Abstract
Probiotics are non-pathogenic living microorganisms which, when administered in adequate amounts, confer a health benefit on the host. Probiotic microorganisms most often are constituents of special foodstuffs, such as fermented milk products or food supplements. They are also employed as feed additives in livestock breeding and as active components of medical remedies in human and veterinary medicine. In the food industry, probiotics traditionally are lactic acid bacteria (LAB), especially lactobacilli and lactococci, although bifidobacteria and strepcococci (enterococci) are also used. In livestock breeding and medicine, besides lactic acid bacteria other non-pathogenic microorganisms with health-promoting characteristics, e.g. certain strains of yeast (‘Saccharomyces boulardii’) and Escherichia coli (E. coli Nissle 1917), are also applied. This review focuses on the probiotic E. coli strain Nissle 1917 (EcN), its origin and medical history, microbiology, genetics, biological activities, safety, and toxicological aspects. Furthermore, clinical trials performed with this special E. coli strain will be summarized. The EcN strain was detected and isolated by Alfred Nissle in 1917 because of its antagonistic activity against some pathogenic entero-bacteria. With respect to its metabolic capacities, EcN is a typical E. coli strain. It is a non-pathogenic member of the Escherichia coli family, because it does does not carry pathogenic adhesion factors and does not produce any enterotoxins or cytotoxins, it is not invasive, not uropathogenic, and is rapidly killed by non-specific defense factors of blood serum. Besides this, EcN has some special characteristics that distinguish it from other pathogenic and non-pathogenic E. coli strains and are thought to be important for its probiotic activities. Thus, EcN carries so-called genomic islands (GEIs), integrated into its chromosome. On these GEIs, gene clusters coding for several fitness factors are present, e.g. genes for the production of microcins which inhibit the growth of other enterobacteria. Another strain-specific feature of EcN is a special lipopolysaccharide (LPS) in its outer cell membrane, being responsible for the fact that EcN exhibits immunomodulating properties without showing immunotoxic effects. The immunomodulating properties mainly focus on anti-inflammatory activities that are important for the clinical efficacy in remission maintenance of ulcerative colitis. Also of importance is the bacterial–epithelial crosstalk between EcN and the intestinal epithelial cells, leading to a strengthening of the epithelial barrier and the curing of ‘leaky gut’ phenomena. The restoration of a disturbed gut barrier by EcN is thought to be due to the stimulation of epithelial defensin production as well as to a ‘sealing effect’ on the tight junctions of the enterocytes. EcN has also been shown to induce the development of the gut immune system in animal models and human newborns. In addition, it has been found that products of EcN metabolism, probably acetic acid, promote colonic motility that might be helpful for therapeutic application in chronically constipated patients. Randomized controlled clinical trials (RCTs) have shown EcN to be therapeutically effective in rather diverse indications, such as ulcerative colitis, chronic constipation, and acute and protracted diarrhea.
Introduction
Microbial ecology of the gut
The intestinal ecosystem has been described as ‘a precarious alliance among epithelium, immunity and microbiota’ (Citation1) and, under normal conditions, the commensal microorganisms indeed fulfill a wide array of beneficial tasks for the host, including protection from pathogenic microorganisms and modulatory effects on the development and functions of the gastrointestinal tract and the gut immune system (Citation2–7). Important functions of the indigenous intestinal microbiota are given in .
Table I. Important functions of the intestinal microbiota.
A breakdown of the microecological balance in the gut may be caused by different exogenous and endogenous factors and will result in changes of composition, numbers, and activities of indigenous microbial communities. This may be followed by destruction of the physiological barriers of the body, enhanced epithelial irritation, inflammation and permeability, and bad consequences for the health of the host organism (Citation1,Citation3,Citation8–13). Intestinal dysfunctions and diseases that are associated with or caused by disturbances of the indigenous microbiota are summarized in .
Table II. Gastrointestinal diseases associated with disturbances of the intestinal microbiota.
