistically significant with any clinical or histological criteria. In eight (21.6%) patients interlobular arterial C4d staining was shown. Although glomerular C4d staining was not associated with cellular crescent formation, interlobular arterial C4d staining was associated with both cellular crescent formation (p = 0.027) and an E1 score of endocapillary hypercellularity (p = 0.014). There was no significant association between extraglomerular and glomerular C4d staining.
Discussion
In the current study, we showed that glomerular C4d staining is associated with more severe endocapillary hypercellularity and interstitial fibrosis/tubular atrophy. T2 score was an independent risk factor for progression to ESRD in the study of Spanish Glomerular Disease Study Group.Citation12 It is clearly known that T2 score of tubular atrophy is the strongest histopathologic criteria which predicts progression to end-stage renal failure.Citation16 In our study, glomerular C4d staining was significantly associated with a score of T2. This reflects the importance of glomerular C4d staining on predicting renal survival.
Our results suggested that patients with glomerular C4d staining clearly had lower GFR, more severe proteinuria and higher rates of hypertension at the time of diagnosis. Thus, confirming the previous studies we have shown that glomerular C4d staining is associated with worse clinical findings and more severe histologic grade.
Of 16 patients with glomerular C4d-positive staining, four were also positive for interlobular arterial C4d staining. However, four patients were also positively stained for interlobular arterial C4d in glomerular C4d-negative group. In glomerular C4d-positive group, three patients’ renal biopsies were stained for tubular C4d. In glomerular C4d-negative group number of patients with positively tubular C4d staining was 1. Neither of the data about concurrence of glomerular and extraglomerular C4d were statistically significant. Since the number of patients with extraglomerular staining was small, it would be unreliable to comment on association of glomerular and extraglomerular C4d staining, and role of extraglomerular staining in disease prognosis.
No statistically significant association between gender and glomerular C4d-staining was found. Mean follow-up was 24.3 months and excluding two patients who had ESRD at the time of diagnosis, no patient had ESRD in follow-up. Only three patients were in a worse state of glomerular filtration rate compared to baseline eGFR. This regression in eGFR in three patients was not significant and did not cause to categorize the patients as in a more advanced kidney failure stage. We think that since IgAN is usually an indolent disease, this may count for this result. Thus, we could not analyze the effect of complement staining on renal survival.
Interestingly, we could not show an association between glomerular C4d deposition with another predictor of worse prognosis, crescent formation. Results were still statistically insignificant when analyzed for cellular and fibrous crescents, separately. However, it is known that cellular immunity has a major role on crescent formation rather than the complement system.Citation17 On the other hand, we found that interlobular C4d staining is associated with cellular crescent formation and both were associated with only one criteria of Oxford classification, endocapillary hypercellularity. We speculate that this reflects the endothelial damage, the first hit necessary for the formation of crescents.
An interesting result of our study was about the association of mesangial IgM deposition with glomerular C4d staining which is a novel finding. In the study of Espinosa et al.Citation11 the number of patients with mesangial IgM deposition was very small and there was no patient with mesangial IgM deposition in C4d positive group. This is important because although IgM is known to activate the classic pathway of the complement system as IgG does, it also has the potential to activate the lectin pathway both in vitro and in vivo.Citation18 Mesangial IgM deposition was associated with both scores of E1 and T2, and with the presence of nephrotic syndrome. Although this difference on IgM deposition between our data and previous study results may be due to technical issues in detecting IgM, paucity of patients with mesangial IgM deposition in previous studies may also be the consequence of geographical differences. There is no data to claim a role for IgM in disease pathogenesis. Speculatively, IgM may aggravate detrimental effects of lectin pathway in kidney which was started by IgA1 immune complexes in circulation. In a GWAS performed by Kiryluk et al.Citation19 newly identified seven single-nucleotide polymorphisms were differently distributed between ethnicities. Dissimilar results between previous studies and ours for IgM deposition could be a result of various candidate gene actions between diverse patient populations. On the other hand, since our results reflect an association, a cause-and-effect about IgM and IgAN cannot be proven solely with these findings.
