Abstract
Background: Due to the long-term and chronic exposure to the peritoneal dialysis fluid, patients could develop peritoneal fibrosis and ultrafiltration failure which compromises treatment efficacy and outcome, and fibrosis is the major cause of peritoneal dialysis (PD) withdraw among patients. Methods: Twenty-one male WISTAR rats were randomly assigned to three groups, namely saline group, standard peritoneal dialysis fluid (PDF) group, and panax notoginseng saponins (PNS) group. Peritoneal fibrosis was induced by daily injection of PDF for 4 weeks. After execution, multiple histological techniques including HE and Masson's trichrome staining and transmission electron microscopy (TEM) were applied to observe the pathological changes and concentrations of multiple cytokines may involve in the process of fibrosis were determined by enzyme-linked immune sorbent assay (ELISA). Biochemistry parameters were determined by automated chemistry analyzer. Results: PNS can significantly inhibit the expression of transforming growth factor beta (TGF-β1), connective tissue growth factor (CTGF), and monocyte chemoattractant protein (MCP-1) in the peritoneum of rats. Furthermore, pathological damages, including extracellular matrix deposition, vascularization, and fibroblast, were ameliorated in PNS group when being compared with standard PDF group. Peritoneal functions were improved by regular PNS treatment with significantly elevated ultrafiltration. Conclusion: PNS is capable of improving peritoneal function in subjects with PDF exposure and can possibly applied in patients with PD after further verification.
Introduction
Peritoneal dialysis (PD) is an effective self-care treatment for patients with server chronic kidney disease (CKD), it has several advantages, including better life quality, improved survival rate, stabilized cardiovascular function and can be easily performed by patient at home. A study performed among New Zealand end-stage renal disease (ERSD) patients commencing dialysis between 1997 and 2011 showed an overall equivalent survival when comparing PD and facility hemodialysis (HD); however, the reduction of approximately 20% mortality risk was associated with PD during the first 3 years of dialysis.Citation1 Furthermore, PD also maintains lower cost when comparing with HD, according to the data reported by United States, the annual cost for dialysis care is $24,293 higher for patients treated with facility HD compared to PD;Citation2 therefore, PD has great economic advantage when comparing with HD. Despite of the advantages mentioned previously, the usage of PD varies significantly between regions, in United Kingdom, South Korea, and Mexico, the usage of PD was greater than world average level, and the proportion of dialysis patients on PD reached as high as 79% in Hong Kong;Citation3 on the other hand, Japan and Germany had rates lower than global average level. Among all the complications caused by PD, peritoneal fibrosis is the major cause of PD withdraw among patients with long-term PD. Due to the long-term and chronic exposure to the peritoneal dialysis fluid, patients could develop peritoneal fibrosis and ultrafiltration failure which compromises treatment efficacy and outcome.Citation4 Therefore, study on the mechanism of PD-related peritoneal fibrosis and the treatment against it has great clinical significance to the improvement of PD efficacy and prognosis.
Transforming growth factor-β1 (TGF-β1) is a multifunctional cytokine which critically involved in the process of fibrosis through its potential effects on fibroblast differentiation, extracellular matrix formation,Citation5,Citation6 and epithelial-to-mesenchymal transition (EMT).Citation7 In chronic renal diseases, TGF-β1 has been identified as the most potent mediator and convergent pathway in inducing EMT and renal fibrosis, mainly via the TGF-β/Smads signal transduction pathway.Citation8 Among the procedure of PD, TGF-β1 has been considered as the master factor in the development of peritoneal dysfunction due to its over-expression has been associated with poor PD outcome.Citation9 Animal experiment conducted in a rodent model with intraperitoneal administration of adenoviral vector driving the active form of TGF-β1 observed the thickening of peritoneum, along with cellular proliferation and increased vascularization.Citation10 Another animal experiment applied the blocking peptides of TGF-β1 revealed that blocking peptides can lead to significant ameliorated fibrosis and angiogenesis, improved peritoneal function, and reduced the number of FSP-1+cells, especially in the Cyto+/FSP-1+subpopulation.Citation11 Connective tissue growth factor (CTGF) is a kind of matricellular protein of the CCN family, it has important roles in many biological processes, including cell adhesion, migration, proliferation, angiogenesis, skeletal development, and tissue wound repair, and also is critically involved in fibrotic disease and several forms of cancers. A cell assay observed significantly elevated CTGF expression in human peritoneal mesothelial cells (HPMC) after cultured in human peritoneal