For a long period of time, roughly for the second half of the last century, the use of antibiotics has generally been recommended in human medicine for changing an unwelcome microbial colonization of distinct sites of the body, i.e. in cases of diseases caused by infectious microorganisms. However, the increasing recognition of adverse effects and the development and spread of resistance to a wide range of antibiotics by a growing number of pathogens (Citation14–18) has led to the renaissance of a therapeutic concept from the pre-antibiotic era: the prevention or treatment of specific diseases with living non-pathogenic microorganisms, i.e. probiotics. In recent years our knowledge concerning the regulatory processes within the gut ecosystem in health and disease has grown enormously (Citation1,Citation3–5,Citation7,Citation13,Citation19). The knowledge of particular characteristics and activities of members of the indigenous intestinal microbiota and their interactions with the host is fundamental to the application of living microorganisms as health-promoting or therapeutic agents (Citation12,Citation19–25).
The term ‘probiotics’ – development of definitions
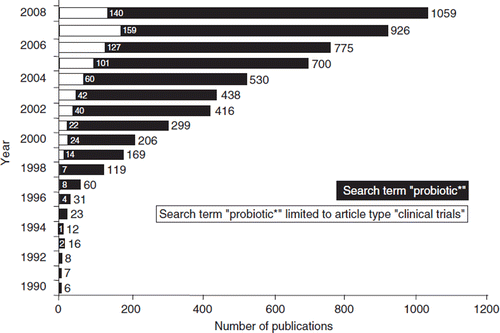
Today, probiotics are defined as non-pathogenic living microorganisms which, when administered in adequate amounts, confer a health benefit on the host. This definition was coined by an expert committee of a joint FAO/WHO meeting on probiotics in 2001 (Citation26). The word ‘probiotic’ has been derived from the Greek language and can roughly be translated into ‘for life’. According to Vergin (1954) (Citation27), in Germany the term ‘Probiotika’ was originally coined by the physician and dietician Werner Kollath shortly after the Second World War, meaning ‘food ingredients with health-promoting characteristics beyond their nutritional value’. Later and independently, the term ‘probiotic’ was introduced into the international literature by Lilly and Stillwell (1965) (Citation28), saying that a probiotic is a microbial substance that is produced by one microorganism and has a growth-promoting effect on another microorganism, thus being the exact opposite of an antibiotic. However, this definition did not gain wide acceptance and failed to survive. Since that time, the meaning of the term ‘probiotic’ has changed considerably. Parker (1974) (Citation29) was the first to use the word ‘probiotic’ in a sense that is very similar to today's use. He adopted the term to describe ‘organisms and substances which contribute to intestinal microbial balance’. This definition associated the use of probiotics with an effect on gut microecology. Since Parker's definition would also include antibiotics (‘substances’), Fuller in 1989 redefined a probiotic as ‘a live microbial feed supplement which beneficially affects the host animal by improving its intestinal microbial balance’ (Citation30). In 1995, a workshop hosted by the Lactic Acid Bacteria Industrial Platform (LABIP) and sponsored by the European Community issued a consensus definition of the term probiotics, saying ‘oral probiotics are living micro-organisms, which upon ingestion in certain numbers, exert health benefits beyond inherent basic nutrition’ (Citation31). Accordingly, probiotics may be either consumed as food components or applied as non-food preparations. The latest definition of the FAO/WHO expert committee (see above) broadened the meaning by omitting the relation to solely oral administration and intestinal effects as well as to nutrition. According to this definition, also microbial preparations for medical use and for extraintestinal, e.g. vaginal, applications can be termed probiotics, provided that they contain living microorganisms and confer a health benefit on the host. For microorganisms with specific therapeutic properties the term ‘biotherapeutic agent’ (instead of probiotic) has been proposed by McFarland and colleagues (Citation32,Citation33). This may be helpful to differentiate between food and feed probiotics and pharmaceutical probiotics, but has not gained broader acceptance in the scientific and medical literature, mainly because this term is close to the terms ‘biologicals’ or ‘biological agents’, which include native and recombinant biological preparations, such as vaccines, immunomodulators, interleukins, growth hormones, specific antibodies, and others (Citation34–37). The growing scientific and medical interest in probiotics is reflected by the steadily increasing number of publications on this topic, as shown in .
