Abstract
Background: Gentamicin (GM) is the commonly used antibiotics against Gram-negative infection, but the nephrotoxic potential of drug limit its clinical interest. The aim of this study was to investigate the protective effect of berberine (BER) against GM-induced nephrotoxicity and possible underlying mechanisms. Material and methods: The rats were divided into various group, namely normal, GM-control, GM + BER (10, 20, and 40 mg/kg). Nephrotoxicity was induced by intraperitoneal administration of GM (120 mg/kg) for 7 consecutive days. BER (10, 20, and 40 mg/kg; p.o.) was also administered for the 7 days. Various biochemical, molecular, and histological parameters were assessed in serum and kidney. Results: GM-administration significantly increased (p < 0.001) the serum creatinine and blood urea nitrogen (BUN) as well as renal malonaldehyde (MDA), nitric oxide (NO) along with Kidney Injury Molecule-1 (KIM-1), Neutrophil gelatinase-associated lipocalin (NGAL), and nuclear factor-kappa B (NF-KB) renal mRNA expressions. In addition, GM also significantly decreased (p < 0.001) the renal superoxide dismutase (SOD), reduced glutathione (GSH), B-cell lymphoma 2 (Bcl-2) mRNA expression, and mitochondrial enzymes (NADH dehydrogenase and cytochrome c oxidase) activities. Rats treated with BER (20 and 40 mg/kg; p.o.) significantly and dose-dependently (p < 0.05 and p < 0.01) restore the altered levels of antioxidant, inflammatory, apoptosis, AKI markers as well as depleted mitochondrial enzymes. Histopathological abbreviations were also ameliorated by BER administration. Conclusion: Berberine exerts renoprotective effects through its anti-oxidant, anti-inflammatory, and anti-apoptotic properties.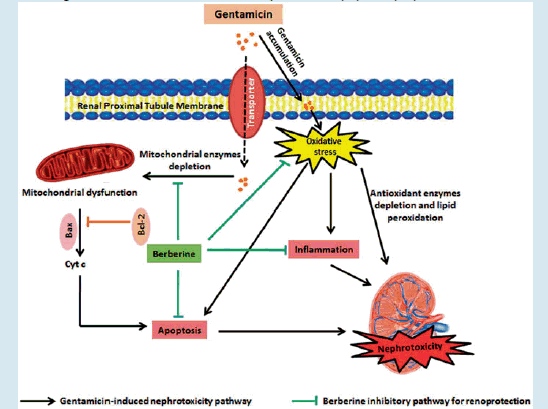
Introduction
Gentamicin (GM) is an aminoglycoside antibiotic commonly used in clinical practice for the treatment of life-threatening Gram-negative bacterial infections. The prescription has been restricted due to serious nephrotoxicity side effect.Citation1 Nevertheless, it has been reported that approximately 30% of patients administered with GM results in renal impairment. Although the exact mechanism underlying GM-induced nephrotoxicity are not completely explicated, renal proximal tubule cells are the primary target site for the aminoglycoside antibiotics where it accumulates and cause nephrotoxicity through specific transporter.Citation2,Citation3 However, several other mechanisms that contribute to the pathogenesis of GM-induced nephrotoxicity include the excess production of reactive oxygen species (ROS) such as superoxide anions, hydroxyl radicles, and hydrogen peroxide,Citation4 reactive nitrogen species (RNS),Citation5 Na+–K+–ATPase inhibition, and inhibition of mitochondrial oxidative phosphorylation.Citation4–6 Accumulating evidence has also suggested that apoptosis, necrosis, and oxidative stress augmentation could be associated with the GM-induced nephrotoxicity.Citation7,Citation8 Thus, a promising therapeutic approach may be required to curb or protect renal damage against antibiotics adverse effects, and GM-induced nephrotoxicity is an excellent animal model for studying pathogenesis and therapeutic approach development.Citation9
In the past few years, much attention has been laid on the role of naturally occurring dietary substances for the control and management of various acute and chronic diseases, one such compound berberine has been used since ancient times for promoting human health. Berberine is an isolated bioactive alkaloid moiety obtained from the Hydrastis canadensis (goldenseal), Berberis aquifolium (Oregon grape), Berberis vulgaris (berberry), and Berberis aristata (tree turmeric).Citation10 Berberine possesses an array of pharmacological activities such as antioxidant, hypolipidemic, hypoglycemic, anti-tumor activity, anti-inflammatory, cardioprotective, and anti-atherogenic.Citation11–14 Recently, results from our laboratory suggested a renoprotective effect of berberine through apoptotic and mitochondrial-dependent pathway modulation in renal ischemia-reperfusion model of rats.Citation15 Similarly, another rodent model also described that berberine treatment improves renal interstitial fibrosis of obstructed kidney.Citation16 In addition, it has also been documented that berberine ameliorates hypertension-induced renal damage in spontaneous hypertensive rats.Citation17 However, the effect of berberine in association with GM-induced nephrotoxicity has been not explored. Hence, this study was designed to evaluate the antioxidative, anti-apoptotic, and anti-inflammatory potential of the berberine against GM-induced nephrotoxicity in rats.