fibroblasts conditioned medium, and the synthesis of CTGF can be partly suppressed by blocking TGF-β1.Citation12 In addition, a study performed among patients receiving PD indicated that CTGF expression was positively correlated with peritoneal fibrosis and ultrafiltration failure.Citation13 Monocyte chemotactic protein-1 (MCP-1) currently has been recognized as an angiogenic chemokine. Animal study showed that MCP-1 activation can be inhibited by astragalus treatment, and consequently, the TGF-β1 protein concentration has significantly decreased.Citation14 A prospective study showed that elevated MCP-1 in dialysate was associated with higher all-cause and cardiovascular mortality in PD patients, indicating that MCP-1 may contribute to poor clinical outcomes.Citation15
Panax notoginseng saponins (PNS) consist of major therapeutically active gradients of panax notoginseng, in detail, PNS have been proved to possess remarkable effects of improving blood circulation and removing blood stasis,Citation16 and reversing multidrug resistance of cancer.Citation17 In an animal experiment, researchers observed that PNS demonstrated the protective effect against cisplatin-induced nephrotoxicity, with the reduction in blood urea nitrogen and serum creatinine, also PNS can reduce the rate of cellular apoptosis by inhibiting the expression of Bax and caspase 9, while elevating the expression of Bcl-2.Citation18 In the aspect of inhibiting fibrosis, it has been reported that PNS is capable of reducing the pulmonary fibrosis by downregulating the expression of TGF-β1 in rats.Citation19 However, currently, there were only few studies regarding the PNS and peritoneal fibrosis conducted.
In our present study, we aimed to investigate the relationship between PNS application and peritoneal fibrosis in rat model that peritoneal fibrosis was induced by daily intraperitoneal injection of peritoneal dialysis fluid (PDF). We attempted to observer the changes in peritoneal fibrosis and TGF-β1 expression and other cytokines related with TGF-β1 by applying PNS in PDF. Weight, peritoneal function, biochemistry parameters, and pathological changes were compared between three groups of rats in our study.
Materials and methods
Laboratory animals
Twenty-one male WISTAR rat of specific pathogen free (SPF) level with weight ranging from 160 g to ∼190 g were provided by Shanghai SLAC Laboratory Animal Company (Shanghai, China). Each rat was house in an individual cage under air-conditioned animal facility. All rats were subjected to 12-h dark/Light cycle at a temperature of 24 °C ± 2 °C and a relative humidity of 45% ± 10%. Before the commencement of experiment, rats were allowed 1 week of acclimatization with standard rat diet and free access to tap water. The rats were handled by qualified personnel with the certification of laboratory animal care. The animal experiments were approved by the Ethic Committee of the First Affiliated Hospital of Xiamen University.
Animal treatments
All 21 male WISTAR rats were randomly assigned to the following three groups and the treatment in total lasted for 4 weeks:
Saline group: Seven rats received 100 mL/kg bodyweight of sterile saline via daily intraperitoneal injection.
Standard PDF group: Same amount of rats received 4.25% PDF (Baxter Healthcare, Guangzhou, China) at a dose of 100 mL/kg bodyweight via daily intraperitoneal injection. In addition, about 0.6 mg/kg bodyweight of lipopolysaccharide (LPS, Sigma, St. Louis MO, USA) were added into PDF for intraperitoneal injection in a 2-day interval over the first week of experiment.
PNS group: Also seven rats received 4.25% PDF at a dose of 100 mL/kg bodyweight mixed with PNS (Wuzhou Pharmaceutical, Guangxi, China) via daily intraperitoneal administration. LPS at a dose of 0.6 mg/kg bodyweight was given in a 2-day interval over the first week of experiment. Furthermore, PNS and LPS were injected into PDF with separated sterilized syringes of 1 mL.
Sample collection and preparation
After 4 weeks of preparation and daily intraperitoneal injection, peritoneal function test was performed followed 2 days of cessation of dialysis. Briefly, 20 mL of 4.25% PDF was administered intraperitoneally to the rats before being euthanized. After injection, rats have free access to tap water and movement inside cage for 1 h. About 10% of chloral hydrate was applied to anesthetize all rats, and tube was placed through a section of the abdomen carefully. All procedures have practiced with caution and no PDF was leaked during the placement of tube. Net ultrafiltration was the volume of fluid removed after 4 h minus the volume of fluid administered. Ascites samples were collected by using sterilized syringe and then subjected to white cell count analysis and biochemistry test. Blood sample was collected by cardiac puncture directly. Peritoneal fluid samples were also collected and centrifuged at 1500 rpm for 5 min. Peritoneal fluid urea nitrogen (Durea), plasma urea nitrogen (Purea), PDF glucose (Do), and peritoneal fluid glucose (Di) were measure by end-point assay on a 7180 Hitachi automated chemistry analyzer (Hitachi, Tokyo, Japan).