Figure 1. The increase in basic scientific and clinical publications on probiotics during the years 1990–2008, indicating the growing interest of the scientific and the medical community in this topic. The black bars (and the corresponding numbers on top of the bars) represent the total numbers of scientific and clinical publications in the respective year. The inserted open bars (and the corresponding numbers) represent the numbers of published clinical trials with probiotics. Source: National Library of Medicine Database MEDLINE; search and graphics performed by M. Schiemann, Herdecke, Germany.
Application of probiotics
Probiotic microorganisms are often found as food ingredients, e.g. in fermented milk products and food supplements, but are also employed as feed additives in livestock breeding and as active components of medical remedies in human and veterinary medicine [19,38–42]. Usually, especially in the food industry, probiotics mostly are lactic acid bacteria (LAB), such as lactobacilli, lactococci, bifidobacteria, and streptococci (enterococci) (Citation43). However, other non-pathogenic microorganisms, e.g. certain yeast strains (‘Saccharomyces boulardii’) and Escherichia coli strains, like E. coli Nissle 1917 (EcN), are also used, not in food processing, but rather in human and veterinary medicine (Citation19,Citation21,Citation33,Citation44–47).
Origin and medical history of E. coli strain Nissle 1917
E. coli strain Nissle 1917 (EcN) is the active component of the pharmaceutical preparation Mutaflor®, a microbial drug licensed for use in human medicine in Germany and some other European countries today. This drug has been traditionally used since 1917 (Citation48,Citation49) to treat various diseases and dysfunctions of the intestinal tract (Citation19,Citation21,Citation47,Citation50–52).
The Mutaflor® story began in the early years of the last century when the physician and bacteriologist Alfred Nissle () of Freiburg, Germany, screened human intestinal E. coli strains for growth-inhibiting (antagonistic) activity against Salmonella, Shigella, and other enteropathogens. Searching for a novel therapeutic approach to combat virulent enter-obacteria, Nissle recognized that some fecal E. coli isolates showed antagonistic action against these pathogens in vitro, while others failed to inhibit their growth. This led Nissle to the idea that these antagonistically active E. coli strains might be useful as therapeutics in diarrheal diseases. Nissle isolated the EcN strain in 1917 during the First World War from the feces of a member of the armed services who took part in the military campaign on the Balkan peninsula. In contrast to his companions, this soldier did not develop infectious diarrhea when staying in the region of Dobrudja, which was heavily contaminated with enteropathogens at that time (Citation49). Nissle assumed that this soldier harbored a ‘strong, antagonistically active’ E. coli strain in his gut that had protected him from infection. This was in fact the case, and Nissle – after performing laboratory tests and some self-experiments with this E. coli isolate – introduced the EcN strain into medical practice. From the beginning, the Mutaflor® preparation contained (and still contains) the Nissle strain in viable form. [Note that, today, E. coli Nissle 1917 (EcN) is deposited as an industrially used strain under ‘patent deposit’ rules at the Deutsche Sammlung von Mikroorganismen und Zellkulturen (German Collection of Microorganisms and Cell Cultures) (DSMZ, Braunschweig) as E. coli DSM 6601.] In the pre-antibiotic era, it was first used as an anti-infective agent against infectious diarrhea and its clinical consequences, later on, non-infectious gastrointestinal disturbances and diseases, such as chronic constipation or inflammatory bowel diseases, have also been treated.
