Abstract
Objectives: The role of SOST/sclerostin in mediating tissue fibrogenic response to injury/inflammation remains largely unknown. Thus, we conducted this study to determine whether SOST/sclerostin plays a role in renal interstitial fibrosis (RIF) for the first time. Methods: Unilateral ureteral obstruction (UUO) was performed to create obstructive kidney injury model. Twelvemale SOST knockout (SOST KO) mice and 12 age-matched wild-type (WT) mice were divided into three groups: sham surgery, UUO 3 d and UUO 7 d. The mice were sacrificed at each time point and kidney tissues were collected. Histopathological changes were evaluated by hematoxylin and eosin and Masson staining, while α-smooth muscle actin (α-SMA), type I collagen (Col-I) and fibronectin (FN) expression levels were detected by RT-PCR and western-blot. Results: In sham control group, neither WT nor SOST KO exhibited fibrotic change. On 3 days after UUO, total renal histopathological score and fibrotic area were aggravated and α-SMA, Col-I and FN expressions were upregulated, but no difference was observed between WT and SOST KO. On 7 days after UUO, compared with WT, SOST KO mice showed higher total renal histopathological score and fibrotic area percentage, as well as a higher level of fibrogenic marker mRNA/protein expression (except for α-SMA mRNA and FN mRNA). Conclusion: It is supposed that SOST gene is involved in the regulation of RIF progression. In obstructive kidney injury, SOST gene deletion would probably enhance renal fibrogenic response and promote the progression of RIF. But more evidences are needed to further identify the role of SOST/sclerostin in mediating RIF progression.
Introduction
Fibrosis of internal organs is a global health problem with limited therapeutic option.Citation1 Renal interstitial fibrosis (RIF), also known as renal tubular interstitial fibrosis, is one of the most important pathological features of chronic kidney disease (CKD) resulting in end-stage renal disease. It is tightly linked to the progression of nearly all causes of renal diseases.Citation2,Citation3 The critical role of Wnt signaling pathway in kidney fibrogenic response and tissue repair has been identified.Citation4,Citation5 Recently, Wnt ligand associated low-density lipoprotein receptor-related proteins 6 (LRP-6) receptor has been identified as a coreceptor for multiple fibrogenic signaling pathways, including MAPK and JNK pathways. It is shown that Dickkopf-1 (Dkk1), by binding to LRP-6 coreceptor to block fibrogenic signals, can effectively inhibit the progression of RIF. Thus, LRP-6 is considered to be an attractive therapeutic target for treating RIF.Citation6
Sclerostin, encoded by SOST gene, can directly bind to LRP-6 receptor and inhibit the activation of LRP-6 related signal like Wnt pathway.Citation7,Citation8 However, since SOST was once thought to exclusively expressed by osteocytes in bone and mineral tissue,Citation9 the role of SOST/sclerostin in mediating tissue repair and fibrogenic response to injury/inflammation remains largely unknown. In recent years, a high expression of SOST gene has been detected in kidney tissue,Citation10 as well as the expression of sclerostin protein in dimeric form.Citation11 We hypothesized that in kidney tissue, SOST/sclerostin is essential for inhibiting RIF. Thus, using a well-characterized obstructive kidney injury model, we aimed to detect the fibrogenic response of kidney tissue when SOST gene was deleted, to determine whether SOST/sclerostin plays a role in RIF.
Material and methods
Experimental animal and procedure
SOST knockout (SOST KO) mice were generated as previously described, and identified by tail DNA genotyping.Citation12,Citation13 Ten-month-old male SOST KO mice on a C57BL background were obtained from Baylor College of Dentistry, Texas A&M University, Dallas, TX. Age-matched wild-type (WT) C57BL mice were used as control. Obstructive kidney injury was performed using a well-characterized unilateral ureteric obstruction (UUO) model.Citation14 Groups of four SOST KO mice and four WT mice were sacrificed on days 3 and 7 after UUO surgery. As sham controls, additional groups of SOST KO and WT mice (four mice each) were sacrificed on the same time point on days 3 and 7 after sham surgery. Kidney tissues were collected and prepared for histopathological examination and related gene/protein expression analyses. The animal experiment protocol was approved by the Ethics Committee of State Key laboratory of Oral Diseases, Sichuan University.