Peritoneum tissue was obtained from the section in abdomen and then fixed with 10% formalin for 16 to 18 hours. Tissue was subjected to dehydration by graded ethanol treatment, and then, paraffin wax infiltration was performed. Finally, the tissue was cut into section of 4 μm and was stained with hematoxylin and eosin (H&E) reagent for pathological histological examination. In addition, Masson's trichrome stain was also performed.
General status & biochemistry analysis
The weight of rats included in our study was carefully recorded weekly, and the initial weight and the weight before execution were compared between groups. The blood glucose, triglyceride, total cholesterol, low-density lipoprotein, plasma urea nitrogen, and albumin were measure in the blood sample obtained by cardiac puncture by using 7180 Hitachi automated chemistry analyzer. As for peritoneal fluid samples, urea nitrogen and glucose concentration were determined. White blood cell count was calculated by using SF-300 hematology analyzer (Sysmex, Kobe, Japan). All performed laboratory procedures were strictly in accordance with the manufactures' instruction.
Enzyme-linked immunosorbent assay (ELISA) for the TGF-β1, CTGF, and MCP-1 protein concentration
Mouse TGF-β1, CTGF, and MCP-1 ELISA kits (Boster, Wuhan, China) were used to determine the corresponding cytokines concentration in peritoneal fluid samples. Following procedure is for the determination of TGF-β1 level: (1) Firstly, pipette 100 µL of the standard diluent buffer to the well reserved for the standard blanks. (2) Then, pipette 100 µL of standards and diluted samples to the appropriate microtiter wells and incubated for 90 min in 37 °C. (3) Pipette 100 µL of biotin-conjugated detection antibody solution into each well and tap gently on the side of the plate to mix, followed with an incubation for 60 min in 37 °C. (4) Wash wells with 0.01 M tris buffer solution for three times. (5) Pipette 100 µL of avidin-biotin complex in to each well and incubate for 30 min in 37 °C. (6) Wash wells with 0.01 M tris buffer solution for five times. (7) Pipette tetramethylbenzidine (TMB) into each well and cover the wells with plate cover and incubate for 30 min at room temperature. (8) Add stop solution into each well. (9) Read the absorbance of each well at 450 nm having blanked the plate reader against a chromogen blank composed of 100 µL each of stabilized chromogen and stop solution. Plot the absorbance of the standards against the standard concentration. Draw the best smooth curve through these points to construct the standard curve and read the TGF-β1 protein concentrations for all samples and controls from the standard curve plotted.
ELISA procedure for CTGF protein concentration
(1)Set a blank well without any solution. Add 50 µL of standard or sample per well. (2) Add 50 µL of HRP-conjugate to each well (not to blank). Incubate for 1 hour at 37 °C. (3) Aspirate each well and wash, repeating the process three times for a total of three washes. Wash by filling well with ddH2O (200 µL) using an auto washer. After the last wash, remove any remaining solution by aspirating or decanting. Invert the plate and blot it against clean paper towels. (4) Add 50 µL of substrate A and 50 µL substrate B to each well. Incubate for 15 min at 37 °C. Keeping the plate away from drafts and other temperature fluctuations in the dark. (5) Add 50 µL of stop solution to each well when the first four wells containing the highest concentration of standards develop obvious blue color. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. (6) Determine the optical density of each well within 30 min, using a microplate reader set to 450 nm. (7) Using the software provided by manufacturer to make a standard curve and read the CTGF protein concentrations for all samples.
