Abstract
Background: The syndrome of rapid onset end-stage renal disease (SORO-ESRD) was first described in the journal Renal Failure in 2010. This is an acute precipitate unpredictable yet irreversible ESRD following acute kidney injury (AKI), as distinct from “classic” ESRD where chronic kidney disease (CKD)-ESRD progression was linear, time-dependent, and predictable. The overall impact of SORO-ESRD on ESRD outcomes in the adult US ESRD population remains speculative and called for larger studies. Methods: A retrospective investigation of an incident adult ESRD population, Mayo Clinic, Rochester, 2001–2013. Results: One hundred and forty-nine of 1461 (10%) incident patients with ESRD had SORO-ESRD – M:F = 76:73, age 62 (19–95) years, 139 (93%) native kidneys, and 10 (7%) renal transplant recipients (RTRs). The modal age group was 71–80 years. A total of 147 (99%) SORO-ESRD patients started first hemodialysis treatment via a dialysis catheter. Kidney biopsy in 10 RTRs and 34 native kidneys revealed acute tubular necrosis (ATN) as the commonest pathology. Cardiac arrest remained the leading cause of death among SORO-ESRD patients. Conclusions: SORO-ESRD accounted for 149 (10%) of 1461 incident ESRD patients. There was no gender disparity. The older population was more susceptible. Ninety-nine percent (99%) of SORO-ESRD patients started their first hemodialysis treatment via a dialysis catheter, a major negative impact on AV fistula first programs. ATN was the leading pathologic diagnosis. We conclude that SORO-ESRD contributes significantly to incident ESRD here in the USA including renal allograft loss. Efforts to reduce AKI incidence or renoprevention demand more attention and priority.
Introduction
We described the syndrome of rapid onset end-stage renal disease (SORO-ESRD), in the journal, Renal Failure, in 2010.Citation1 This is an acute precipitate unpredictable yet irreversible ESRD following acute kidney injury (AKI), in contradistinction from “classic” ESRD where CKD to ESRD progression was progressive, linear, time-dependent, and predictable over years to sometimes decades.Citation1–6 In “classic” ESRD, patients with CKD demonstrate predictably increasing serum creatinine levels, simultaneously falling eGFR values, associated increasing proteinuria where applicable ultimately leading inexorably to terminal symptomatic ESRD and the subsequent need for renal replacement therapy (RRT).Citation7–11 In a June 2011 retrospective study of SORO-ESRD in four Northwestern Wisconsin Mayo Clinic Dialysis Services Outpatient Hemodialysis Units, SORO-ESRD had accounted for 31 (34%) of 91 incident adult ESRD patients.Citation6 Nevertheless, the plausible differential impact of this newly described syndrome on ESRD outcomes, including patient mortality, longevity on dialysis, and causes of death remains uncertain.Citation1–5 Moreover, the underlying pathologic features of the renal pathology in these patients is mostly unknown and often is undiagnosed.Citation1–6,Citation12 Besides, the potential negative impact of the phenomenon of SORO-ESRD on AV fistula first programs and the resulting increase in the use of hemodialysis catheters for unanticipated and therefore unplanned initiation of RRT in these patients has been previously highlighted in our previous reports.Citation1–6,Citation12 In fact, a 2014 report by Chin et al, from UC Davis, Sacramento, California, had identified SORO-ESRD patients with late acceleration of glomerular filtration loss before needing permanent RRT as a risk factor for hemodialysis catheter use in patients with established CKD care.Citation13
We therefore sought to answer some of these questions by studying the incidence, features, and clinical outcomes of patients with SORO-ESRD among a larger incident US ESRD cohort seen at Mayo Clinic, Rochester. This report represents the results of a retrospective 13-year Mayo Clinic, Rochester, investigation of SORO-ESRD, with emphasis on the impact on initial hemodialysis vascular access, mortality outcomes, and the renal pathology of the precipitating AKI.