Figure 2. Photographic portrait of Professor Alfred Nissle MD (1874–1965), discoverer of the antagonistic action of certain commensal E. coli strains against enteropathogens. In 1917, Alfred Nissle isolated the specific E. coli strain, now called E. coli Nissle 1917 (EcN), from the feces of a healthy young man (Citation48,Citation49). Photograph courtesy of Alfred-Nissle-Gesellschaft, Hagen, Germany.

Great effort has been made in the past two decades to elucidate in detail the microbiological characteristics and the molecular genetic background of EcN. At the same time its toxicological profile and its pharmacological and immunological modes of action, as well as its clinical efficacy for the treatment of specific intestinal diseases and dysfunctions have been studied extensively.
Microbiological properties and phenotypic strain characteristics
General properties
The E. coli strain Nissle 1917 has been thoroughly analyzed by means of microbiological, biochemical, and molecular genetic methods (Citation53–56). Its basic characteristics are shown in . The strain does not exhibit any virulence factors (see below), but has gene clusters located on genomic islands (GEIs) on its chromosome responsible for the synthesis of several so-called ‘fitness factors’, which contribute to the strain's probiotic nature. Serologically, EcN belongs to the E. coli O6 group and is of serotype O6:K5:H1 (Citation54,Citation55). EcN is a typical gram-negative enterobacterium containing lipopolysaccharide (LPS) as a structural component of its outer cell membrane. The O6 surface antigen represents the outer part of the strain's LPS and exhibits some peculiar features.
Table III. Basic microbiological and molecular genetic characteristics of E. coli strain Nissle 1917 (EcN, serotype O6:K5:H1).
The molecular structure of the LPS of EcN has been completely resolved (Citation55). The LPS of EcN differs in several molecular aspects from all other LPS types of E. coli (Citation57). For instance, the O6 polysaccharide side-chain is very short, consisting of only one single ‘repeating unit’ of the oligosaccharide building block typical of the O6 antigen, giving the strain a so-called ‘semi-rough’ phenotypic appearance when grown on solid nutrient media. The reason for this is a point mutation introducing a stop codon in the gene for the O6 antigen polymerase (Citation55). Also, modifications previously unknown for E. coli LPS were found in the oligosaccharide core segment of the molecule. The specific features of the LPS of EcN are likely to explain the phenomenon whereby the strain exhibits immunomodulating properties without showing immunotoxic effects (Citation55).
As indicated by its serotype (O6:K5:H1), EcN is able to form an extracellular capsule of the K5 sero-type. This kind of capsule is present in only about 1% of E. coli isolates. The gene loci on the chromosomal DNA responsible for the synthesis of the K5 capsule have been detected by using a gene probe specific for the K5 capsule gene cluster (Citation58). In many extraintestinal pathogenic E. coli (ExPEC) strains the existence of a capsule protects the pathogen from attacks by non-specific defense components of blood serum (e.g. complement), thus making the bacterium serum-resistant (Citation59). Serum resistance lengthens the time of survival in the bloodstream and increases the virulence of a pathogen (Citation59). Interestingly, this is not the case with EcN (Citation55). Despite its capability to form a capsule, in the classic serum resistance test (Citation60) the EcN strain is nevertheless serum-sensitive and is rapidly killed in the presence of human serum or sera of other mammalian species (bovine and porcine sera).
EcN possesses flagella of serotype H1 and is thus quite mobile. The possession of flagella enables the microbe to actively move within the viscous intestinal mucus layer, e.g. in the direction of the intestinal mucosa, which serves as an oxygen source, useful for aerobic catabolism of substrates by E. coli. Besides their function as driving apparatus, the flagella are important in bacterial crosstalk with the epithelium (see below) and have also been described as bacterial sensors for humidity (Citation61,Citation62).
EcN possesses three different types of fimbriae: F1A and F1C fimbriae (Citation54,Citation63,Citation64), as well as so-called ‘curli’ fimbriae (Citation56,Citation65,Citation66), which mediate adhesion to intestinal epithelial cells in cell culture experiments or to the mucus layer of the intestinal wall in vivo (see below), facilitating colonization of the gut.