ELISA procedure for MCP-1 protein concentration
(1) Add 100 µL of Standard, Blank, or Sample per well. Cover with the adhesive strip. Incubate for 2 h at 37 °C. (2) Remove the liquid of each well, do not wash. (3) Add 100 µL of biotin-antibody working solution to each well. Incubate for 1 h at 37 °C. Biotin-antibody working solution may appear cloudy. Warm up to room temperature and mix gently until solution appears uniform. (4) Aspirate each well and wash, repeating the process three times for a total of three washes. Wash: Fill each well with 200 µL of wash buffer and let it stand for 2 min, then remove the liquid by flicking theplate over a sink. The remaining drops are removed by patting the plate on a paper towel. (5) Add 100 µL of HRP-avidin working solution to each well. Cover the microtiter plate with a new adhesive strip. Incubate for 1 h at 37 °C. (6) Repeat the aspiration and wash three times as step 4. (7) Add 90 µL of TMB substrate to each well. Incubate for 10 to 30 min at 37 °C. Keeping the plate away from drafts and other temperature fluctuations in the dark. (8) Add 50 µL of stop solution to each well when the first four wells containing the highest concentration of standards develop obvious blue color. If color change does not appear uniform, gently tap the plate to ensure thorough mixing. (9) Determine the optical density of each well within 30 min, using a microplate reader set to 450 nm.
Histological analysis and transmission electron microscopy (TEM) examination
Roughly, the morphologic changes were observed by eyes as soon as the peritoneum tissue removed from laboratory animals. Peritoneum tissues were embedded in paraffin and cut into slices (6 μm) and dehydrated by using dimethylbenzene, followed the slices were stained with H&E and Masson's trichrome for light microscopic observation, respectively. Measurement of the peritoneal thickness was performed by image analysis at × 400 original magnification in each slice, peritoneal thickness was measured at random 10 different points and average value was used to evaluate the peritoneal thickness of each slice.
Peritoneum tissue blocks ranging from 0.5 to 1.0 mm3 were cut and fixed in 2.5% glutaraldehyde in 1 min. After the process of dehydration, embedding, sectioning, and staining, ultrathin sections were observed under JEM2100HC electron microscope (JEOL, Tokyo, Japan).
Statistical analysis
All data obtained from this study are expressed in the form of mean ± SD. Statistical analysis was performed by SPSS version 19.0 (Chicago, IL), using one-way analysis of variance (ANOVA) to compare the weight, peritoneal function, biochemistry parameters, peritoneal thickness, and the concentration of TGF-β1 between three groups. A p-value less than 0.05 was considered significant.
Results
General status
During 4 weeks of daily intraperitoneal injection and dialysis, 21 male WISTAR rats maintained normal activity and food intake. With the consumption of diet and water, the rats gained weight gradually. Induration in injection site was presented in few rats and dissolved after the change of injection site. One-way ANOVA was used to compare the weight between groups, and no significant was observed between groups both in initial weight and the weight before execution. After execution, we observed no encapsulation inside the abdomen in all rats. The data regarding weight of rats in all group was presented in .
Table 1. The change of body weight of rats in three groups.
Changes on peritoneal function
As can be seen in , the ultrafiltration and Di/Do were significantly decreased in both standard PDF and PNS groups, when being compared with saline group, while dialysate-to-plasma urea concentration ratio (D/Purea) was significantly elevated in both standard PDF and PNS groups (p < 0.05). It is noted that the ultrafiltration and Di/Do were higher in PNS group when being compared with standard PDF group, and significant reduction in D/Purea of PNS group was observed when compared with standard PDF group (p<0.05).
Table 2. The results of peritoneal function test of rats in three groups.
Changes on biochemistry parameters
After 4 weeks of experiment, the statistical analysis showed no significant differences in all biochemistry parameters that we tested between three groups, including blood glucose, triglyceride, total cholesterol, low-density lipoprotein, and albumin. The data were presented in .
Table 3. The results of peritoneal function test of rats in three groups (mmol/L).
Histology analysis
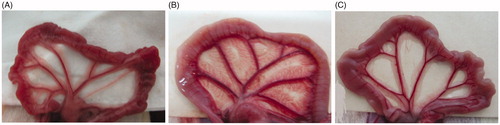
We attempted to observe morphologic changes by eyes as soon as the peritoneum tissue removed from laboratory animals. As demonstrated in the photographs in , the peritoneum tissue of saline group was normal and clear, while the samples of standard PDF group was thicken and unclear, also with extra vascularization. The structure and morphologic changes in PNS group were considered worse than saline group, but with improvement when compared with standard PDF group.
Figure 1. The morphologic changes of rat peritoneum on the effects of standard PDF and PNS. (A) Saline group, (B) Standard PDF group, and (C) PNS group.