Methods
In November 2014, we completed a retrospective investigation of the incidence of SORO-ESRD among the incident ESRD population seen and treated at Mayo Clinic, Rochester, between January 2001 and December 2013 to ascertain and clarify further, the clinical features of this syndrome, to analyze the impact of SORO-ESRD on initial hemodialysis vascular access, mortality outcomes, and the renal pathology of the precipitating AKI in a larger population study as contrasted with “classic” ESRD.Citation1–13
The Mayo Clinic Health System provides a comprehensive integrated health care network in an area with 395,000 residents in Southeast Minnesota, Northern Iowa, and Southwest Wisconsin. The Mayo Clinic Dialysis Services provides all hemodialysis care in the Mayo Clinic Health System through eight community-based outpatient hemodialysis facilities and is staffed solely by Mayo Clinic nephrologists who also provide the inpatient hemodialysis care. All adults (age 18 years; n = 1461) in the Mayo Clinic Health System initiating hemodialysis therapy between 1 January 2001 and 31 December 2013, with Minnesota Research Authorization were identified. The primary objective was to establish the incidence of the syndrome of rapid onset end-stage renal disease (SORO-ESRD) in this incident hemodialysis cohort and to further characterize the features of presentation and patient outcomes in this specific group of patients. Trained nurse abstractors reviewed all relevant electronic medical records and databases to extract needed patient demographics, clinical information, and follow-up outcomes. The Mayo Clinic Institutional Review Board approved this study.
Diagnosis of the Syndrome of Rapid Onset End-Stage Renal Disease (SORO-ESRD) in this Study
The diagnosis of the syndrome of rapid onset end-stage renal disease (SORO-ESRD), centered on our previous reported experiencesCitation1–6,Citation12 was based on the following three diagnostic criteria:
eGFR of ≥30 mL/min/1.73 sq. m BSA or a serum creatinine equivalent of ≤1.75 mg/dL in the last year before first hemodialysis treatment.
The patient remaining on maintenance hemodialysis or other forms of RRT for ≥90 days from first hemodialysis treatment without evidence of renal recovery at the time of reporting.
Adult patient, age 18 years and older, at first hemodialysis treatment.
Results
One thousand, four hundred and sixty-one patients constituted the entire incident hemodialysis cohort seen at Mayo Clinic, Rochester, from 1 January 2001 to 31 December 2013. Of this number, 149 (10%) patients fulfilled the diagnostic criteria for the syndrome of rapid onset end-stage renal disease (SORO-ESRD), in contradistinction to 1312 (90%) patients with “classic” ESRD.Citation1–13
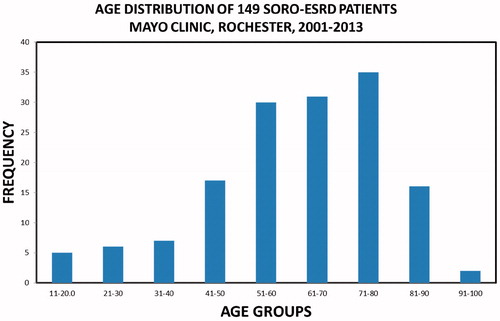
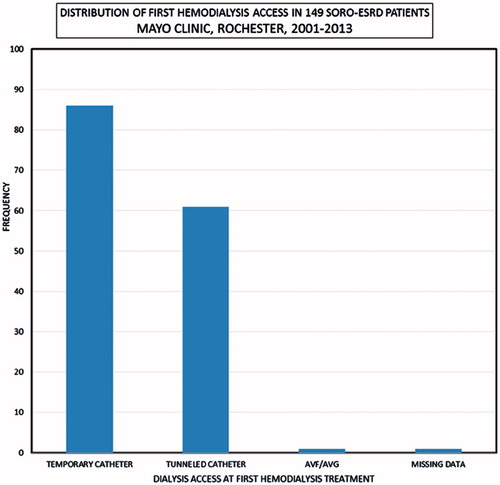
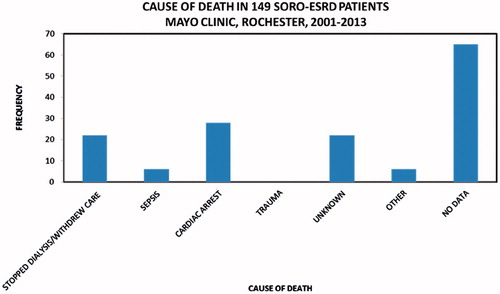
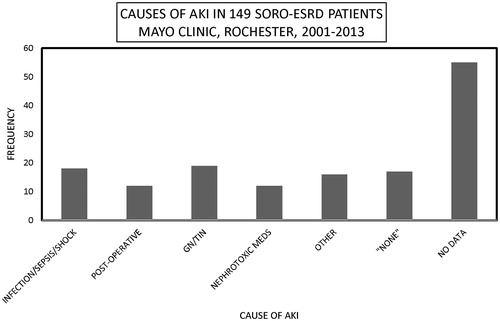
One hundred and forty-nine patients with SORO-ESRD consisted of 76 males and 73 females, mean age 62 (19–95) years, and included 139 (93%) native kidneys and 10 (7%) renal transplant recipients (RTRs). The 71–80-year age group constituted the modal group, accounting for 35 (23%) patients (). Infections including septic shock, the nephritides—glomerulonephritides, and tubulo-interstitial nephritides—and exposure to nephrotoxic agents were the leading causes of AKI among the 149 SORO-ESRD patients (). The first hemodialysis treatment was initiated among the 149 SORO-ESRD patients, in the hospital setting in 126 (85%) patients versus the outpatient setting in 23 (15%) patients. One hundred and forty-seven (99%) of the 149 SORO-ESRD patients started their first hemodialysis treatment via a dialysis catheter—temporary dialysis catheters in 86 (58%), and tunneled dialysis catheters in 61 (41%) (). Only one of the 149 (1%) patients had an AV fistula as vascular access at the time of initiation of RRT. Vascular access data