Materials and methods
Drugs and chemicals
Gentamicin and methylprednisolone were obtained from Symed Pharmaceuticals Pvt. Ltd., Hyderabad, India. Berberine (purity: 96%), NADH, cytochrome-C, mannitol and bacterial protease were purchased from Sigma-Aldrich Co., St Louis, MO. 1,1′,3,3′-Tetraethoxypropane, crystalline beef liver catalase, and 5,5′-dithiobis (2-nitrobenzoic acid) were purchased from SD Fine Chemicals, Mumbai, India. Sulfanilamides, naphthylamine diamine HCl, and phosphoric acid were obtained from Loba Chemie Pvt. Ltd., Mumbai, India. Creatinine and BUN kits were purchased from Accurex Biomedical Pvt. Ltd., Mumbai, India. Total RNA Extraction kit and One-step RT-PCR kit was purchased from MP Biomedicals India Private Limited, Mumbai, India.
Experimental animals
Adult male Sprague–Dawley rats (220–250 g) were purchased from the National Institute of Biosciences, Pune (India). They were maintained at 24 °C ± 1 °C with a relative humidity of 45–55% and 12:12 h dark/Light cycle. The animals had free access to standard pellet chow (Pranav Agro-industries Ltd., Sangli, India) and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00 h. The experimental protocol (CPCSEA/PCL/6/2014–2015) was approved by the Institutional Animal Ethics Committee (IAEC) of Poona College of Pharmacy, Pune and performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA), Government of India on animal experimentation.
Experimental design
Rats were randomly divided into following groups (n = 6) as follows.
Group I: Normal (N): Rats were orally treated with distilled water (10 mg/kg, p.o.) and received an intraperitoneal (i.p.) injection of normal saline daily for 7 consecutive days.
Group II: Gentamicin control (GM-control): Rats were orally treated with distilled water (10 mg/kg, p.o.) and an i.p. injection of GM (120 mg/kg, i.p.) daily for 7 consecutive days.
Group III: Gentamicin + berberine (10 mg/kg) [GM + BER (10)]: Rats were treated daily with both berberine (10 mg/kg, p.o.) and GM (120 mg/kg, i.p.) at an interval of 1 h for 7 consecutive days.
Group IV: Gentamicin + berberine (20 mg/kg) [GM + BER (20)]: Rats were treated daily with both berberine (20 mg/kg, p.o.) and GM (120 mg/kg, i.p.) at an interval of 1 h for 7 consecutive days.
Group V: Gentamicin + berberine (40 mg/kg) [GM + BER (40)]: Rats were treated daily with both berberine (40 mg/kg, p.o.) and GM (120 mg/kg, i.p.) at an interval of 1 h for 7 consecutive days.
Group VI: Gentamicin + methylprednisolone (12.5 mg/kg) [GM + MP (12.5)]: Rats were treated daily with both methylprednisolone (12.5 mg/kg, i.p.) and GM (120 mg/kg, i.p.) at an interval of 1 h for 7 consecutive days.
Nephrotoxicity was induced in rats (except normal) by GM at a dose of 120 mg/kg, intraperitoneally for 7 days.Citation18 GM was dissolved in normal saline. Doses of berberine (20 mg/kg and 40 mg/kg) were selected based on the previous study carried out in our laboratory.Citation15 The dose of methylprednisolone (12.5 mg/kg) was selected based on the previous study.Citation19 At the end of the study, whole blood samples were collected from retro-orbital plexus to obtain serum for renal function parameters (creatinine and BUN). Body weights and kidney weights of all animals were recorded, and animals were sacrificed by cervical dislocation. Kidney tissues were harvested, fatty, and conjunctive tissue layer were removed, rinsed in normal saline and stored in −80 °C freezer for further biochemicals and RT-PCR studies. A kidney of rat from each group was isolated and fixed in 10% formalin solution for histopathological examination.
Serum biochemistry
The serum was separated by centrifugation using Eppendorf Cytocentrifuge (model No. 5810, Germany), maintained at 4 °C and run at a speed of 7000 rpm for 15 min. Serum creatinine and BUN were measured by a spectrophotometer (UV–Visible spectrophotometer, V-530, Japan) using reagent kits according to the procedure provided by the manufacturer (Accurex Biomedical Pvt. Ltd., Mumbai, India).
Biochemical estimation
Kidney tissue homogenate preparation, antioxidants, lipid peroxidation (MDA), and NO estimation
A known weight of the kidney tissue homogenates was prepared with 0.1 M Tris–HCl buffer (pH 7.4), and supernatant of homogenates was employed to estimate superoxide dismutase (SOD), reduced glutathione (GSH), lipid peroxidation (MDA), and nitric oxide (NO) as described previously.Citation20–26
Mitochondrial enzymes estimation
Renal mitochondria were isolated and mitochondrial Complex (I–IV) activity was measured spectrophotometrically according to previously described method.Citation27–30
Determination of KIM-1, NGAL, NF-κB, and Bcl-2 by reverse transcriptase-PCR in kidney
The levels of mRNA were analyzed in renal tissue using a reverse transcription (RT)-PCR approach as described previously.Citation20,Citation31–33 Briefly, single-stranded cDNA was synthesized from 5 μg of total cellular RNA using reverse transcriptase (MP Biomedicals India Private Limited, India) as described previously.Citation20 Amplification of β-actin served as a control for sample loading and integrity. The primer sequences for KIM-1, NGAL, NF-kB, Bcl-2, and β-actin were selected according to the previously reported methodCitation15 (Supplementary material). PCR products were detected by electrophoresis on a 1.5% agarose gel containing ethidium bromide. The size of amplicons was confirmed using a 100-bp ladder as a standard size marker. The amplicons were visualized, and images were captured using a gel documentation system (Alpha Innotech Inc., San Leandro, CA). Gene expression was assessed by generating densitometry data for band intensities in different sets of experiments, by analyzing the gel images on the Image J program Version 1.33 (Wayne Rasband, National Institutes of Health, Bethesda, MD) semi-quantitatively. The band intensities were compared with constitutively expressed β-actin. The intensity of mRNAs was standardized against that of the β-actin mRNA from each sample, and the results were expressed as PCR-product/β-actin mRNA ratio.
