Abstract
Purpose The aim of the present study was to investigate oxidative stress and apoptosis in kidney tissues of male Wistar rats that pre- and postnatally exposed to wireless electromagnetic field (EMF) with an internet frequency of 2.45 GHz for a long time.
Methods The study was conducted in three groups of rats which were pre-natal, post-natal. and sham exposed groups. Oxidative stress markers and histological evaluation of kidney tissues were studied.
Results Renal tissue malondialdehyde (MDA) and total oxidant (TOS) levels of pre-natal group were high and total antioxidant (TAS) and superoxide dismutase (SOD) levels were low. Spot urine NAG/creatinine ratio was significantly higher in pre- and post-natal groups (p < 0.001). Tubular injury was detected in most of the specimens in post-natal groups. Immunohistochemical analysis showed low-intensity staining with Bax in cortex, high-intensity staining with Bcl-2 in cortical and medullar areas of pre-natal group (p values, 0.000, 0.002, 0.000, respectively) when compared with sham group. Bcl2/Bax staining intensity ratios of medullar and cortical area was higher in pre-natal group than sham group (p = 0.018, p = 0.011).
Conclusion Based on this study, it is thought that chronic pre- and post-natal period exposure to wireless internet frequency of EMF may cause chronic kidney damages; staying away from EMF source in especially pregnancy and early childhood period may reduce negative effects of exposure on kidney.
Introduction
The rapid increase in the use of wireless communication devices in recent years has been accompanied by a significant amount of research in potential health effects of high exposure to radiofrequency (RF) energy emitted by these devices.Citation1–5 The World Health Organization (WHO) has classified mobile phone radiation as possibly carcinogenic, so additional research into the long-term and heavy use of wireless devices needs to be conducted.Citation6
Children are more willing to use wireless devices than adults. They may also be more vulnerable to toxic effects of wireless electromagnetic field, because of their developing organ systems, the greater absorption of energy in the tissues, and longer lifetime of exposure. In childhood period, the most sensitive periods are fetal, neonatal, and infancy period to toxic agents. Recent researches showed that a significant mortality increase has been found from cancer of kidney and renal pelvis in some occupational categories with a more frequent exposure to magnetic field than the general population. To the best of our knowledge, there are restricted studies about toxic effects of electromagnetic field (EMF) on fetal kidneys.Citation7,Citation8
Previous limited data about harmful effects of different doses of EMF on fetal and post-natal kidney has revealed possible developmental abnormalities like primitive tubules, fetal glomeruli, cystic changes on renal parenchyma; in addition, post-natal exposure caused damage in the glomerulus, dilatation in Bowman’s capsule, tubular degeneration, perivascular edema and inflammatory cell infiltration, increased congestion, thickening in the basal membrane, and severely interrupted connections between tubules.Citation9–11
Widespread uses of 2.45 GHz EMF in industrial, medicinal, technical, and daily lives exist. Pregnant women and young children are constantly exposed to wireless devices. So it is important to clarify the possible harmful effects of 2.45 GHz EMF generating technologies, such as wireless internet. The aim of this study was to investigate the effect of long-time exposure to wireless electromagnetic field (EMF) with a frequency of 2.45 GHz during pre- and post-natal period on renal tissues of male Wistar albino rats, in terms of oxidative stress that promotes production of reactive oxygen species (ROS) and apoptosis markers Bcl-2 and Bax immuno histopathologically.
Materials and methods
The study was approved by the Institutional Review of Animal Research Board of Suleyman Demirel University Faculty of Medicine (Isparta, Turkey) and was conducted in accordance with the institutional guidelines and EC Directive 6/609/EECfor animal experiments (European Council, 2010).