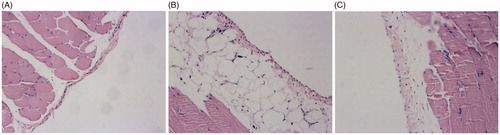
H and E staining revealed that the structure of mesothelial cells of saline group was intact and with little extracellular matrix (ECM) deposition, the presence of inflammatory cell , vascularization and fibroblast were seldom observed. In the rats of standard PDF group, we observed remarkable shedding, inflation in mesothelial cells and increased ECM thickness and deposition, also the presence of inflammatory cell, vascularization, and fibroblast were significantly increased. The pathological damages of peritoneum tissue in PNS group were improved when compared with standard PDF group ().
Figure 2. Effects of PNS on the peritoneum tissue of rats examined by HE staining. (A) Saline group, (B) Standard PDF group, and (C) PNS group. Original magnification 200x.
According to the data from Masson's trichrome staining, the mean thickness of peritoneum tissue of saline group, standard PDF group and PNS group were 15.4 μm, 55.3 μm, and 33.6 μm, respectively. Statistical comparison showed significant elevation on the thickness in standard PDF group and PNS group when compared with saline group. However, the reduction of thickness in PNS group when compared with standard PDF group was also observed with significance. The details of Masson's trichrome analysis were presented in and .
Figure 3. Effects of PNS on the peritoneum tissue of rats examined by Masson's trichrome staining. (A) Saline group, (B) Standard PDF group, and (C) PNS group. Original magnification 200x.
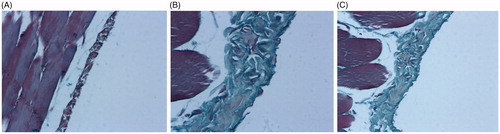
Figure 4. Effects of PNS on the peritoneum tissue of rats examined by transmission electron microscope. (A) Saline group, (B) Standard PDF group, and (C) PNS group.
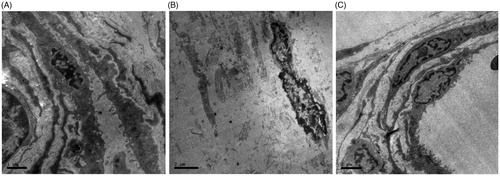
Table 4. The thickness of peritoneum tissue in 3 groups.
Transmission electron microscopy (TEM) analysis
We observed normal cell structure of tissue obtained from saline group under TEM and rich in microvillus, no denudation in nucleus and no collagenous fiber synthesis were observed. Obvious inflation and denudation observed in standard PDF group with the loss of microvillus, also massive collagenous fiber synthesis detected. The damages in PNS group were improved when compared with standard PDF group, while the reduction in both inflation in nucleus and in the loss of microvillus was observed, and the synthesis of collagenous fiber was less obvious.
Analysis on cytokines concentration
The results of statistical analysis on the cytokines concentration between groups were displayed in . The concentrations of all cytokines tested were significantly elevated in both standard PDF and PNS group when being compared with saline group. Significant reductions in the concentrations of all cytokines were also found in PNS group when compared with standard PDF group. The images obtained by using TEM were presented in .
Table 5. The concentration of cytokines of rats in three groups (pg/mL).