Histological examination
The dissected kidney tissue specimens were fixed in 10% formaldehyde, processed routinely for embedding in paraffin. Sections were stained with hematoxylin–eosin stain and Masson’s trichrome stain as described previously.Citation34 Kidney sections were analyzed qualitatively under a light microscope (40× and 100×) for various histopathological alterations.
Statistical analysis
Data was expressed as mean ± standard error mean (SEM). Data analysis was performed using Graph Pad Prism 5.0 software (Graph Pad, San Diego, CA). Data was analyzed by one-way analysis of variance (ANOVA), and Dunnett’s tests were applied for post-hoc analysis. A value of p < 0.05 was considered to be statistically significant.
Results
Effect of berberine on GM-induced alterations in relative kidney weight
Gentamicin (GM) administration significantly (p < 0.01) increased the relative kidney weight when compared to normal rats. Co-administration of berberine (20 and 40 mg/kg, p.o.) with GM significantly (p < 0.05) decreased the relative kidney weight compared to GM control rats. Whereas, berberine (10 mg/kg, p.o.) treatment did not show significant changes in relative kidney weight ratio compared to control rats. Administration of methylprednisolone (12.5 mg/kg, p.o.) showed a significant reduction (p < 0.001) in relative kidney weight as compared to GM control rats ().
Table 1. Effect of berberine on GM-induced alterations in relative kidney weight, serum creatinine, BUN, and creatinine clearance in rats.
Effect of berberine on GM-induced alterations in renal function parameters
The levels of serum renal function parameters such as creatinine and BUN were significantly (p < 0.01 and p < 0.001, respectively) increased in GM control rats compared to normal rats. Creatinine clearance was significantly (p < 0.001) decreased in GM treated rats compared to normal rats. Oral administration of berberine (20 and 40 mg/kg) to GM-treated rats significantly and dose-dependently (p < 0.05 and p < 0.01) decreased the serum creatinine and BUN levels compared to GM control rats. However, treatment of berberine (20 and 40 mg/kg, p.o.) significantly (p < 0.01) increased the creatinine clearance compared to GM alone treated rats. In addition, methylprednisolone (12.5 mg/kg, p.o.) significantly (p < 0.001) decreased the serum creatinine and BUN levels whereas it significantly increased (p < 0.001) the creatinine clearance as compared to GM control rats ().
Effect of berberine on GM-induced alterations in oxidative stress
Renal SOD and GSH levels were significantly (p < 0.05 and p < 0.01, respectively) decreased in GM control rats when compared to normal rats. Co-administration of berberine (20 and 40 mg/kg, p.o.) with GM significantly (p < 0.05 and p < 0.01, respectively) increased the renal SOD and GSH levels compared to GM control rats. However, the berberine (10 mg/kg, p.o.) administration failed to produce a significant effect on the renal SOD and GSH levels in GM administered rats. Administration of methylprednisolone (12.5 mg/kg, p.o.) significantly (p < 0.01) increased renal SOD and GSH levels as GM control rats ().
Table 2. Effect of berberine on GM-induced alterations in oxido-nitrosative stress in rats.
Effect of berberine on GM-induced lipid peroxidation and NO alteration
Gentamicin (GM) administration produced a significant (p < 0.01) increase in kidney tissue MDA and NO levels as compared to normal rats. Administration of berberine treatment (40 mg/kg, p.o.) significantly (p < 0.01) restored the levels of kidney tissue MDA and NO compared to GM alone treated rats. Furthermore, administration of berberine (10 and 20 mg/kg, p.o.) did not show a significant effect on kidney tissue MDA and NO levels as compared to GM control rats. However, methylprednisolone (12.5 mg/kg, p.o.) treatment significantly (p < 0.001) decreased renal MDA and NO levels as GM control rats ().
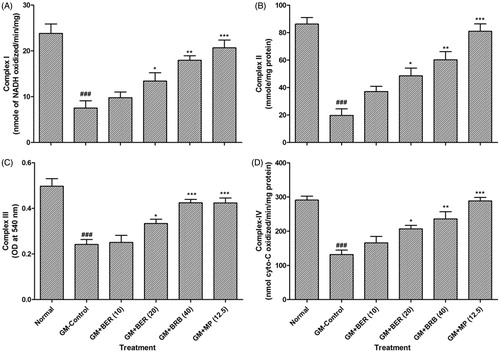
Effect of berberine on GM-induced renal mitochondrial dysfunction
The activities of the mitochondrial complex (I–IV) was significantly (p < 0.001) reduced in renal mitochondria isolated from GM-treated control rats when compared to normal rats. Oral administration of berberine (20 and 40 mg/kg) along with GM significantly and dose-dependently (p < 0.05 and p < 0.01) restored the mitochondrial complex (I–IV) activities as compared to GM control group. Methylprednisolone (12.5 mg/kg, p.o.) treatment also showed significant attenuation (p < 0.001) in GM-induced rats decreased in mitochondrial complex (I–IV) activities when compared with GM control rats ().