Fitness factors
Pathogenic bacteria are able to produce so-called virulence factors, which enhance the infectious capacity of the respective strains. Virulence factors are often encoded by genes representing special DNA sequences located on the bacterial chromosome, probably obtained by horizontal gene transfer in the evolution of the pathogen (Citation67). These DNA sequences are called pathogenicity islands (PAIs) (Citation68). Non-pathogenic (avirulent) commensal bacteria may also produce specific factors that enable them to compete with other strains in the ecological system of the gut and to effectively communicate with the host organism. These factors are designated fitness factors, which are also often encoded by special DNA sequences (genomic islands, GEIs) on the bacterial chromosome (Citation69,Citation70). Different fitness factors have been detected in the EcN strain in recent years.
Siderophores are iron-chelating substances needed for bacterial iron uptake. EcN produces an unexpectedly wide array of siderophores: aerobactin, enterobactin, salmochelin, and yersiniabactin, as well as a hemin- and a citrate-dependent iron acquisition system. Besides the siderophore ferric iron uptake systems, EcN also possesses an elemental ferrous iron uptake system (EfeU) (Citation71). Regarding the siderophores aerobactin and yersiniabactin as well as the hemin and the citrate system of EcN, the chromosomal DNA of the strain has been checked for the existence of the corresponding genes by using specific DNA probes (Citation72–75). Positive signals for all four iron acquisition systems could be obtained on its genomic DNA.
EcN was detected by Nissle in 1917 because of its growth-inhibiting (antagonistic) activities against enteropathogenic microorganisms. The antagonistic actions of EcN are due, at least in part, to the formation of microcins against which the producer strain itself is immune. One of these microcins (microcin H47) had already been described before for another E. coli strain (Citation76–78), whereas the second microcin was unknown so far. The latter has been termed ‘microcin M’ (M from Mutaflor) by Klaus Hantke and colleagues (Citation79).
In addition to the production of microcins, EcN belongs to that phylogenetic subgroup of E. coli bacteria (group B2) that contains strains able to synthesize peptide/polyketide hybrids (Citation80,Citation81). These substances belong to a heterogeneous group of molecules with antimicrobial and antitumor activities. To date it is not known whether the peptide/polyketide hybrides of EcN contribute to the antagonistic action against other bacteria.
Metabolic properties
EcN has been characterized biochemically by analysis of its strain-specific metabolic capacities and fermentation properties, using commercially available identification kits for Enterobacteriaceae in which a range of biochemical tests are carried out simultaneously. As far as its metabolic capacities are concerned, EcN is a typical E. coli strain, with the exception that it is capable of metabolizing arginine (). This property is seen in just about 7% of all E. coli isolates.
Table IV. Taxonomically important enzymes present in and biochemical reactions performed by E. coli strain Nissle 1917 (EcN); comparison with the corresponding characteristics typical of the enterobacterial species Escherichia coli in general.
Gas chromatographic analysis of spent culture supernatants (Citation82) has revealed that EcN produces short-chain fatty acids as end products of carbohydrate metabolism under aerobic and anaerobic growth conditions as well. These are mainly acetic acid and formic acid, as well as minor amounts of propionic and butyric acid (G. Sollorz and U. Sonnenborn, unpublished results).