Discussion
As previously discovered, the long-term exposure to bioincompatible PDF with the properties of high glucose and low pH and episodes of infections causes inflammation and injury to peritoneum which progressively undergoes fibrosis and angiogenesis,Citation20 and the progress of fibrosis is positively correlated with the duration of PD. Study conducted by Williams et al.Citation21 investigated 130 patients with PD and increased thickness of the submesothelial compact collagenous zone and higher prevalence of vascular changes were identified when compared with healthy individuals and patients with HD, also the loss of the mesothelial cell layer has been identified as a major pathological change in the long-term PD patients. Both renal failure and dialysis would render exposure to inflammatory mediators on peritoneum,Citation22 and this exposure will possibly induce the expression of profibrotic cytokines such as TGF-β1. Current knowledge on this issue indicated that mesothelial cell may play a key role in this process, because the expression of TGF-β1 can be induced in mesothelial cells by high-concentration glucose solutions and spent dialysate.Citation23 Therefore, the TGFβ/Smad pathway plays a crucial role in the development of peritoneal fibrosis, latest study attempted to inhibit TGFβ/Smad pathway and MCP-1 in a rat model by administrating Astragalus membranaceus, the results indicated that the reduced productions on TGF-β1 and MCP-1 can leaded to improved pathological damages.Citation14
PNS is the active gradients extracted from TCM herb panxa notoginseng, it contains various of chemical components, including notoginseng saponin R1 and ginseng saponin Rg1, Rb1, Re, Rd, and so on. Although so far no report on the relationship between fibrosis and PNS treatment published, a cell assay demonstrated that PNS can significantly inhibit the proliferation in vascular smooth muscle cells by inhibiting the activation of extracellular regulated protein kinases (ERK)-signaling pathway.Citation24 In our study, we managed to induce morphological and functional damages of peritoneal membrane in rats similar to those observed in patients with long-term PD by daily exposure of PDF. H and E and Masson's trichrome analysis showed typical peritoneal damages in standard PDF group, including the presence of vascularization and fibroblast, and increased thickness. Above-mentioned facts indicated that the rat model that we established was solid and can be employed to study the peritoneal fibrosis. The pathological damages in standard PDF group also involved ECM deposition and collagenous fiber synthesis. Combined all damages together, that eventually lead to peritoneal fibrosis and ultrafiltration failure. Although there was no study reported regarding the protective effect against PD induced by PDF, however, an animal experiment demonstrated that PNS was capable of inhibiting the oral submucous fibrosis induced by areca nut extract in mice. Moreover, this study also observed the significant reduction in both TGF-β1 and CTGF among the subjects that have been applied with PNS treatment.Citation25 Previous cell assay has demonstrated that an active ingredient in PNS, namely ginseng saponin Rg1, can inhibit the expression of P-ERK1/2 in rat renal tubular epithelial cells induced by TGF-β1,Citation26 which is an important signal pathway in cell proliferation. Similarly, investigators also observed the antifibrotic effect of PNS in rat hepatic fibrosis model induced by carbon tetrachloride, along with reduction in TGF-β1 reduction among PNS treatment group.Citation27 Unlike the specific and acute toxicity of carbon tetrachloride rendered to liver cell, the fibrotic effect of PDF in peritoneum tissue can be chronic. However, above-mentioned evidence suggested that PNS can provide protective effect against fibrosis in different tissues induced by different chemical toxin and also can suppress the expression of TGF-β1.
In our study, TGF-β1 and other two cytokines were induced by peritoneal damages and were significantly higher when compared with saline group. Statistical analysis showed that the ultrafiltration improvement and TFG-β1 and CTGF reduction in PNS group when compared with standard PDF group, along with reduction in tissue thickness. Those findings suggested that PNS can inhibit the expression of TFG-β1 and CTGF and consequently improve the peritoneal function under the regular exposure of PDF in rat model. MCP-1 is mainly involved in the pathogenesis of inflammation and fibrotic diseases, including renal fibrosis,Citation28 cardiac fibrosisCitation29 and diabetic nephropathyCitation30. MCP-1 has also been revealed to have a functional role in the PD-related EMT and ECM synthesis, and which is partly mediated by TGF-β1. Similarly, our study also suggested that regular PNS treatment can significantly downregulate the expression of MCP-1 in rats, with the improved of ultrafiltration and ameliorated pathological damages by histological and TEM analysis.
In summary, we conducted an animal study attempted to investigate the relationship between PNS treatment and peritoneal fibrosis with a relatively large sample size. Multiple histological techniques, including transmission electron microscopy, were applied to observe the pathological changes and concentrations of multiple cytokines may involve in the process of fibrosis were determined by ELISA. Evidence suggested that PNS can significantly inhibit the expression of TGF-β1, CTGF, and MCP-1 in the peritoneum of rats. Furthermore, pathological damages and peritoneal function were ameliorated by the regular PNS treatment. Therefore, PNS is capable of improving peritoneal function in subjects with PDF exposure and can possibly applied in patients with PD after further verification.
Declaration of interest
The authors have no conflict of interest to declare. And they are grateful to the Scientific Research Program (No. 3502Z20104027) of Xiamen Bureau of Science and Technology for supporting this research.