Figure 1. Effect of berberine on GM-induced alterations in renal mitochondrial enzyme activities in rats. Data are expressed as mean ± SEM (n = 5) and analyzed by one-way ANOVA followed by post-hoc Dunnett's tests. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with GM-control group and ###p < 0.001 as compared with normal group. BER: berberine; BUN: blood urea nitrogen; GM: gentamicin; MP: methylprednisolone.
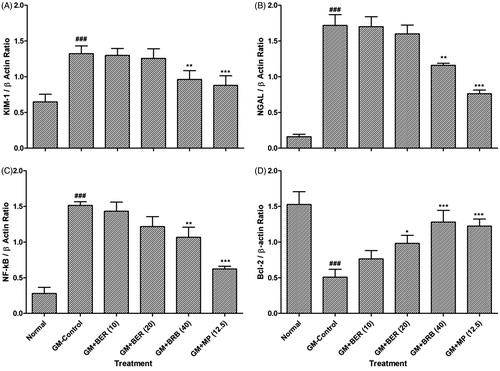
Effect of berberine on GM-induced alteration in renal KIM-1, NGAL, NF-KB, and Bcl-2 mRNA expressions
Gentamicin (GM) administration produced a significant (p < 0.001) upregulation in renal KIM-1, NGAL, and NF-KB mRNA expressions, whereas a significant down-regulation (p < 0.001) in renal Bcl-2 mRNA expression as compared to normal rats. Administration of berberine (40 mg/kg, p.o.) significantly (p < 0.01) down-regulated the levels of renal KIM-1, NGAL, and NF-KB mRNA expressions as compared to GM alone treated rats. Furthermore, administration of berberine (20 and 40 mg/kg, p.o.) showed significant upregulation in renal Bcl-2 mRNA expression as compared to GM control rats. When compared with GM control rats, methylprednisolone (12.5 mg/kg, p.o.) treatment significantly (p < 0.001) inhibited GM-induced alteration in renal KIM-1, NGAL, NF-KB, and Bcl-2 mRNA expressions ().
Figure 2. Effect of berberine on GM-induced alterations in renal KIM-1, NGAL, NF-kB, and Bcl-2 mRNA expression in rats. Data are expressed as mean ± SEM (n = 5) and analyzed by one-way ANOVA followed by post-hoc Dunnett's tests. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with GM-control group and ###p < 0.001 as compared with normal group. BER: berberine; BUN: blood urea nitrogen; GM: gentamicin; MP: methylprednisolone.
Effect of berberine on GM-induced histological alteration in renal tissue
depicted normal architectures (intact glomerulus basement membrane and tubules) of kidney tissue from a normal rat which is devoid of any congestion, necrosis, and inflammatory infiltration. GM administration of showed the intrinsic lesions (grade 4) within the glomeruli and epithelium along with glomerular hypertrophy (grade 4) reflected renal damage. It showed the presence of intracellular edema (grade 3) and inflammatory infiltration (grade 3) (). Kidney tissue from methylprednisolone (12.5 mg/kg)-treated rats showed reduced inflammatory infiltration, intracellular edema and necrosis (grade 1) reflected a reduction in GM-induced renal damage (). Renal tissue from berberine (20 and 40 mg/kg)-treated rats showed the presence of inflammatory infiltration with mild glomerular hypertrophy and necrosis (, respectively) ().
Figure 3. Effect of berberine on GM-induced alterations in kidney histology in rats. Photomicrograph of sections of the kidney of normal (A), GM-control rats (B), methylprednisolone (12.5 mg/kg)-treated rats (C), berberine (20 mg/kg) treated rats (D), and berberine (40 mg/kg) treated rats (E). Necrosis (Yellow arrow), inflammatory infiltration (Green arrow) and increased thickness of basement membrane (Red arrow) H & E staining at 40× and 100 × (inset).
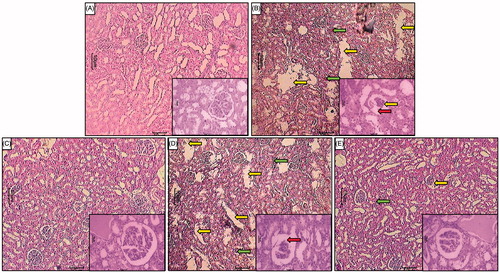
Table 3. Effect of berberine on GM-induced alterations in kidney histology in rats.
Discussion
Gentamicin (GM) is an aminoglycoside antibiotic used against Gram-negative bacterial infections. In the clinical setting, serum creatinine and BUN concentrations are important renal functional biochemical indices and GM-induced notable elevation of serum creatinine and BUN concentration are signs of renal damage and dysfunction.Citation35 Various studies revealed that oxidative stress induced by GM play a major role in the renal structural and functional deterioration by altering biochemical indicators.Citation18 In this study, GM-induced nephrotoxicity was manifested by elevated serum creatinine and BUN levels. Although berberine an antioxidant attenuates the elevated serum creatinine and BUN levels in GM-induced renal toxicity. Protective effect of antioxidants over GM-induced altered renal biochemical indices has been reported.Citation36 In addition, decreased body weight and organ weight was observed in GM administered group compared to the normal group, which was reversed by berberine treatment. Reduction in body weight and organ weight could be due to inhibition of food intake and protein synthesis caused by GM administration.