Molecular genetic properties
Genome structure
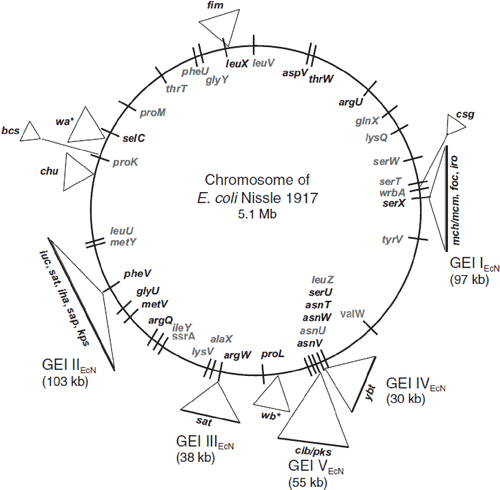
The genome structure of E. coli Nissle 1917 has been elucidated by three different approaches: (Citation1) DNA sequence analysis of the gene loci for tRNA genes as potential integration sites for DNA sequences (GEIs) obtained by horizontal gene transfer during evolution; (Citation2) DNA sequence analysis of the detected genomic islands; and (Citation3) comparison of the EcN genome with those of other E. coli strains by DNA– DNA hybridization (Citation56). PCR-based screening of 324 pathogenic and non-pathogenic E. coli strains of different origin revealed distinct chromosomal regions occurring in non-pathogenic E. coli and in intestinal and extraintestinal pathogenic E. coli as well. In EcN, five large genomic islands and some smaller genomic islets have been detected so far, carrying gene clusters for different known fitness factors of this special E. coli strain (). These insertions into the chromosome are thought to originate from horizontal gene transfer (Citation56). In a dissertation by Schmidt, additional DNA sequences were found on the chromosome of the Nissle strain that also might have been acquired by horizontal gene transfer during the evolution of EcN (Citation83). Comparison of the genome structure of EcN with those of the E. coli K-12 strain MG1655 and the uropathogenic E. coli (UPEC) O6 strains 536 and CFT073 revealed similarities in gene structure and organization between the different E. coli strains, especially between the isolates of sero-type O6. With respect to the genome size of the EcN strain, with 5.1 Mb its genome is larger than that of E. coli K-12 strain MG1655 (4.7 Mb) and UPEC strain 536 (5.0 Mb), but a bit smaller than that of UPEC strain CFT073 (5.23 Mb).
Figure 3. Functional genomic map of E. coli Nissle 1917 (EcN) (Citation56,Citation80). Five large genetic islands (GEI I to GEI V) and some smaller genetic islets coding for different so-called ‘fitness factors’ are inserted at distinct sites (mainly next to tRNA-encoding sequences) into the chromosome of the EcN strain. The bars on the chromosome circle mark the positions of tRNA genes (in gray: tRNA genes with sequence contexts identical to those of the completely sequenced non-pathogenic E. coli K-12 strain MG1655; in black: tRNA genes with sequence contexts identical to the completely sequenced uropathogenic E. coli O6 strain CFT073). The following genes and gene clusters of the EcN strain have been identified and sequenced: fim encoding F1A fimbriae (‘common type-1 fimbriae’); csg encoding curli fimbriae; mch/mcm encoding microcin H47 and microcin M synthesis; foc encoding F1C fimbriae; iro/ybt/iuc encoding the siderophores salmochelin, yersiniabactin, and aerobactin; clb/pks (colibactin gene cluster) encoding non-ribosomal peptide synthetases, polyketide synthases and accessory proteins; sat encoding Sat protease; iha encoding Iha adhesion; sap encoding Sap-like autotransporter; kps encoding capsule synthesis; chu encoding hemin iron uptake system; bcs encoding cellulose biosynthesis; wa*/wb* encoding LPS biosynthesis.
The lack of gene clusters coding for classical pathogenicity traits of ExPEC, e.g. α-hemolysin, P-fimbriae, S-fimbriae, combined with the existence of genes coding for fitness factors (e.g. iron acquisition systems, microcins) and the genetically fixed synthesis of a semi-rough LPS may together be of importance for the probiotic nature of EcN (Citation84). First results of the annotation of DNA sequences obtained after shotgun sequencing of the whole EcN genome have been published, focusing on genes representing the metabolic capacities of the Nissle strain (Citation85) and on the search for genes coding for suspected pathogenicity or fitness traits (Citation83).