Histopathological examination
All kidneys tissues were stored in 10% neutral buffered formaldehyde solution for 24 h. Formaldehyde fixed tissues were subsequently paraffin embedded and cut into 3 μm sections. After deparaffinized with xylene, sections were stained with hematoxylin and eosin (H&E) and Masson trichrome. Ten non-repeated fields were randomly selected under microscope (E600; Nikon, Tokyo, Japan) at 200× magnification, and examined by two pathologists independently. The total renal histopathological score (ranging from 0 to 3: 0 – normal, 1 – mild impairment, 2 – moderate impairment and 3 – severe damage) was calculated depending on eight aspects: interstitial fibrosis, tubular atrophy, interstitial infiltration, interstitial edema, red tube, protein casts, tubular dilatation and tubule vacuolar degeneration.Citation15 In addition, blue stained (fibrotic) area was quantified by morphometric analysis using Image-Pro Plus 6.0 software (Media cybernetics, Silver Spring, MD). The average percentage of fibrotic area accounting for the total area was calculated.Citation16
RT-PCR analysis
Total RNA was extracted from the kidney tissue by using Trizol Reagent (Invitrogen, Carlsbad, CA). The cDNA synthesis and PCR procedures were performed using an RT-PCR kit (Takara, Tokyo, Japan). Primers and TaqMan probes were designed according to GenBank database. The primer sets were 5′-CTGACAGAGGCACCACTGAA-3′ (forward) and 5′-GAAATAGCCAAGCTCAG-3′ (reverse) for α-smooth muscle actin (α-SMA); 5′-TGCCGTGACCTCAAGATGTG-3′ (forward) and 5′-CACAAGCGTGCTGTAGGTGA-3′ (reverse) for type I collagen (Col-I); 5′-GAAGGTTTGCAACCCACTGT-3′ (forward) and 5′-TCTGCAGTGTCGTCTTCACC-3′ (reverse) for fibronectin (FN). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was set as housekeeping gene, and its primer set was 5′-CAGATCCACAACGGATATATTGGG-3′ (forward) and 5′-CATGACAACTTTGGCATTGTGG-3′ (reverse). The relative amount of mRNA was calculated using the comparative CT (ΔΔCT) method.
Western-blot
Kidney tissue total protein was extracted in a lysis buffer, separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred onto a polyvinylidene fluoride membrane. Membranes were blocked with 5% bovine serum albumin for 1 h and subsequently incubated overnight with primary antibodies of α-SMA, Col-I, FN and GAPDH (Abcam, Cambridge, UK), respectively. After subsequent washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Millipore, Billerica, MA). The immunoreactive bands were detected by enhanced chemiluminescence (Santa Cruz Biotechnology, Dallas, TX). The density of bands was analyzed using Quantity One 1-D software on a Chemi Doc XRS System (Bio-Rad, Hercules, CA); all protein expressions were normalized to GAPDH.
Statistical analysis
Data were presented as mean ± standard deviation. As parameters were not normally distributed, non-parametric test was performed: the Kruskal–Wallis H test was used for multiple comparison and then the Mann–Whitney U test was used to determine the difference between each two groups. All statistical analyses were performed using the SPSS 17.0 software (PASW Inc., Chicago, CA) and α = 0.05 was set for significance.
Results
Histopathological changes of kidney tissue
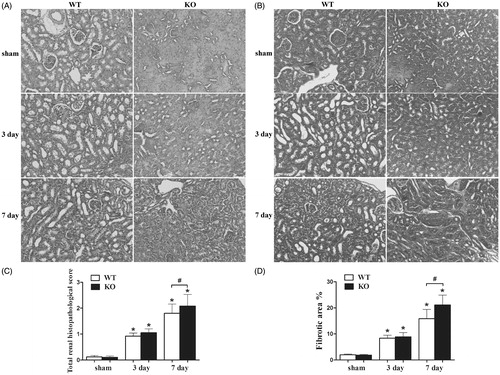
Semi-quantitative analysis results for H&E and Masson staining are presented in . Kidneys tissue harvested from WT and SOST KO mice subjected to sham surgery exhibited no fibrotic changes. On 3 days after UUO, although both SOST KO and WT mice presented mild renal tissue impairment, the value of total renal histopathological score and fibrotic area percentage showed no difference. On 7 days after UUO, WT mice exhibited moderate renal tissue impairment. However, a higher total renal histopathological score (2.08 ± 0.45, p < 0.05, ) and a higher fibrotic area percentage (21.10 ± 3.80, p < 0.05, ) were detected in SOST KO mice, indicating a severer degree of tubulointerstitial inflammation and fibrosis.