Exposure setup
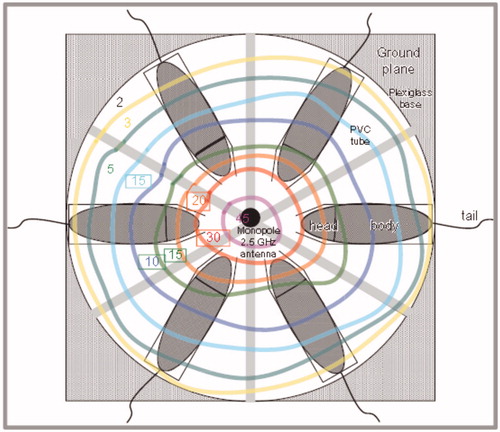
We used six rats in the exposure system in the same time (). A 217 Hz pulse RF source (SET ELECO, Set Elektronik, Istanbul, Turkey) and its monopole antenna were used for the 2450 MHz experimental exposure. Using this equipment, 45.5 V/m electric field intensity could be obtained with 1 W of antenna power in the near-field region. However, antenna output power was limited to 0.8 W to obtain a realistic value of 0.1 W/kg of whole body SAR. During all exposure stages, rats were kept in PVC restrainer tubes which were custom made in three different sizes according to animal size. The setup was designed to apply equal exposure to six animals at the same time (). The exposure procedure was applied for 60 min on consecutive days from 11:00 AM to 12:00 AM until puberty. Animals that reached puberty were sacrificed, and their tissue and blood samples were collected. The exposure process was conducted in an EMF-shielded room so as not to affect the control group. The room was large enough: approximately over 10 lambda according to the IEEE recommendations (IEEE, C.95). The EMF-shielded room was constructed with stainless steel sheets covering the walls in the Experimental Animal Laboratory of Suleyman Demirel University (Isparta, Turkey). Average shielding effectiveness value was 100 dB on the frequency band of 1–6 GHz. Electromagnetic measurements were conducted inside the room by colleagues from the Department of Electronics and Communication Engineering of Suleyman Demirel University. The repetition time and frequency were monitored using a spectrum analyser (Promax, MC-877C, Barcelona, Spain). Unwanted reflection and noise were monitored using an RF portable survey system (HI-4417, Holaday, Eden Prairie, MN). All subjects in the sham-exposed group were kept in the rat restrainers for 1 h per day to equalize stress among the groups. The finite-difference time-domain (FDTD) method was used to evaluate RF energy absorbed in the biological tissues.Citation12,13 SAR values were calculated using the FDTD method and MATLAB software (MATLAB 5.0, Mathworks, Natick, MA).Citation13,14 Whole body average SAR can be tuned to a value of 0.1 W/kg (exactly 0.143 W/kg). The exposure setup was designed to maintain the same distance from the monopole antenna in six rats at one time. Here, whole body exposure of all rats was the same because the antenna was omnidirectional, and the full physical symmetry produced equal exposure for all rats. Equifield circles around the central antenna showed the average electric field intensity values measured. At 2.45 GHz, the dielectric permittivity r”, conductivity, and specific mass of rat tissues were determined from tables in the literature.Citation15–17
Animal selection
Twelve 3-month-old female Wistar albino rats were mated with 12 male rats at the beginning of the study. Each rat was placed in a cage overnight with a male rat, and the first 24 h period following the mating procedure was designated as day 0 of pregnancy. All pregnant female rats were placed in one cage and randomly distributed among three groups: pre-natal, post-natal, and sham exposed (control). The ‘‘pre-natal group’’ was exposed to EMF in utero (mother rats were exposed to a 2.45 MHz EMF (0.1 W/kg, 1 h/day) during the time of pregnancy and then from 18 days of age (2.45 MHz, 0.1 W/kg, 1 h/day) till 12th week. The ‘‘post-natal group’’ was exposed to EMF (2.45 MHz, 0.1 W/kg, 1 h/day) from 18 days of age till 12th week. The third group was sham exposed. The mother rats of ‘‘post-natal’’ and ‘‘sham’’ groups were also sham-exposed. As the pups remained with their dams through the suckling period and could not be placed in separate cages, pre-natal and post-natal groups were exposed to EMF after 18 days of suckling. This time was also proper for gender assignment. At 18 days of age, eight male rats from each group (two from each rat) were randomly selected and isolated. The male pups from the pre-natal and post-natal groups continued to be exposed to 2.45 GHz EMF, SAR 0.1 W/kg for 1 h/day. Male pups selected from the sham exposed were sham-exposed until 12th week. All pups were fed breast milk of the dams, which were fed standard food during the first 21 days of the pups’ lives. After weaning, rats in all groups were continually fed with standard food. All animals were fed ad libitum and subjected to normal daylight cycles (08.00 AM to 08.00 PM) under normal room temperature (21–22 °C). During experiments, all animals were provided the same feeding and watering regime. Groups were kept under the same environmental conditions [humidity (55–60%), temperature, light intensity, and EMF]. The sham exposure and exposure groups were separated in the same room by a custom-made stainless steel shield. All animals were sacrificed under anesthesia at the end of 12th week. The rat kidneys were removed immediately for biochemical and histological examination. Before scarification, spot urine samples from all rats were obtained by spontaneous micturition.
Biochemical analysis
Kidney tissues were weighed and homogenized in an ice-cold phosphate buffer (50 mM, pH 7.4, at 1:5 ratio).