Plasmid characterization
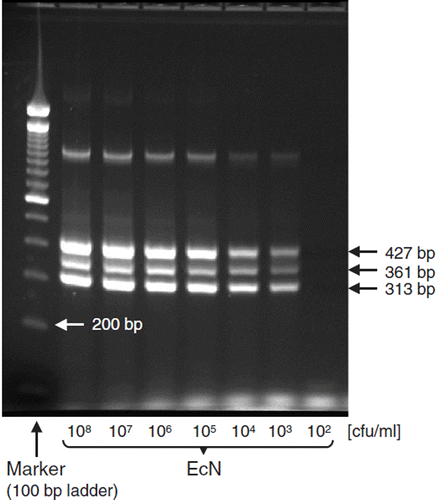
EcN possesses two small cryptic plasmids, termed pMUT1 and pMUT2 (Citation53). Both plasmids, with molecular masses of Mr = 2.1 and 3.7 Md (megadaltons), respectively, are genetically stable and are not transferable to other E. coli strains. The smaller plasmid (pMUT1) consists of 3173 bp (base pairs), the larger one (pMUT2) consists of 5526 bp. The circular DNAs of these two strain-specific plasmids have been completely sequenced (Citation53). Strain-specific DNA sequences from both plasmids, which have not been found so far in other E. coli strains, can be used in a multiplex PCR method developed to specifically identify the EcN strain (Citation53) (). The function of these cryptic plasmids in EcN is not known as yet, since plasmid-free clones of EcN do not show morphological, cultural, or metabolic differences compared to the original strain carrying pMUT1 and pMUT2. There is, however, some evidence that cryptic plasmids per se may protect the carrier bacterium from attacks by mobile genetic elements such as bacteriophages, thus having an influence on the genetic stability of the strain (Citation86).
Figure 4. Strain-specific multiplex PCR for identification of E. coli Nissle 1917 (EcN). PCR and agarose gel electrophoresis were performed as described (Citation53). The PCR yields three distinct DNA products of Mr 313, 361, and 427 bp length. Using this test system, the detection limit of EcN is between 102 and 103 bacterial cells/ml. Photograph: K. Eiteljörge, Herdecke.
Modes of action
Antagonistic activities against other microorganisms
E. coli Nissle 1917 has been tested for its antagonistic activities (antimicrobial effects) against other microorganisms, in particular against microbial pathogens, both in vitro and in vivo. In vitro, antagonistic effects of EcN have been determined by means of co-cultivation with different enteropathogenic, uropathogenic, and other E. coli strains, as well as with individual strains of the species Proteus vulgaris, Salmonella enteritidis, Shigella dysenteriae, Yersinia enterocolitica, Vibrio cholerae, and Candida albicans (Citation87,Citation88). Co-cultivation experiments with EcN and the respective test strain have been performed either in suitable liquid media, where the number of viable bacteria of each strain has been determined after aerobic growth at 37°C, or on solid media by means of an agar diffusion test (similar to the classic test for antibiotic sensitivity), where EcN produced an inhibition zone against the test strain (in vitro tests according to Halbert (Citation89) and Mayr-Harting et al. (Citation90), ). Co-cultivation of EcN with Shiga toxin-producing E. coli (STEC) in liquid cultures resulted in a dose-dependent decrease of Shiga toxin levels (Citation87).
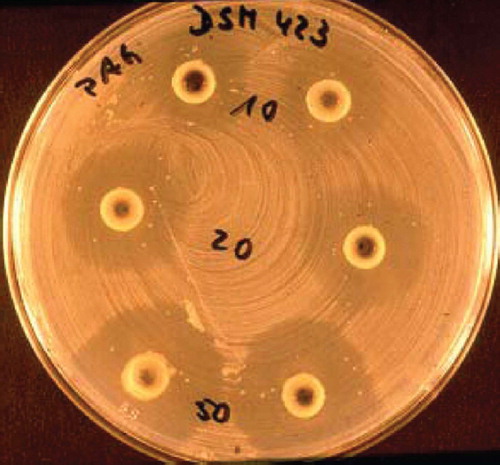
Figure 5. Determination of antagonistic activity of E. coli Nissle 1917 (EcN) against another E. coli strain (DSM 423) in vitro. A suspension of E. coli DSM 423 was homogeneously spread across the surface of an agar plate (PAG minimal medium). Small cylinders of agar were punched out using a punching tool and the resulting cavities were filled with 10, 20 or 50 μl of an overnight culture of EcN (2 × 109 cfu/ml). Tests were performed in duplicate. The picture was taken after 24 h of aerobic incubation at 37°C. Antagonistic activity becomes visible by the development of inhibition zones (halos) around the cavities containing the EcN suspension. Photograph: U. Sonnenborn, Herdecke.