Compared to monoclonal antibody, using polyclonal antibody is an inexpensive and quick way on recognizing multiple epitopes on an antigen. However, monoclonal antibodies are more specific. One of our aims was to analyze the deposition of C4d in regions other than mesangium. Polyclonal antibody for C4d may lack the signal in these areas.Citation20
It is still unknown whether there is a direct role of lectin pathway on pathogenesis of IgAN or it is only a result of the main cause. We think it would be wisely to assess immunoglobulin deposition and C4d staining in origins of antigen presentation like intestinal tissue layers, although classical pathway may also be activated in these cites. This may render new opinions in pathogenesis of IgAN. Moreover, secondary IgAN patients were excluded in previous studies. In forthcoming studies including those patients and analyzing their C4d staining status would show possible different effects of different facilitators of IgAN.
In conclusion, glomerular C4d staining is shown to be associated with worse clinical and histopathologic findings once again in a different geographical region. Association of mesangial IgM deposition with glomerular C4d staining is a new finding. Since IgM has the potential to activate lectin pathway, the discrepancy of results between previous studies and ours should be re-evaluated in forthcoming studies. IgAN has a two-sided pathway in its pathogenesis: alternative and lectin pathways of the complement system. In these well-defined ways, studying the role of mesangial IgM deposition, effects of secondary causes of IgAN on lectin pathway and the role of complement staining in antigen-presentation sites like intestinal tissue layers would construct new avenues on this two-sided pathway.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- D’Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Quart J Med. 1987;64(245):709–727
- Galla JH. IgA Nephropathy. Kidney Int. 1995;47(2):377–387
- Donadio JV, Grande JP. IgA nephropathy. N Engl J Med. 2002;347:738–748
- Wyatt RJ, Julian BA. IgA nephropathy. N Engl J Med. 2013;368:2402–2414
- Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097
- Li PK, Ho KK, Szeto CC, et al. Prognostic indicators of IgA nephropathy in the Chinese – Clinical and pathological perspectives. Nephrol Dial Transplant. 2002;17:64–69
- Moriyama T, Tanaka K, Iwasaki C, et al. Prognosis in IgA nephropathy: 30-Year analysis of 1012 patients at a single center in Japan. PLoS One. 2014;9(3):e91756
- Xie J, Kiryluk K, Wang W, et al. Predicting progression of IgA nephropathy: New clinical progression risk score. PLoS One. 2012;7(6):e38904
- Lundberg S, Qureshi AR, Olivecrona S, et al. FGF23, albuminuria, and disease progression in patients with chronic IgA nephropathy. Clin J Am Soc Nephrol. 2012;7(5):727–734
- Roos A, Rastaldi MP, Calvaresi N, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–1734
- Espinosa M, Ortega R, Gómez-Carrasco JM, et al. Mesangial C4d deposition: A new prognostic factor in IgA nephropathy. Nephrol Dial Transplant. 2009;24:886–891
- Espinosa M, Ortega R, Sanchez M, et al. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9(5):897–904
- Sahin OZ, Yavas H, Taslı F, et al. Prognostic value of glomerular C4d staining in patients with IgA nephritis. Int J Clin Exp Pathol. 2014;7(6):3299–3304
- Nasri H, Ahmadi A, Rafieian-Kopaei M, Bashardoust B, Nasri P Mubarak M. Association of glomerular C4d deposition with various demographic data in IgA nephropathy patients; a preliminary study. J Nephropathol. 2015;4(1):19–23
- Faria B, Henriques C, Matos AC, Daha MR, Pestana M, Seelen M. Combined C4d and CD3 immunostaining predicts immunoglobulin (Ig)A nephropathy progression. Clin Exp Immunol. 2015;179(2):354–361
- Roberts ISD. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10:445–454
- Kitching AR, Holdsworth SR, Tipping PG. IFN-gamma mediates crescent formation and cell-mediated immune injury in murine glomerulonephritis. J Am Soc Nephrol. 1999;10:752–759
- McMullen ME, Hart ML, Walsh MC, Buras J, Takahashi K, Stahl GL. Mannose-binding lectin binds IgM to activate the lectin complement pathway in vitro and in vivo. Immunobiology. 2006;211:759–766
- Kiryluk K, Li Y, Sanna-Cherchi S, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genet. 2012;8(6):e1002765
- Suzuki T, Horita S, Kadoya K, et al. C4d Immunohistochemistry in glomerulonephritis with different antibodies. Clin Exp Nephrol. 2007;11(4):287–291