Mechanical and Teflon homogenizers (at 18–20 beats per sample) were used, and then homogenization of the tissues was completed with sonication for 1 min with HD 2070 Sonopuls Ultrasonic homogenizer (Bandelin Electronic, Berlin, Germany). The homogenate was centrifuged at 10,000 rpm for 10 min at 4 °C (Centrifuge 5415-R, Eppendorf, Hamburg, Germany). After obtaining the supernatant fractions, an aliquot was taken for protein determination. The remainder of the supernatant was portioned into five Eppendorf tubes and frozen at −80 °C until assay.
Determination of lipid peroxidation levels
Lipid peroxidation (LPO) (as malondialdehyde, MDA) levels in serum samples were measured using the thiobarbituric acid reaction method of Draper and Hadley.Citation18 Quantification of thiobarbituric acid reactive substances was determined at 532 nm by comparing the absorption to the standard curve of MDA equivalents generated by acid-catalyzed hydrolysis of 1,1,3,3-tetramethoxypropane. Values of MDA were expressed as nmol/mL.
Determination of SOD activity
Superoxide dismutase activity determination: total (Cu—Zn and Mn) SOD (EC1.15.1.1) activity was determined according to the method of Woolliams et al.Citation19 The test is based on the inhibition of nitroblue tetrazolium (NBT) reduction by the xanthine–xanthine oxidase system as a superoxide generator. Activity was assessed in the ethanol phase of the supernatant after 1.0 mL ethanol/chloroform mixture (5/3, v/v) was added to the same volume of sample and centrifuged at 4000g. One unit of SOD was defined as the enzyme amount causing 50% inhibition in the NBT reduction rate. SOD activity was also expressed as units per gram of hemoglobin in erythrocyte samples.
Determination of GPx activity
Glutathione peroxidase (GPx) (EC 1.6.4.2) activity was measured by the method of Paglia and Valentine.Citation20 The enzyme reaction in the tube, which contains NADPH, reduced glutathione, sodium azide, and glutathione reductase, was initiated by addition of hydrogen peroxide, and the change in absorbance at 340 nm was monitored by a spectrophotometer. Activity was given as units per gram of hemoglobin in erythrocyte samples and all samples were assayed in duplicate.
Determination of TOS levels
Rel Assay, a novel automated colorimetric kit, which was developed by ErelCitation21 was used for determination of TOS of tissue samples. Principle of assay is described as follows: oxidants present in the sample oxidize the ferrous ion-chelator complex to ferric ion. The oxidation reaction is prolonged by enhancer molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of mM hydrogen peroxide equivalent per g liter (mmol H2O2 Equiv/L, mmol H2O2 Equiv/mg protein).
Determination of TAS level
Rel Assay, a novel automated colorimetric kit, developed by ErelCitation22 was used for the determination of total antioxidant status of tissue samples. Principle of assay is described as follows: antioxidants in the sample reduce dark blue-green colored ABST radical to colorless reduced ABST form. The change of absorbance at 660 nm is related with the total antioxidant level of the sample. The assay is calibrated with a stable antioxidant standard solution traditionally named as Trolox Equivalent, which is a vitamin E analog. Levels were expressed as mmol Trolox Eq/mg protein. TOS and TAS procedure was applied to Olympus AU2700 autoanalyzer system, so the automated measurement was performed at Olympus AU2700.
Determination of urinary excretion of NAG
Spot urine NAG level was measured according to the method of Yakata et al.Citation23 at 580 nm as 3-cresol sulphonphthalein released from 3-cresol sulphonphthaleinyl b-d-glucosaminide. Creatinine level in the urine was measured using standard spectrophotometric methods (autoanalyzer, Abbott Aeroset, Chicago, IL) in the hospital clinical laboratory. Urinary NAG excretion was expressed as U/g urinary creatinine to rule out the influence of urinary dilution or concentration. In addition, the final value of microalbuminuria was represented by the ratio of albumin measured in urine versus creatinine in urine (lg/mg creatinine).
Histopathological evaluation
Staining with hematoxylin–eosin
After sacrifice, kidney tissues of the animals were taken and fixed in 10% neutral formalin. The tissues were embedded in paraffin blocks. Four-5 μm of dissections were taken from the paraffin blocks, and stained with hematoxylin–eosin. After the staining procedure, all dissections were examined under light microscope (Nikon ellipse CX-41 Research Microscope, Europe & Africa).