were missing in one patient. Kidney biopsy was carried out in 10 RTRs and in 34 native kidneys, at the time of diagnosis of AKI. Among the RTRs, the commonest pathologic diagnoses included acute tubular necrosis (ATN) in 3 (30%), polyoma virus nephropathy in 2 (20%) and acute rejection in 2 (20%). For native kidneys, the commonest pathologic diagnoses were ATN in seven (21%), glomerulonephritis in six (18%), insufficient tissue in six (18%), focal segmental glomerusclerosis (FSGS) in four (12%), and myeloma cast nephropathy in three (9%). Diabetic glomerulosclerosis, Ig A nephropathy, and oxalate nephropathy accounted for two (6%) patients, each. There was recorded a total of 64 deaths, with cardiac arrest being the leading cause of death among the 149 SORO-ESRD patients ().
Figure 2. Causes of acute kidney injury among the 149 SORO-ESRD patients, Mayo Clinic, Rochester, 2001–2013.
Conclusions
The syndrome of rapid onset end-stage renal disease or SORO-ESRD is not unlike “classic ESRD” in requiring permanent RRT.Citation1–6,Citation12 Nevertheless, it is acute precipitate and unanticipated yet irreversible ESRD following AKI in a priori otherwise stable CKD.Citation1–6,Citation12 A tale indeed, of two different cities, as was noted in our recent publication.Citation4 SORO-ESRD accounted for 10% of the incident hemodialysis ESRD cohort at Mayo Clinic Rochester, 2001–2013, in this study. Thus, SORO-ESRD, or precipitate yet irreversible ESRD in a priori stable patients with CKD following AKI is not rare among incident adult patients with ESRD on maintenance hemodialysis. Indeed, recent reports of acute precipitate irreversible ESRD from other centers in the USA and Canada, outside of the Mayo Clinic Health System and outside of the Mayo Clinic, Rochester, support this view that SORO-ESRD contributes significantly to the adult ESRD population in North America.Citation13–16 In a 2011 report, Lee et al., had studied all consecutive patients initiated on maintenance hemodialysis or peritoneal dialysis over several years at two dialysis units in Northern California and demonstrated that eight (7.6%) of 105 incident chronic dialysis patients in one dialysis unit and nine (12.7%) of 71 incident patients at another dialysis unit had experienced rapid decline in kidney function that was the immediate precipitant for the need for permanent RRT.Citation14 Similarly, O'Hare et al., in an analysis of the trajectories of eGFR during the 2-year period before dialysis initiation in 5606 Veterans Affairs patients who initiated long-term dialysis between 2001 and 2003 revealed that 9.5% of the patients with ESRD had accelerated loss of eGFR from levels >60 mL/min/1.73 m2 (mean eGFR slope, 32.3 ± 13.4 mL/min/1.73 m2 per year) and another 3.1% experienced catastrophic loss of eGFR from levels >60 mL/min/1.73 m2 within 6 months or less to reach irreversible ESRD.Citation15 In a related Canadian report from 2012, Siddiqui et al showed that among 1294 patients who received acute dialysis following cardiac surgery and survived beyond 90 days, 352 (27%) patients subsequently required chronic dialysis,Citation16 a diagnosis that is synonymous with the syndrome of rapid onset end stage renal disease or SORO-ESRD.Citation17
Older age with renal senescence appears to be one unmodifiable factor in the pathogenesis of this syndrome of rapid onset ESRD as the 71–80 years age group constituted the modal age group, followed by the 61–70 years age group.Citation1–6,Citation12,Citation18,Citation19 However, other factors that precipitate or aggravate AKI such as intraoperative hypotension, infections including septic shock, and exposure to nephrotoxic agents may be significant predisposing but modifiable risk factors for SORO-ESRD.Citation1–6,Citation12 The latter modifiable risk factors should be amenable to manipulation and management by the medical team to mitigate the incidence of AKI, thereby limiting the scourge of ESRD following AKI.Citation1–6,Citation12 Thus, as we have reported variously, the concept of renoprevention, must include the pre-emptive avoidance of nephrotoxics including NSAIDs, diuretics, angiotensin inhibition, iodinated contrast in certain hospitalized patients, especially the older (>65-year old) patients with later stage CKD.Citation1–6,Citation12,Citation20,Citation21