It is well known that overproduction of ROS due to GM nephrotoxicity alter the oxidant–antioxidant balance and disrupt the membrane lipid composition through lipid peroxidation and subsequently increase the MDA, a final metabolite product of lipid peroxidation.Citation18,Citation37–40 GSH is a non-enzymatic antioxidant and act as a free radical scavenger for the cells protection against the detrimental effect of ROS.Citation41–44 Several lines of studies reported that GM-induced GSH level depletion might be due to excess generation of free radicals or increased consumption in the protection of –SH group-containing proteins and decreased SOD antioxidant enzymes associated with overproduction of superoxide anions and hydrogen peroxide.Citation18,Citation45–47 In consistent with the previous finding, our results showed that GM administration increased MDA level as an end product of lipid peroxidation while decreased GSH and SOD levels in renal tissue.Citation48 The present finding indicated that berberine treatment restore the MDA, GSH and SOD levels in renal tissue of GM-induced toxicity groups via its free radical scavenging and/or increasing antioxidant property.
Nitric oxide (NO) is produced by nitric oxide synthase (NOS) and involve in various pathophysiological processes such as cellular signaling, inflammation, and host defence.Citation49–53 It has been reported that GM-induced oxidative stress results in NO production and its reaction with superoxide radicals generates highly cytotoxic reactive oxygen species, i.e., peroxynitrite, which in turn cause renal failure.Citation54,Citation55 The result of this study shows that GM administration increased the NO levels in renal tissue of rats may be attributed to oxidative stress. Berberine treatment decreased the NO levels and ameliorates the renal impairment caused by the GM-induced oxidative stress.
Mitochondrial dysfunction thought to be a key factor in the pathogenesis of kidney diseases,Citation56 and an important consequence of GM-induced nephrotoxicity, which promotes excess ROS formation from respiratory chain and results in structural and functional alteration.Citation57 In addition, few studies underlined that GM inhibits oxidative phosphorylation and slow down the ATP levels in tubular cells of kidney.Citation58 In this study, GM-induced mitochondrial dysfunction was demonstrated by inhibition of NADH dehydrogenase (complex-I) and cytochrome c oxidase (complex-IV) enzymes activities of the respiratory chain. Indeed, it has been reported that GM administration hampers the mitochondrial function in the kidney through reduction of complex-I and complex-IV enzyme activities.Citation18 Treatment with berberine favorably ameliorates the altered mitochondrial respiratory chain enzymatic activities from cytotoxic effect of GM-induced renal damage by preserving mitochondrial integrity. Besides, many studies have shown the probable protective effect of berberine on the respiratory chain complexes.Citation15
It has been documented that GM-induced nephrotoxicity leads to acute kidney injury (AKI) or acute renal failure (ARF).Citation59 Kidney injury molecule-1 [KIM-1 or T cell immunoglobulin and mucin-1 (TIM-1)] and neutrophil gelatinase-associated lipocalin (NGAL, also known as Lipocalin-2) are promising markers used clinically for the assessment of AKI or ARF.Citation60,Citation61 KIM-1 is a type 1 membrane glycoprotein greatly expressed in proximal tubular cells after nephrotoxic or ischemic insults.Citation60,Citation62 On the other hand, NGAL is a cytosolic protein found in the urine, blood, renal, and proximal-distal tubules in the case of renal ischemia, nephrotoxins, kidney parenchymal damage, and renal transplant denial.Citation63 In line with previous findings,Citation64,Citation65 we also observed significantly increased renal KIM-1 and NGAL mRNA expression in GM-induced nephrotoxicity. Enhanced KIM-1 and NGAL mRNA expression might be associated with regeneration or proliferation of cells in response to toxic abuse.Citation66,Citation67 Previously, it has been shown that berberine might be renoprotective by decreasing KIM-1 and NGAL mRNA expressions.Citation15 Similarly, in this study, we also found the renoprotective effect of berberine through downregulation of KIM-1 and NGAL mRNA expressions in GM-induced nephrotoxicity.
It has been formerly reported that GM-induced renal tubular necrosis encourages inflammatory actions and promotes monocytes and macrophages migration at the site of tissue injury.Citation5 Activation of NF-κB, in response to GM-induced oxidative stress, thought to be a key transcription factor and play a pivotal role in the renal inflammatory events through regulating various gene expressions of cytokines, adhesion molecules, and growth factors.Citation68 Earlier studies have shown that NF-κB activation responsible for the GM-induced inflammation and apoptosis in renal tissue.Citation18,Citation69 In our study, upregulated NF-κB mRNA expression was found in renal tissue after GM treatment. Thus, inhibition of NF-κB activation may be an efficient approach for the management of nephrotoxicity caused by GM. Interestingly, in our study, berberine treatment remarkably attenuated NF-κB activation which further confirms its protective role through anti-inflammatory action against GM-induced renal toxicity. Anti-inflammatory effect of berberine on NF-κB activation corroborate with the previously reported literature.Citation70
Apoptosis is a programmed cell death and a key mechanism in the GM-induced renal injury.Citation71 Thus, apoptosis inhibition is very important for the maintenance of renal integrity and Bcl-2 proteins play a central role in the GM-induced renal apoptosis through its anti-apoptotic action.Citation69,Citation72–74 Bax, a pro-apoptotic protein endorse cytochrome c release from mitochondria in response to toxic stimuli and thereby indulge in apoptosis.Citation75–78 Conversely, Bcl-2 inactivates cytochrome c efflux from mitochondria by blocking Bax activity and suppresses apoptosis process.Citation79,Citation80 Interestingly, data from this study suggest that GM reduced the mRNA expression of Bcl-2 in renal tissue, which was reversed by berberine treatment. In addition, the anti-apoptotic potential of berberine against renal injury is in agreement with the previous findings.Citation81
Conclusion
In conclusion, this study demonstrated that berberine attenuates GM-induced nephrotoxicity. Renoprotective effect of berberine against GM-induced nephrotoxicity could be partially mediated through its antioxidant, anti-inflammatory, and anti-apoptosis action (Graphical abstract). Berberine might be a better therapeutic approach against GM renal toxicity. However, further studies need to be performed to investigate the exact molecular mechanisms and to explore the biological properties that will explain the renoprotective effect of berberine.