Immunohistochemical studies
Dissections of 4-5 μm thickness were taken from the kidney tissues in paraffin blocks and placed on slides coated with poly-l-lysine. They were kept in xylene for 20 min for deparaffinization process and kept in absolute alcohol for 20 min and rinsed with distilled water. The dissections were heated at 98 °C for 20 min within citrate tampon in P1 module (Labvision, Freemont, CA) equipment. After the process, it was cooled at room temperature for 20 min in citrate tampon and rinsed with distilled water. Hydrogen peroxide was dropped onto the dissections and they were incubated for 20 min, and rinsed with “phosphate buffer saline (PBS)” solution (pH: 7.6). Ultra V blocking was made and they were incubated for 5 min. They were incubated in 1/100 dilution for 60 min at room temperature for Bax monoclonal that is adaptable with the rats (sc-748 – Santa Cruz, CA) and Bcl-2 monoclonal (sc-783 – Santa Cruz, CA), and rinsed with PBS. As a secondary antibody, Biotinylated Goat Anti-Polyvalent (TP-125-BN Labvision, Freemont, CA) was dropped. Strepavidin Peroxydase (TS-125-HR Labvision, Freemont, CA) was dropped and they were incubated for 20 min and rinsed with PBS. DAB Chromogen was made. They were incubated for 5-15 min and rinsed with distilled water. Contrast staining was made with Mayer’s hematoxylin for 15 s to 1 min and rinsed with distilled water. They were rinsed with alcohol and air dried and placed in xylene. Later on, the closing process was carried out by using entelen. The dissections were evaluated with Nikon Ellipse CX-41 research microscope. Intensity of the staining was taken as the basis in the evaluation of the immunohistochemical staining. Intensity of the cortical medullar and glomerular cytoplasmic immune staining was scored semi-quantitatively from 0 to +2. They were classified as 0: negative, 1: weakly positive, and 2: strongly positive.
Evaluation of immunohistochemical staining in digital setting
The dissections were reevaluated in the computer setting by “Image processing and analysis in java” (Image J, US National Institute of Health, Bethesda, MD) method. It is used to process microscopic, macroscopic, or radiological images obtained in especially the medical field. The dissections of 300 dpi image resolution were transferred to the computer setting by a scanner and red, blue, and green staining scale intensity in the cortical and medullar fields was measured automatically by Image J Program. The numerical values of the image color scale range between 0 and 255. 0 represents samples that are stained darker and 255 represents the most lightly stained ones. We used Bcl2/Bax staining intensity ratio for determining the rate of apoptosis. The increase in apoptotic rate was associated with a decrease in the Bcl/Bax ratio.
Statistical analysis
Data were presented as means ± SD. A “Software Package System” computer program (SPSS 15.0, SPSS Inc., Chicago, IL) was used for statistical analysis. A one-way ANOVA test was applied to data to detect significant differences initially. At the second step, Tukey’s post-hoc test was used to compare the groups. For non-numerical data comparisons chi-square test was used. Differences were considered significant at p < 0.05.
Results
Biochemical findings
There was no significant difference in all groups in terms of birth and post-natal 12th week weights (p > 0.05). Biochemical results are shown in . Malondialdehyde level in the group that was applied EMF in pre-natal period was found to be significantly higher than it was in the post-natal and sham exposed groups (p = 0.025, p = 0.04). Kidney tissue SOD level in the pre-natal group was significantly lower than that of the sham exposed group (p = 0.017). GSH-Px levels of all groups were not statistically different (p > 0.05). Kidney tissue TAS value in the pre-natal group was lower in comparison to the sham exposed group (p = 0.043). Kidney tissue TOS value of the pre-natal group was higher than those of the sham exposed and post-natal group (p = 0.046, p = 0.049, respectively). There was no significant difference found between the sham exposed and post-natal group (p > 0.05). Spot urine NAG/creatinine ratio of the pre-natal group was found to be significantly higher than those of the sham and post-natal groups (p < 0.001, p < 0.05, respectively). Moreover, spot urine NAG/creatinine ratio of the post-natal group was significantly higher than that of the sham exposed group (p < 0.05).
Table 1. Biochemical results of kidney homogenates and urinary samples (mean ± SE).