Major impact of SORO-ESRD on AV fistula first programs—high hemodialysis catheter rates persist in the US
Classic teaching has highlighted reduced infection risk, fewer hospitalizations, and lower total costs associated with hemodialysis that is initiated via AVF in comparison with hemodialysis catheter (HC), and indeed, the 1997 National Kidney Foundation–Kidney Disease Outcomes Quality Initiative practice guidelines regarding permanent hemodialysis access creation and maintenance targeted a 50% or greater incidence rate for AVF.Citation22 Despite these obvious implications, Malas et al in a just published retrospective analysis of 510,000 patients with ESRD from the USRDS database demonstrated that 82.6% initiated hemodialysis via HC, 14.0% via AVF, and 3.4% via AVG.Citation23 Indeed, AVF use only increased only marginally from 12.2% to 15.0% between 2006 and 2010.Citation23 Moreover, patients initiating hemodialysis with AVF had 35% lower mortality than those with HC (adjusted hazard ratio, 0.65; 95%CI, 0.64–0.66; p < 0.001) and survival at 1 year was 78% in the HC group compared with 84% for the AVG group and 89% for the AVF group (Wilcoxon p < 0.001).Citation23 AVF was associated with a 38% lower hazard of cardiovascular mortality (aHR, 0.62; 95%CI, 0.61–0.64; p < 0.001).Citation23 AVF was associated with a 44% lower hazard of sepsis-related mortality (aHR, 0.56; 95%CI, 0.53–0.59; p < 0.001).Citation23 Our study demonstrated that 147 (99%) of the 149 SORO-ESRD patients in this Mayo Clinic incident ESRD cohort started hemodialysis with a dialysis catheter and without an AV fistula nor an AV graft. Indeed, similar conclusions have been drawn from experiences at two centers in Northern California with Lee et al, and Chin et al, showing that 100% of SORO-ESRD patients began hemodialysis via a dialysis catheter.Citation13,Citation14 Clearly, SORO-ESRD is a risk factor for hemodialysis catheter use among patients with ESRD and efforts to mitigate AKI and thereby reduce the incidence of SORO-ESRD must be more vigorously pursued in our hospitals and clinics.Citation24
A case for alternate RRT options for patients with SORO-ESRD
In a just published commentary by the AKI Advisory Group of the American Society of Nephrology on promoting kidney function recovery in patients needing RRT, concerns were vigorously raised regarding the potential contribution of the hypotensive episodes that occur during hemodialysis toward the perpetuation of renal damage and therefore more prolonged and sometimes permanent need for RRT following AKI.Citation25 As a result, this commentary had called for research in RRT innovations that promote renal recovery and therefore avoid further kidney damage.Citation25 There is accumulating evidence incriminating hypotension in the causation of AKI in hospitalized patients especially in the perioperative periods.Citation26–28 Arguably, we posit that preventive pre-emptive measures that would limit perioperative hypotension may turn out to be one of the most effective means of preventing postsurgical AKI.Citation26–28 Consequently, we have therefore hypothesized that since “urgent-start” peritoneal dialysis (PD) following AKI requiring RRT is less likely to precipitate intradialytic hypotensive episodes, that PD initiation for AKI may represent an innovative approach to RRT that may lead to higher renal recovery and improved renal outcomes in patients presenting with dialysis-requiring AKI.Citation29–36 More studies are warranted to further confirm the validity of this hypothesis.