Acknowledgements
The authors would like to acknowledge Dr S. S. Kadam, Vice-Chancellor and Dr K. R. Mahadik, Principal, Poona College of Pharmacy, Bharati Vidyapeeth Deemed University, Pune, India, for providing necessary facilities to carry out the study. The authors would also like to acknowledge Dr Pralhad Wangikar, CEO-PRADO, Pune, India, for providing necessary facilities to carry out histological analysis. The authors would also like to acknowledge Invaluesys Research Group to carry out statistical analysis of the study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Salgado C, Hernandes F, Novoa J. Glomerular nephrotoxicity of amino nucleosides. Toxicol Appl Pharmacol. 2007;223:86–98.
- Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011;79:33–45.
- Wedeen RP, Batuman V, Cheeks C, Marquet E, Sobel H. Transport of gentamicin in rat proximal tubule. Lab Invest. 1983;48:212–223.
- Baliga R, Ueda N, Walker PD, Shah SV. Oxidant mechanisms in toxic acute renal failure. Drug Metab Rev. 1999;31:971–997.
- Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–186.
- Weinberg JM, Harding PG, Humes HD. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. II. Effects on mitochondrial monovalent cation transport. Arch Biochem Biophys. 1980;205:232–239.
- Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253.
- Bledsoe G, Crickman S, Mao J, et al. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21:624–633.
- Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL. Patentability of animal models: India and the Globe. Inter J Pharm Bio Arc. 2011;2:1024–1032.
- Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66:227–233.
- Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351.
- Vuddanda PR, Chakraborty S, Singh S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin Invest Drugs. 2010;19:1297–1307.
- Lee S, Lim H-J, Park J-H, Lee K-S, Jang Y, Park H-Y. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–857.
- Holy EW, Akhmedov A, Lüscher TF, Tanner FC. Berberine, a natural lipid-lowering drug, exerts prothrombotic effects on vascular cells. J Mol Cell Cardiol. 2009;46:234–240.
- Visnagri A, Kandhare AD, Bodhankar SL. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail. 2015;37:482–493.
- Wang F-M, Yang Y-j, Ma L-l, Tian X-j, He Y-q. Berberine ameliorates renal interstitial fibrosis induced by unilateral ureteral obstruction in rats. Nephrology. 2014;19:542–551.
- Guo Z, Sun H, Zhang H, Zhang Y. Anti-hypertensive and renoprotective effects of berberine in spontaneously hypertensive rats. Clin Exp Hypertens. 2015;37:332–339.
- Sahu BD, Tatireddy S, Koneru M, et al. Naringin ameliorates gentamicin-induced nephrotoxicity and associated mitochondrial dysfunction, apoptosis and inflammation in rats: Possible mechanism of nephroprotection. Toxicol Appl Pharmacol. 2014;277:8–20.
- Kakalij RM, Alla CP, Kshirsagar RP, Kumar BH, Mutha SS, Diwan PV. Ameliorative effect of Elaeocarpus ganitrus on gentamicin-induced nephrotoxicity in rats. Indian J Pharmacol. 2014;46:298–302.
- Kandhare AD, Shivakumar V, Rajmane A, Ghosh P, Bodhankar SL. Evaluation of the neuroprotective effect of chrysin via modulation of endogenous biomarkers in a rat model of spinal cord injury. J Nat Med. 2014;68:586–603.
- Adil M, Visnagri A, Kumar VS, Kandhare AD, Ghosh P, Bodhankar SL. Protective effect of naringin on sodium arsenite induced testicular toxicity via modulation of biochernical perturbations in experimental rats. Pharmacologia. 2014;5:222–234.
- Badole SL, Chaudhari SM, Jangam GB, Kandhare AD, Bodhankar SL. Cardioprotective activity of Pongamia pinnata in streptozotocin-nicotinamide induced diabetic rats. Biomed Res Int. 2015;2015:403291.
- Ghule AE, Kandhare AD, Jadhav SS, Zanwar AA, Bodhankar SL. Omega-3-fatty acid adds to the protective effect of flax lignan concentrate in pressure overload-induced myocardial hypertrophy in rats via modulation of oxidative stress and apoptosis. Int Immunopharmacol. 2015;28:751–763.
- Honmore V, Kandhare A, Zanwar AA, Rojatkar S, Bodhankar S, Natu A. Artemisia pallens alleviates acetaminophen induced toxicity via modulation of endogenous biomarkers. Pharm Biol. 2015;53:571–581.
- Kamble H, Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of low molecular weight galactomannans from fenugreek seeds on animal models of diabetes mellitus. Biomed Aging Pathol. 2013;3:145–151.