Histopathological findings
When the hematoxylin–eosin stained preparations were examined, there were mild tubular degeneration and tubular cylinders and casts in the prenatal group (). Histopathological findings were normal in the sham exposed and post-natal group. Kidney tissue dissections received from each group were stained with monoclonal Bcl-2 and Bax immunohistochemically. There was no difference in terms of Bcl-2 and Bax staining intensity in the three groups when they were evaluated under microscope (p > 0.05, p > 0.05) ( and ). Immunohistochemical results according to Image J analysis are shown in . Bcl-2 immunohistochemical staining intensity was higher in the cortical and medullary areas in the pre-natal group in comparison to the sham exposed group (p = 0.002, p = 0.000, respectively). Post-natal group cortical area Bcl-2 immunohistochemical staining intensity is also higher than that of the sham exposed group (p = 0.002). Bax immunohistochemical staining intensity in the cortical area were lower in the prenatal and postnatal groups in comparison to the sham exposed (p < 0.001, p = 0.007, respectively). Bcl-2/Bax staining intensity ratio in the pre-natal group was found to be higher in the medullar and cortical area in comparison to the sham exposed group (p = 0.018, p = 0.011, respectively).
Figure 3. Photomicrographs of theBcl-2 immunohistochemical stained kidneys from (a) control, (b) pre-natal, and (c) post-natal group.
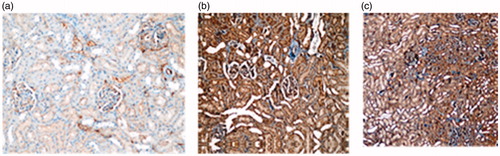
Figure 4. Photomicrographs of the Bax immunohistochemical stained kidneys from (a) sham exposed and (b) pre-natal group.
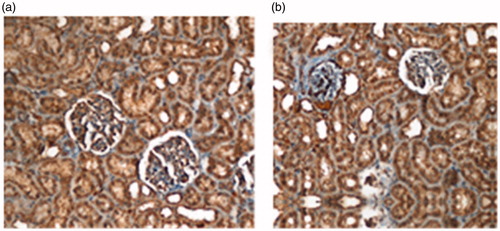
Table 2. Immunohistochemical results of image J analyze program (mean ± SE).
Discussion
There are some concerns and assumptions related with wireless (2.45 GHz) electromagnetic fields (EMFs) and their possible adverse effects on pregnant and children. In our daily lives, it is nearly impossible to unexpose wireless technology. Because of those reasons, we used 2.45 GHz EMF exposure set up for evaluating the possible harmful effects of wireless EMF on in utero and post-natal kidneys. Age-related dose and risk determination of EMF’s is reviewed and electrical conductivity and transmissions of the tissues in young rats was found higher than those of the adults.Citation24 Thinner skin and skull, and large head compared to body in children when matched with adults led to higher SAR values after exposure to EMF. Conil et al.Citation25 reported mean resonance frequency as 60 MHz in adults, while 80, 100 and 120 MHz, respectively, in 12, 8 and 5 years old models. Children may be more sensitive to EMF exposure than adults; however, all the side effects of EMF and protection principles have not been fully clarified today.
Studies examining the effects of EMF on kidney tissue revealed different results. These different results may be due to various factors, such as different application methods, durations, cumulative concentration of exposure, and gender. It should be taken into consideration that response to EMF may vary between individuals and species. Increase in MDA and GSH-Px levels in kidney tissue was found in Sprague-Dawley male rats after exposure to 50 Hz EMF.Citation26 Rat kidney tissue GSH-Px levels decreased after 60 Hz EMF exposure. 900 MHz EMF exposure led to decrease in SOD, GSH-Px and catalase levels, and increase in MDA and NO levels in kidney tissue and in addition, increase in urine NAG levels were also noted in two different studies.Citation27,Citation28 In our study, we found higher levels of TOS and MDA, lower levels of TAS and SOD levels in kidney tissue after exposure to 2.45 GHz EMF prenatally. Post-natal exposure caused higher TOS levels and lower TAS and SOD levels in kidney tissues, but differences were not statistically significant. Effects of 900 MHz EMF exposure were studied in a recent study. They showed that the antioxidative defense system enzymes, catalase, and SOD, were decreased in the pre-natal 900 MHz exposure group. They found that exposure to 900 MHz EMF during the pre-natal period caused oxidative stress in the developing rat embryos and it persisted after birth. Our results were similar with previous studies that oxidative stress negatively affects 2.45 GHz EMF exposed rat kidneys.Citation11
The experimental study that investigated action of electromagnetic waves (84 mW/cm2 and 395 mW/cm2) on functional-morphological features of healthy kidney of female rats showed a marked disturbance of intra-renal circulation. This involves disorders of hemodynamic stability, stromal edema, dystrophy of tubular epithelium, and periglomerular sclerosis causing interstitial inflammation.Citation29 Tubular involvement was also demonstrated with two different studies by showing high levels of urine NAG levels.Citation27,Citation28 Bedir et al.Citation9 found mild pathological changes as tubular dilatation, vacuolization, epithelial desquamation, degenerations of cells, and Bowman’s capsule dilatation in kidneys of prenatally 900 MHz exposed rats. A recent study that investigated the harmful effects of 900 MHz EMF is showed glomerular dilatation and damage in tubules.Citation10 They also showed basal membrane thickening, capillary endothelial irregularity upon examining the specimens under the electron microscope.