In summary, we conclude that SORO-ESRD is not uncommon in the incident ESRD population here in the USA and Canada. Similar observations will most likely be duplicated outside of North America. This syndrome of acute yet irreversible ESRD is a significant contributor to the increasing incident ESRD population, involving both native kidneys and renal allografts. Every effort to limit, if not eliminate AKI, by the practice of more preventative nephrology paradigms (renoprevention) is warranted.Citation19–21 The impact of the phenomenon of SORO-ESRD on the rising use of hemodialysis catheters due to unplanned hemodialysis starts constitutes a major unintended consequence.Citation23,Citation24 The place of “urgent-start” PD in such patients presenting with SORO-ESRD following AKI is one tantalizing hypothesis as discussed in the preceding sectionCitation29–36. This is yet another research question that calls for urgent attention.
Acknowledgments
This study is dedicated to the millions of Nigerians who suffer in the midst of plenty, especially with a dearth of affordable and veritable healthcare. We hope for a day when a government-sponsored effective and efficient universal healthcare insurance coverage will be in place in Nigeria for all the Nigerians.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Onuigbo MA. Syndrome of rapid-onset end-stage renal disease: A new unrecognized pattern of CKD progression to ESRD. Ren Fail. 2010;32(8):954–958
- Onuigbo MAC, Onuigbo NTC. The syndrome of rapid onset end-stage renal disease (SOROESRD) – A new Mayo clinic dialysis services experience, January 2010-February 2011. In: Di Iorio B, Heidland A, Onuigbo M, Ronco C, eds. Hemodialysis: How, When and Why. Italy: NOVA Science Publishers; 2012:443–485
- Onuigbo MA, Onuigbo NT, eds. In: Chronic kidney gisease and RAAS blockade: A new view of renoprotection. London, England: Lambert Academic Publishing GmbH 11 and Co. KG; 2011
- Onuigbo MA, Achebe NJ, Musso CG. The syndrome of rapid onset ESRD in the last 100 consecutive incident Northwestern Wisconsin Mayo Clinic chronic hemodialysis patients, 2010–2011: Results of the analysis of individual patient-level serum creatinine trajectories to ESRD – Can there be a link with angiotensin inhibition and renal senescence in older CKD patients? In: Onuigbo MAC, ed. ACE Inhibitors: Medical Uses, Mechanisms of Action, Potential Adverse Effects and Related Topics. New York: NOVA Publishers; 2013:109–125
- Onuigbo MA. Syndrome of rapid onset end-stage renal disease in two consecutive renal transplant recipients. Indian J Nephrol. 2013;23(3):222–225
- Onuigbo MA, Onuigbo NT, Musso CG. Syndrome of rapid onset end stage renal disease in incident Mayo Clinic chronic hemodialysis patient. Indian J Nephrol. 2014;24:75–81
- The GISEN Group. Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet. 1997;349:1857–1863
- O'Hare AM, Choi AI, Bertenthal D, et al. Age affects outcomes in chronic kidney disease. J. Am. Soc. Nephrol. 2007;18:2758–2765
- Chiu YL, Chien KL, Lin SL, Chen YM, Tsai TJ, Wu KD. Outcomes of stage 3-5 chronic kidney disease before end-stage renal disease at a single center in Taiwan. Nephron Clin Pract. 2008;109:c109–c118
- Yoshida T, Takei T, Shirota S, et al. Risk factors for progression in patients with early-stage chronic kidney disease in the Japanese population. Intern Med. 2008;47:1859–1864
- Conway B, Webster A, Ramsay G, et al. Predicting mortality and uptake of renal replacement therapy in patients with stage 4 chronic kidney disease. Nephrol Dial Transplant. 2009;24:1930–1937
- Onuigbo MA. Evidence of the syndrome of rapid onset end-stage renal disease (SORO-ESRD) in the acute kidney injury (AKI) literature—Preventable causes of AKI and SORO-ESRD—A call for re-engineering of nephrology practice paradigms. Ren Fail. 2013;35(6):796–800