Figure 1. Histopathological changes of kidney tissue. (A) H&E staining, magnification × 200, (B) Masson trichrome staining, magnification × 200, (C) total renal histopathological score and (D) fibrotic area percentage. WT, wild-type; KO, SOST knockout. Each bar represented the mean ± standard deviation. *p < 0.05 versus shame control. #p < 0.05 time-matched UUO WT versus KO.
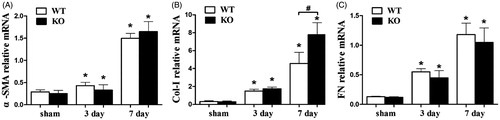
Fibrogenic marker mRNA expression level
As shown in , mRNA expression of α-SMA, Col-I and FN were enhanced with the increased duration of obstruction. However, on 3 days after UUO, for α-SMA, Col-I and FN, no difference was detected between WT and SOST KO mice. On 7 days after UUO, a higher level of Col-I was observed in SOST KO mice (p < 0.05), while α-SMA and FN expression showed no difference between WT and SOST KO.
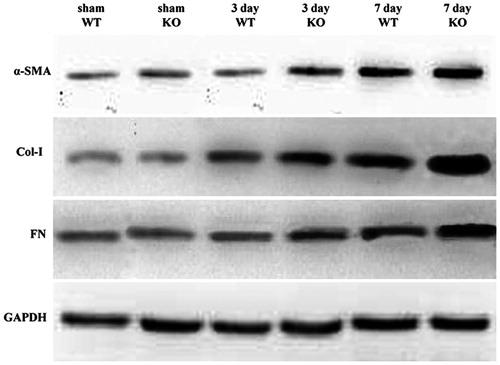
Fibrogenic marker protein expression level
Western-blot result is presented in . Based on density analysis, compared with sham controls, protein expression levels of α-SMA, Col-I and FN were upregulated in both WT and SOST KO mice on 3 days after UUO, but no difference was observed between WT and SOST KO. On 7 days after UUO, the upregulation of α-SMA, Col-I and FN was more remarkable in both WT and SOST KO mice, but the expressions in SOST KO mice were higher than that in WT.
Discussion
CKD can be initiated by many insults to kidney including toxic, ischemic, infectious, endocrine and immunological diseases.Citation17 RIF, characterizing by activation of renal fibroblasts and accumulation of excessive amounts of extracellular matrix, acts as a common pathway in the pathogenesis of CKD initiated by nearly all kinds of insults.Citation18 Despite the high prevalence of CKD, there is limited specific therapy at present.Citation17,Citation19 Myofibroblasts and their pericyte progenitors play a crucial role in the development of CKD. In response to kidney injury, pericytes detach from capillaries, differentiate into myofibroblasts and regulate myofibroblast activation, causing renal fibrosis and inflammation.Citation20
In pericytes and myofibroblasts, LRP-6 has been identified as a coreceptor for multiple fibrogenic signaling pathways activated by TGF-β, CTGF and PDGF.Citation6 Blocking LRP-6 by systemic delivering Dkk1 can effectively inhibit pericyte detachment/transition to myofibroblasts, and attenuate established myofibroblasts proliferation/activation, resulting in reduced renal fibrosis and inflammation.Citation6 Thus, targeting LRP-6 is a promising strategy for treating RIF. In this case, Sclerostin ought to have the potential of preventing RIF for similar molecular mechanism with Dkk1.Citation7,Citation8 Since sclerostin was once reported to be an osteocyte-specific protein, the role of SOST/sclerostin in the regulation of fibrogenesis has little report. Until detection of SOST gene expression in kidney, lung, heart, aorta, as well as dimeric sclerostin protein existence in non-skeletal soft tissues, it is suggested that SOST/sclerostin widely exists in tissues and cells.Citation10,Citation11