Pre-natal exposure to 900 MHz EMF caused tubular and glomerular developmental abnormalities under the evaluation with transmission electron microscopy, which was shown in a previous study.Citation11 In our study, both pre-natal and post-natal exposure to EMF at 2.45 GHz resulted in high levels of 24-hour urinary NAG in rats. In addition, examination of sections stained with hematoxylin and eosin showed that pre-natal EMF exposure caused tubular damage.
Electromagnetic field exposure of pregnant women and possible adverse effects of EMF in intrauterine life received significant concerns. Few studies were published in literature related with the effects of electromagnetic fields on fetuses.Citation29–31 It may be due to difficulty of planning and performing of fetal studies. It is found that extremely low-frequency EMF (50 Hz) exposure for 2 weeks in preimplantation stage caused damaging effects on embryo development by decreasing the number of blastocysts and increasing the blastocysts DNA fragmentation.Citation29 In some other studies they found no teratogen effect on embryos.Citation30,Citation31
Great levels of oxidative stress in cells may lead to oxidation of macromolecules and have been implicated in DNA mutations, aging, and cell death. Mitochondria-associated oxygen radical productions play an important role in the release of pro-apoptotic proteins, which can trigger caspase activation and apoptosis.Citation32 There is a close relationship between oxidative stress and apoptosis.Citation33–37 The tight balance between apoptotic and anti-apoptotic factors is very important for embryological development of urinary system, kidney diseases, and malignancies.Citation38–44 The imbalance between natural antioxidant molecules and oxidant molecules are predisposing factors for acute and chronic kidney damage.Citation45 Low levels of inflammatory reactions may cause chronic kidney damage. TNF-α, C-reactive protein, adhesion molecules, and IL6 are principal responsible molecules for kidney damage.Citation46
The most sensitive part of kidney to oxidative stress is tubular cells. The number of apoptotic cells rapidly increased by the time of acute kidney injury, and DNA fragmentations occurred. The depletion of Guanosine-triphosphate, stimulates apoptosis through p53 molecule. The p53 molecule is transferred from cytosol to mitochondria and it induces some anti-apoptotic (Bcl-2, Bcl-xL, Mcl-1) and pro-apoptotic proteins (Bax, Bak, Bad). Bax is the main molecule that damages mitochondrial membrane. Bax is induced by Bid, Bim and p53 molecules. Membrane permeability increases, and cytochrome c and caspases are activated. Transition of excess calcium into the cells lead to apoptosis. Therefore, tubular necrosis develops.Citation47 Necrosis and apoptosis coexist in ischemic renal injury. TNF activates apoptosis through outer pathway by nuclear factor-B (NF-kB) activation, enhanced leukocyte migration, and the expression of cytokines and adhesion molecules occur.Citation48 TNF-α is the common member of both external and internal apoptotic pathways. Tumor necrosis factor activates Bid and it also triggers caspase-dependent apoptosis.Citation49
The balance between cell proliferation and cell death is impaired after electromagnetic field exposure. During the first hours of static magnetic field exposure, number of apoptotic cells significantly increase at the advanced hours, and increase in antiapoptotic factors limits apoptosis, and a balance between proliferating and apoptotic cells is established. At the end, proliferating cells are dominated. EMF-associated DNA damage is also related with apoptosis. In an experimental study, it is shown that extremely low frequency EMF exposure causes double strand DNA damages, and increased expression of Bcl2 mRNA.Citation50
A previous report indicated connections between exposures to 50–60 Hz electromagnetic fields and abnormalities in the early stages of chicken embryonic development. Their results showed electromagnetic field exposures have toxic effects on brain embryonic cells by increasing the number of apoptotic cells.Citation51 We found no significant difference in the intensity of immunohistochemical staining among three groups when we examined under the microscope. Nevertheless, the examination of specimens with Image J software program revealed that prenatal exposure caused significant increment in Bcl 2 staining in medullary and cortical areas, and low-intensity staining of Bax in cortical regions. Medullary and cortical Bcl2/Bax staining intensity ratio significantly increased in pre-natal group. There was no difference between post-natal and sham exposure groups. Those findings revealed that only microscopic observation is not enough to come in conclusion that there is no difference between groups. More detailed examinations may be needed for more informative results.