References
- Marshall MR, Walker RC, Polkinghorne KR, Lynn KL. Survival on home dialysis in New Zealand. PLoS One. 2014;9(5):e96847
- Lee H, Manns B, Taub K, et al. Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis. 2002;40(3):611–622
- Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23(3):533–544
- Krediet RT. Peritoneal physiology-impact on solute and fluid clearance. Adv Ren Replace Ther. 2000;7(4):271–279
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358
- Yao HW, Xie QM, Chen JQ, et al. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76(1):29–37
- Willis BC, duBois RM, Borok Z. Epithelial origin of myofibroblasts during fibrosis in the lung. Proc Am Thorac Soc. 2006;3(4):377–382
- Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172
- Lai KN, Lai KB, Lam CW, et al. Changes of cytokine profiles during peritonitis in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;35(4):644–652
- Margetts PJ, Kolb M, Galt T, et al. Gene transfer of transforming growth factor-beta1 to the rat peritoneum: effects on membrane function. J Am Soc Nephrol. 2001;12(10):2029–2039
- Loureiro J, Aguilera A, Selgas R, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol. 2011;22(9):1682–1695
- Leung JC, Chan LY, Tam KY, et al. Regulation of CCN2/CTGF and related cytokines in cultured peritoneal cells under conditions simulating peritoneal dialysis. Nephrol Dial Transplant. 2009;24(2):458–469
- Mizutani M, Ito Y, Mizuno M, et al. Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am J Physiol Renal Physiol. 2010;298(3):F721–F733
- Li Z, Zhang L, He W, et al. Astragalus membranaceus inhibits peritoneal fibrosis via monocyte chemoattractant protein (MCP)-1 and the transforming growth factor-β1 (TGF-β1) pathway in rats submitted to peritoneal dialysis. Int J Mol Sci. 2014;15(7):12959–12971
- Ko KI, Park KS, Lee MJ, et al. Increased dialysate MCP-1 is associated with cardiovascular mortality in peritoneal dialysis patients: A prospective observational study. Am J Nephrol. 2014;40(4):291–299
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng). J Pharm Pharmacol. 2006;58(8):1007–1019
- Zhang JH, Wang JP, Wang HJ. Clinical study on effect of total panax notoginseng saponins on immune related inner environment imbalance in rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2007;27(7):589–592
- Liu X, Huang Z, Zou X, et al. Panax notoginseng saponins attenuates cisplatin-induced nephrotoxicity via inhibiting the mitochondrial pathway of apoptosis. Int J Clin Exp Pathol. 2014;7(12):8391–8400
- Wu H, Feng YZ, Gu ZL, et al. Effects of PNS on the change of pulmonary pathology and expression of TGF-β1 in rats pulmonary fibrosis. SuZhou Da Xue Xue Bao. 2009;29(1):29–32
- Krediet RT. The peritoneal membrane in chronic peritoneal dialysis. Kidney Int. 1999;55(1):341–356
- Williams JD, Craig KJ, Topley N, et al. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol. 2002;13(2):470–479
- Breborowicz A, Oreopoulos DG. Evidence for the presence of chronic inflammation during peritoneal dialysis: Therapeutic implications. Perit Dial Int. 1997;17 (Suppl 2):S37–S41
- Kang DH, Hong YS, Lim HJ, Choi JH, Han DS, Yoon KI. High glucose solution and spent dialysate stimulate the synthesis of transforming growth factor-beta1 of human peritoneal mesothelial cells: Effect of cytokine costimulation. Perit Dial Int. 1999;19(3):221–230
- Zhang W, Chen G, Deng CQ. Effects and mechanisms of total panax notoginseng saponins on proliferation of vascular smooth muscle cells with plasma pharmacology method. J Pharm Pharmacol. 2012;64(1):139–145
- Dai JP, Chen XX, Zhu DX, et al. Panax notoginseng saponins inhibit areca nut extract-induced oral submucous fibrosis in vitro. J Oral Pathol Med. 2014;43(6):464–470
- Xie XS, Yang M, Liu HC, et al. Ginsenoside Rg1, a major active component isolated from Panax notoginseng, restrains tubular epithelial to myofibroblast transition in vitro. J Ethnopharmacol. 2009;122(1):35–41
- Peng XD, Dai LL, Huang CQ, et al. Relationship between anti-fibrotic effect of Panax notoginseng saponins and serum cytokines in rat hepatic fibrosis. Biochem Biophys Res Commun. 2009;388(1):31–34
- Wada T, Furuichi K, Sakai N, et al. Gene therapy via blockade of monocyte chemoattractant protein-1 for renal fibrosis. J Am Soc Nephrol. 2004;15(4):940–948
- Xu J, Lin SC, Chen J, et al. CCR2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin II-induced cardiac fibrosis. Am J Physiol Heart Circ Physiol. 2011;301(2):H538–H547
- Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69(1):73–80