- Kandhare AD, Alam J, Patil MV, Sinha A, Bodhankar SL. Wound healing potential of naringin ointment formulation via regulating the expression of inflammatory, apoptotic and growth mediators in experimental rats. Pharm Biol. 2016;54:419–432.
- Ketkar S, Rathore A, Kandhare AD, et al. Alleviating exercise-induced muscular stress using neat and processed bee pollen: oxidative markers, mitochondrial enzymes, and myostatin expression in rats. Integr Med Res. 2015;4:147–160.
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-kappaB, Muc5ac, TNF-alpha and IL-1beta. Chem Biol Interact. 2015;237:151–165.
- Kandhare AD, Ghosh P, Bodhankar SL. Naringin, a flavanone glycoside, promotes angiogenesis and inhibits endothelial apoptosis through modulation of inflammatory and growth factor expression in diabetic foot ulcer in rats. Chem Biol Interact. 2014;219:101–112.
- Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol. 2014;52:814–828.
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Prophylactic efficacy and possible mechanisms of oligosaccharides based standardized fenugreek seed extract on high-fat diet-induced insulin resistance in C57BL/6 mice. J App Pharma Sci. 2015;5:035–045.
- Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Elucidation of molecular mechanism involved in neuroprotective effect of Coenzyme Q10 in alcohol-induced neuropathic pain. Fundam Clin Pharmacol. 2013;27:603–622.
- Visnagri A, Kandhare AD, Ghosh P, Bodhankar SL. Endothelin receptor blocker bosentan inhibits hypertensive cardiac fibrosis in pressure overload-induced cardiac hypertrophy in rats. Cardiovasc Endocrinol. 2013;2:85–97.
- Kandhare AD, Patil MV, Bodhankar SL. l-Arginine attenuates the ethylene glycol induced urolithiasis in ininephrectomized hypertensive rats: Role of KIM-1, NGAL, and NOs. Ren Fail. 2015;37:709–721.
- Abdelsameea AA, Mohamed AM, Amer MG, Attia SM. Cilostazol attenuates gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2016;68:247–253.
- El-Kashef DH, El-Kenawi AE, Suddek GM, Salem HA. Protective effect of allicin against gentamicin-induced nephrotoxicity in rats. Int Immunopharmacol. 2015;29:679–686.
- Bekheet SH, Awadalla EA, Salman MM, Hassan MK. Prevention of hepatic and renal toxicity with bradykinin potentiating factor (BPF) isolated from Egyptian scorpion venom (Buthus occitanus) in gentamicin treated rats. Tissue Cell. 2013;45:89–94.
- Aswar UM, Kandhare AD, Mohan V, Thakurdesai PA. Anti-allergic effect of intranasal administration of type-A procyanidin polyphenols based standardized extract of cinnamon bark in ovalbumin sensitized BALB/c mice. Phytother Res. 2015;29:423–433.
- Bhilare NV, Dhaneshwar SS, Sinha AJ, Kandhare AD, Bodhankar SL. Novel thioester prodrug of N-acetylcysteine for odor masking and bio availability enhancement. Curr Drug Deliv. 2015;4:4.
- Devkar ST, Kandhare AD, Sloley BD, et al. Evaluation of the bioavailability of major withanolides of Withania somnifera using an in vitro absorption model system. J Adv Pharm Technol Res. 2015;6:159–164.
- Ragab D, Abdallah DM, El-Abhar HS. Cilostazol renoprotective effect: Modulation of PPAR-gamma, NGAL, KIM-1 and IL-18 underlies its novel effect in a model of ischemia-reperfusion. PLoS One. 2014;9:e95313.
- Gosavi TP, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant activity of Argentum metallicum, a homeopathic preparation. Pharm Lett. 2012;4:626–637.
- Goswami S, Kandhare A, Zanwar AA, et al. Oral l-glutamine administration attenuated cutaneous wound healing in Wistar rats. Int Wound J. 2016;13:116–124.
- Honmore VS, Kandhare AD, Kadam PP, et al. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int Immunopharmacol. 2016;33:8–17.
- Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Acute and repeated doses (28 days) oral toxicity study of glycosides based standardized fenugreek seed extract in laboratory mice. Regul Toxicol Pharmacol. 2015;72:323–334.
- Kandhare AD, Bodhankar SL, Singh V, Mohan V, Thakurdesai PA. Anti-asthmatic effects of type-A procyanidine polyphenols from cinnamon bark in ovalbumin-induced airway hyperresponsiveness in laboratory animals. Biomed Aging Pathol. 2013;3:23–30.
- Visnagri A, Kandhare AD, Shiva Kumar V, et al. Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol. 2012;2:157–172.
- Samarghandian S, Azimi-Nezhad M, Mehrad-Majd H, Mirhafez SR. Thymoquinone ameliorates acute renal failure in gentamicin-treated adult male rats. Pharmacology. 2015;96:112–117.
- Erusalimsky JD, Moncada S. Nitric oxide and mitochondrial signaling: From physiology to pathophysiology. Arterioscl Thromb Vasc Biol. 2007;27:2524–2531.
- Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL. Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology. 2012;20:331–341.
- Saraswathi KY, Muthal AP, Kandhare A, Rojatkar S, Bodhankar SL. Study of methanolic extract of Artemisia pallens wall on endurance of laboratory animals. Pharmacologia. 2014;5:298–309.