- Chin AI, Nguyen TA, Dinesh KP, Morfin JA. Late acceleration of glomerular filtration rate decline is a risk for hemodialysis catheter use in patients with established nephrology chronic kidney disease care. Hemodial Int. 2014;19(3):379–385
- Lee P, Johansen K, Hsu CY. End-stage renal disease preceded by rapid declines in kidney function—a case series. BMC Nephrol. 2011;12:5
- O'Hare AM, Batten A, Burrows NR, et al. Trajectories of kidney function decline in the 2 years before initiation of long-term dialysis. Am J Kidney Dis. 2012;59(4):513–522
- Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery–1995 to 2009. CMAJ. 2012;184(11):1237–1245
- Onuigbo MA. Increasing trends in acute dialysis after elective surgery, 1995–2009: How often does AKI directly lead to irreversible ESRD all in one fell swoop? CMAJ. published online July 13, 2012
- Musso CG, Oreopoulos DG. Aging and physiological changes of the kidneys including changes in glomerular filtration rate. Nephron Physiol. 2011;119 (Suppl 1):1–5
- Musso CG, Vilas M, Onuigbo M. Nephroprevention in the oldest old with chronic kidney disease: Special considerations. World J Nephrol. 2015;4(1):1–5
- Onuigbo MAC. Reno-prevention vs. reno-protection: A critical re-appraisal of the evidence-base from the large RAAS blockade trials after ONTARGET—A call for more circumspection. QJM. 2009;102: 155–167
- Onuigbo MA. Renoprevention: A new concept for reengineering nephrology care—An economic impact and patient outcome analysis of two hypothetical patient management paradigms in the CCU. Ren Fail. 2013;35(1):23–28
- National kidney foundation-dialysis outcomes quality initiative. NKF-DOQI clinical practice guidelines for vascular access. Am J Kidney Dis. 1997;30(4)(Suppl 3):S150–S191
- Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA Surg. 2015;150(5):441–448
- Onuigbo M. Major impact of the syndrome of rapid onset ESRD on AV fistula first program—High hemodialysis catheter rates persist in the US. JAMA Surgery, 2015 (in print) (Letter to the Editor)
- Cerdá J, Liu KD, Cruz DN, et al. Promoting kidney function recovery in patients with AKI requiring RRT. Clin J Am Soc Nephrol. 2015. [Epub ahead of print]. doi: 10.2215/CJN.01170215
- Aronson S, Phillips-Bute B, Stafford-Smith M, et al. The association of postcardiac surgery acute kidney injury with intraoperative systolic blood pressure hypotension. Anesthesiol Res Pract. 2013;2013:174091
- Xue FS, Li RP, Liu GP. Modifiable risk factors for acute kidney injury after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2014;148(1):366–367
- Onuigbo M, Agbasi N. Intra-operative hypotension—A neglected causative factor in hospital-acquired acute kidney injury: A Mayo clinic health system experience revisited. J Renal Inj Prev. 2015;4(3)
- Povlsen JV, Ivarsen P. How to start the late referred ESRD patient urgently on chronic APD. Nephrol Dial Transplant. 2006;21 (Suppl 2):ii56–ii59
- Owen PJ, Priestman WS, Sigrist MK, et al. Myocardial contractile function and intradialytic hypotension. Hemodial Int. 2009;13(3):293–300
- McIntyre CW. Recurrent circulatory stress: The dark side of dialysis. Semin Dial. 2010;23(5):449–451
- Ghaffari A. Urgent-start peritoneal dialysis: A quality improvement report. Am J Kidney Dis. 2012;59(3):400–408
- Tennankore KK, Soroka SD, Kiberd BA. The impact of an “acute dialysis start” on the mortality attributed to the use of central venous catheters: A retrospective cohort study. BMC Nephrol. 2012;13:72
- Arramreddy R, Zheng S, Saxena AB, Liebman SE, Wong L. Urgent-start peritoneal dialysis: A chance for a new beginning. Am J Kidney Dis. 2014;63(3):390–395
- Masseur A, Guest S, Kumar V. Early technique success after initiation of treatment with urgent-start peritoneal dialysis. Adv Perit Dial. 2014;30:36–39
- Groenhoff C, Delgado E, McClernon M, et al. Urgent-start peritoneal dialysis: Nursing aspects. Nephrol Nurs J. 2014;41(4):347–352