This is the first study to investigate whether SOST gene plays a role in RIF progression. Based on current results, when SOST gene was deleted, a promoted tendency of RIF progression was observed in general: in normal condition or early obstructive injury (3 days), SOST gene deletion had no impact on RIF; when obstructive injury sustained longer (7 days), SOST gene deletion caused severe tubulointerstitial impairment and fibrosis, as well as higher fibrogenic gene/protein expression (except for α-SMA mRNA and FN mRNA). This may be explained as LRP-6 is a coreceptor for multiple fibrogenic signaling pathways, and may be able to maintain kidney tissue homeostasis in spite of SOST gene deficiency. When injury/toxic/infection sustains for a long time, the compensating ability of kidney declines and the detrimental impact of SOST gene deficiency cannot be balanced, resulting in enhanced fibrogenic response. So, it is likely to suppose that the impact of SOST gene deletion on RIF progression would be more remarkable if obstructive injury sustains longer. Regarding the unexpected expression pattern of α-SMA mRNA and FN mRNA, more explorations are needed to further identify the role of SOST/sclerostin in mediating RIF development.
In conclusion, it is supposed that SOST gene is involved in the regulation of RIF. In obstructive kidney injury, SOST gene deletion would probably enhance renal fibrogenic response and promote the progression of RIF. But, more evidences are still required before SOST/sclerostin can be considered as a therapeutic target for treating RIF.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was supported by National Natural Science Foundation of China grants (No. 11172190, No. 81371171 and No. 81371172).
References
- Lupher ML Jr, Gallatin WM. Regulation of fibrosis by the immune system. Adv Immunol. 2006;89:245–288
- Isaka Y, Takahara S, Imai E. Chronic deteriorating renal function and renal fibrosis. Contrib Nephrol. 2008;159:109–121
- Klahr S, Schreiner G, Ichikawa I. The progression of renal disease. N Engl J Med. 1988;318(25):1657–1666
- Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: Dual roles in renal injury and repair. J Pathol. 2013;229(2):221–231
- Nelson PJ, von Toerne C, Grone HJ. Wnt-signaling pathways in progressive renal fibrosis. Expert Opin Ther Targets. 2011;15(9):1073–1083
- Ren S, Johnson BG, Kida Y, et al. LRP-6 is a coreceptor for multiple fibrogenic signaling pathways in pericytes and myofibroblasts that are inhibited by DKK-1. Proc Natl Acad Sci USA. 2013;110(4):1440–1445
- Li X, Zhang Y, Kang H, et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem. 2005;280(20):19883–19887
- Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280(29):26770–26775
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–615
- Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589
- Hernandez P, Whitty C, John Wardale R, Henson FM. New insights into the location and form of sclerostin. Biochem Biophys Res Commun. 2014;446(4):1108–1113
- Li X, Ominsky MS, Niu QT, et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23(6):860–869
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res. 2010;25(2):178–189
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75(11):1145–1152
- Radford MG Jr, Donadio JV Jr, Bergstralh EJ, Grande JP. Predicting renal outcome in IgA nephropathy. J Am Soc Nephrol. 1997;8(2):199–207
- Choi DE, Jeong JY, Lim BJ, et al. Aliskiren ameliorates renal inflammation and fibrosis induced by unilateral ureteral obstruction in mice. J Urol. 2011;186(2):694–701
- Snyder JJ, Foley RN, Collins AJ. Prevalence of CKD in the United States: A sensitivity analysis using the National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis. 2009;53(2):218–228
- Boor P, Ostendorf T, Floege J. Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010;6(11):643–656
- Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet. 2012;379(9818):815–822
- Campanholle G, Ligresti G, Gharib SA, Duffield JS. Cellular mechanisms of tissue fibrosis. 3. Novel mechanisms of kidney fibrosis. Am J Physiol Cell Physiol. 2013;304(7):C591–C603