- Sarkar A, Sengupta A, Mukhrjee A, et al. Antiulcer potential of morin in acetic acid-induced gastric ulcer via modulation of endogenous biomarkers in laboratory animals. Pharmacologia. 2015;6:273–281.
- Kandhare AD, Ghosh P, Ghule AE, Zambare GN, Bodhankar SL. Protective effect of Phyllanthus amarus by modulation of endogenous biomarkers and DNA damage in acetic acid induced ulcerative colitis: Role of phyllanthin and hypophyllanthin. Apollo Med. 2013;10:87–97.
- Christo JS, Rodrigues AM, Mouro MG, et al. Nitric oxide (NO) is associated with gentamicin (GENTA) nephrotoxicity and the renal function recovery after suspension of GENTA treatment in rats. Nitric Oxide. 2011;24:77–83.
- Ali BH, Al Za'abi M, Blunden G, Nemmar A. Experimental gentamicin nephrotoxicity and agents that modify it: A mini-review of recent research. Basic Clin Pharmacol Toxicol. 2011;109:225–232.
- Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2014;306:F367–F378.
- Morales AI, Detaille D, Prieto M, et al. Metformin prevents experimental gentamicin-induced nephropathy by a mitochondria-dependent pathway. Kidney Int. 2010;77:861–869.
- Simmons CF Jr, Bogusky RT, Humes HD. Inhibitory effects of gentamicin on renal mitochondrial oxidative phosphorylation. J Pharmacol Exp Ther. 1980;214:709–715.
- Ferreira L, Quiros Y, Sancho-Martinez SM, et al. Urinary levels of regenerating islet-derived protein III β and gelsolin differentiate gentamicin from cisplatin-induced acute kidney injury in rats. Kidney Int. 2011;79:518–528.
- Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118:1657–1668.
- Mori K, Nakao K. Neutrophil gelatinase-associated lipocalin as the real-time indicator of active kidney damage. Kidney Int. 2007;71:967–970.
- Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244.
- Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115:610–621.
- Luo QH, Chen ML, Sun FJ, et al. KIM-1 and NGAL as biomarkers of nephrotoxicity induced by gentamicin in rats. Mol Cell Biochem. 2014;397:53–60.
- Zhou Y, Vaidya VS, Brown RP, et al. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–170.
- Bolignano D, Donato V, Coppolino G, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am J Kidney Dis. 2008;52:595–605.
- Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485.
- Bae EH, Kim IJ, Joo SY, et al. Renoprotective effects of the direct renin inhibitor aliskiren on gentamicin-induced nephrotoxicity in rats. J Renin Angiotensin Aldosterone Syst. 2014;15:348–361.
- Chen YC, Chen CH, Hsu YH, et al. Leptin reduces gentamicin-induced apoptosis in rat renal tubular cells via the PI3K-Akt signaling pathway. Eur J Pharmacol. 2011;658:213–218.
- Pandey MK, Sung B, Kunnumakkara AB, Sethi G, Chaturvedi MM, Aggarwal BB. Berberine modifies cysteine 179 of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated antiapoptotic gene products, and potentiates apoptosis. Cancer Res. 2008;68:5370–5379.
- Servais H, Jossin Y, Van Bambeke F, Tulkens PM, Mingeot-Leclercq MP. Gentamicin causes apoptosis at low concentrations in renal LLC-PK1 cells subjected to electroporation. Antimicrob Agents Chemother. 2006;50:1213–1221.
- Kandhare AD, Kumar VS, Adil M, Rajmane AR, Ghosh P, Bodhankar SL. Investigation of gastro protective activity of Xanthium strumarium L. by modulation of cellular and biochemical marker. Orient Pharm Exp Med. 2012;12:287–299.
- Kandhare AD, Raygude KS, Ghosh P, Bodhankar SL. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Inter J Green Pharm. 2011;5:236–243.
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia. 2012;83:650–659.
- Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL. Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neurosci Lett. 2012;511:18–22.
- Kumar VS, Rajmane AR, Adil M, Kandhare AD, Ghosh P, Bodhankar SL. Naringin ameliorates acetic acid induced colitis through modulation of endogenous oxido-nitrosative balance and DNA damage in rats. J Biomed Res. 2014;28:132–145.
- Raygude KS, Kandhare AD, Ghosh P, Bodhankar SL. Anticonvulsant effect of fisetin by modulation of endogenous biomarkers. Biomed Prev Nutr. 2012;2:215–222.
- Sarkate AP, Murumkar PR, Lokwani DK, et al. Design of selective TACE inhibitors using molecular docking studies: Synthesis and preliminary evaluation of anti-inflammatory and TACE inhibitory activity. SAR QSAR Environ Res. 2015;26:905–923.
- Kalkan Y, Kapakin KA, Kara A, et al. Protective effect of Panax ginseng against serum biochemical changes and apoptosis in kidney of rats treated with gentamicin sulphate. J Mol Histol. 2012;43:603–613.
- Patil A, Guru A, Mukhrjee A, et al. Elucidation of gastro-protective activity of Morin in pylorus ligation induced gastric ulcer via modulation of oxidative stress. Pharm Lett. 2015;7:131–139.
- Yu W, Sheng M, Xu R, et al. Berberine protects human renal proximal tubular cells from hypoxia/reoxygenation injury via inhibiting endoplasmic reticulum and mitochondrial stress pathways. J Transl Med. 2013;11:24.