In conclusion, pre-natal electromagnetic field exposure may cause induction of apoptosis in the early periods, then long-term compensation is tried to be established with an increase in anti-apoptotic factors. Secondly, intrauterine exposure of electromagnetic fields may be a serious risk factor for chronic renal diseases and proliferative destructive diseases.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahlbom A, Day N, Feychting M, et al. A pooled analysis of magnetic fields and childhood leukemia. Brit J Cancer. 2000;83:692–698.
- Anderson V, Rowley J. Measurements of skin surface temperature during mobile phone use. Bioelectromagnetics. 2007;28:159–162.
- Adair ER, Mylacraine KS, Cobb BL. Human exposure to 2.45 GHz CW energy at levels outside the IEEE C95.1 standard does not increase core temperature. Bioelectromagnetics. 2001;22:429–439.
- Flyckt VM, Raaymakers BW, Kroeze H, Lagendijk JJ. Calculation of SAR and temperature rise in a high-resolution vascularized model of the human eye and orbit when exposed to a dipole antenna at 900, 1500 and 1800 MHz. Phys Med Biol. 2007;52:2691–2701.
- Adey WR. ELF Magnetic fields and promotion of cancer: Experimental studies. Interaction of low-level electromagnetic fields in living systems. 1992:23–47.
- Röösli M, Frei P, Mohler E, Hug K. Systematic review on the health effects of exposure to radiofrequency electromagnetic fields from mobile phone base stations. Bull World Health Organ. 2010;88:887–896.
- Robert E. Intrauterine effects of electromagnetic fields (low frequency, mid-frequency RF, and microwave): Review of epidemiologic studies. Teratology. 1999;59:292–298.
- Ulubay M, Yahyazadeh A, Deniz OG, et al. Effects of prenatal 900 MHz electromagnetic field exposures on the histology of rat kidney. Int J Radiat Biol. 2015;91(1):35–41.
- Bedir R, Tumkaya L, Şehitoğlu İ, Kalkan Y, Yilmaz A, Şahin OZ. The effect of exposure of rats during prenatal period to radiation spreading from mobile phones on renal development. Ren Fail. 2015;37(2):305–309.
- Koca O, Gökçe AM, Öztürk MI, Ercan F, Yurdakul N, Karaman MI. Effects of intensive cell phone (Philips Genic 900) use on the rat kidney tissue. Urol J. 2013;10(2):886–891.
- Odacı E, Ünal D, Mercantepe T, et al. Pathological effects of prenatal exposure to a 900 MHz electromagnetic field on the 21-day-old male rat kidney. Biotech Histochem. 2015;90(2):93–101.
- Gajsek P, Ziriax JM, Hurt WD, Walters TJ, Mason PA. Predicted SAR in Sprague-Dawley rat as a function of permittivity values. Bioelectromagnetics. 2001;22(6):384–400.
- Gajsek P, Walters TJ, Hurt WD, Ziriax JM, Nelson PA. Empirical validation of SAR values predicted by FDTD modeling. Bioelectromagnetics. 2002;23(1):37–48.
- Taflove A, Hagness SC. Computational Electrodynamics: The FDTD Method. The FDTD Method. Boston, London: Artech House; 2000.
- Sangun O, Dundar B, Darici H, Comlekci S, Doguc DK, Celik S. The effects of long-term exposure to a 2450 MHz electromagnetic field on growth and pubertal development in female Wistar rats. Electromagn Biol Med. 2015;34:63–71.
- Gabriel C, Gabriel S, Corthout E. The dielectric properties of biological tissues: I. Literature survey. Phys Med Biol. 1996;41:2231–2249.
- Peyman A, Rezazadeh AA, Gabriel C. Changes in the dielectric properties of rat tissue as a function of age at microwave frequencies. Phys Med Biol. 2002;46:1617–1629; Erratum in: Phys Med Biol. 47:2187–2188.
- Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431.
- Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–256.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285.
- Yakata M, Sugita O, Sakai T, Uchiyama K, Wada K. Urinary enzyme determination and its clinical significance. C. Enzyme derived from the kidney tubular epithelium-N-acetyl-beta-D-glucosaminidase. 4. Preclinical evaluation of the urinary NAG activity and changes in renal diseases. Rinsho Byori. 1983;56:90–101.
- Dimbylow P, Findlay R. The effects of body posture, anatomy, age and pregnancy on the calculation of induced current densities at 50 Hz. Radiat Prot Dosimetry. 2010;139:532–538.
- Conil E, Hadjem A, Lacroux F, Wong MF, Wiart J. Variability analysis of SAR from 20 MHz to 2.4 GHz for different adult and child models using finite-difference time-domain. Phys Med Biol. 2008;53:1511–1525.
- Nishimura I, Imai S, Negishi T. Lack of chick embryotoxicity after 20 kHz, 1.1 mT magnetic field exposure. Bioelectromagnetics. 2009;30:573–582.
- Lee HJ, Pack JK, Gimm YM, et al. Teratological evaluation of mouse fetuses exposed to a 20 kHz EMF. Bioelectromagnetics. 2009;30:330–333.
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922 (Review).
- Schultz DR, Harrington Jr WJ. Apoptosis: programmed cell death at a molecular level. Semin Arthritis Rheum. 2003;32:345–369.
- Kukhta VK, Marozkina NV, Sokolchik IG, Bogaturova EV. Molecular mechanisms of apoptosis. Ukr Biokhim Zh. 2003;75:5–9.
- Fischer U, Schulze-Osthoff K. New approaches and therapeutics targeting apoptosis in disease. Pharmacol Rev. 2005;57:187–215.
- Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163.
- Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–929.
- Gupta S. Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life Sci. 2001;69:2957–2964.
- Sanz AB, Santamaría B, Ruiz-Ortega M, Egido J, Ortiz A. Mechanisms of renal apoptosis in health and disease. J Am Soc Nephrol. 2008;19:1634–1642.
- Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004;66:500–506.
- Ortiz A, Justo P, Catalán MP, Sanz AB, Lorz C, Egido J. Apoptotic cell death in renal injury: the rationale for intervention. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:181–192.
- Erkan E, Garcia CD, Patterson LT, et al. Induction of renal tubular cell apoptosis in focal segmental glomerulosclerosis: Roles of proteinuria and Fas-dependent pathways. J Am Soc Nephrol. 2005;16:398–407.
- Bates CM. Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol. 2011;26:1373–1379.
- Xu G, Shi Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 2007;17:759–771.
- Cachofeiro V, Goicochea M, de Vinuesa SG, Oubiña P, Lahera V, Luño J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9.
- Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2009;297:F749–F759.
- Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. Am J Physiol Renal Physiol. 2009;297:F316–F326.
- Campbell MT, Dagher P, Hile KL, et al. Tumor necrosis factor-alpha induces intrinsic apoptotic signaling during renal obstruction through truncated bid activation. J Urol. 2008;180:2694–2700.
- Lai H, Singh NP. Magnetic-field-induced DNA strand breaks in brain cells of the rat. Environ Health Perspect. 2004;112:687–694.
- Garaj-Vrhovac V, Horvat D, Koren Z. The effect of microwave radiation on the cell genome. Mutat Res. 1990;243:87–93.
- Garaj-Vrhovac V, Horvat D, Koren Z. The relationship between colony-forming ability, chromosome aberrations and incidence of micronuclei in V79 Chinese hamster cells exposed to microwave radiation. Mutat Res. 1991;263:143–149.
- Lai H, Singh NP. Acute low-intensity microwave exposure increases DNA single-strand breaks in rat brain cells. Bioelectromagnetics. 1995;16:207–210.
- Nikolova T, Czyz J, Rolletschek A, et al. Electromagnetic fields affect transcript levels of apoptosis-related genes in embryonic stem cell-derived neural progenitor cells. FASEB J. 2005;19:1686–1688.
- Lahijani MS, Bigdeli MR, Kalantary S. Effects of sinusoidal electromagnetic fields on histopathology and structures of brains of preincubated white Leghorn chicken embryos. Electromagn Biol Med. 2011;30:146–157.
- Basile A, Zeppa R, Pasquino N, et al. Exposure to 50 MHz electromagnetic field raises the levels of the anti-apoptotic protein BAG3 in melanoma cells. J Cell Physiol. 2011;226:2901–2907.


![Figure 2. Photomicrographs of the hematoxylin–eosin stained kidneys from (a) control, (b) pre-natal [tubular casts (thick arrow), tubular degeneration (thin arrow)], and (c) post-natal group.](/cms/asset/88b2e41c-4cb9-40dc-9492-7da31d48bab6/irnf_a_1148937_f0002_c.jpg)