The antagonistic action of EcN was also confirmed in vivo by means of co-association of EcN with other microorganisms (pathogenic and non-pathogenic E. coli, Salmonella typhimurium, Candida albicans, Lactobacillus johnsonii) in germ-free or gnotobiotic animals (rats, mice, piglets) (Citation91–94) (). Germ-free animals have been inoculated per os either with EcN and the particular pathogenic strain simultaneously, or the test strain has been administered first and EcN several days later. The excrements of the animals have been regularly analyzed microbiologically, whereby the viable cell counts of the administered bacteria have been determined. In gnotobiotic animal models, a decrease or even complete eradication of the pathogenic test strains from the intestines has been achieved by oral administration of EcN, and symptoms of disease occurring due to inoculation with the pathogenic microorganisms disappeared (Citation92–94). In conventional streptomycin-treated CD-1 mice, precolonization of the intestine with EcN limited the growth of E. coli EDL933, an O157:H7 Shiga toxin-producing enterohemorrhagic E. coli (EHEC) strain, when the latter was subsequently administered (Citation95). By contrast, the E. coli K-12 laboratory strain did not inhibit growth of the EHEC strain.
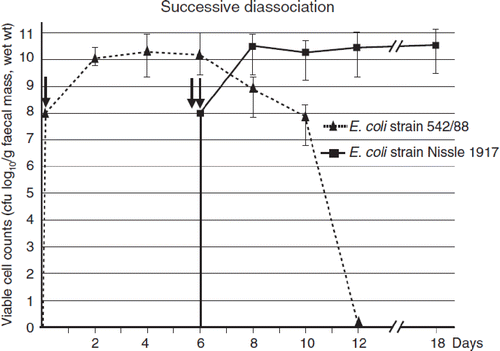
Figure 6. Antagonistic action of E. coli Nissle 1917 (EcN) against enteropathogenic E. coli 542/88 in vivo in gnotobiotic piglets (Citation94). Four 7-day-old germ-free piglets were infected orally with 108 cfu of E. coli 542/88 (arrow). The enteropathogenic E. coli strain quickly colonized the gut (dotted black line) and reached stable bacterial counts (about 1010 cfu/g contents). Shortly after infection the animals showed signs of diarrheal illness. At day 6 of the experiment, when the piglets were visibly ill, they received an EcN suspension p.o. (2 × 108 cfu) (double arrow). Although the pathogenic E. coli strain had already settled in the gut, EcN also colonized the intestine fairly well (straight black curve). Six days later, the pathogen was completely expelled from the gut, whereas EcN continuously showed a high population level, and the piglets recovered.
The inhibitory effects of EcN on facultative and obligate pathogens have also been demonstrated in humans by preventive oral administration of the strain to neonates (colonization prophylaxis) (Citation96,Citation97). The stools of the neonates were analyzed microbio-logically to confirm colonization with EcN. In addition, the effect of colonization with this E. coli strain on the spectrum of other aerobic gut bacteria has been determined. All newborns that received EcN were colonized by the administered strain, most of them showing long-term colonization for several months. The establishment of unwanted microorganisms, i.e. facultative and obligate pathogens, in the children's gut was significantly reduced by intentional colonization with EcN (, see also next section).
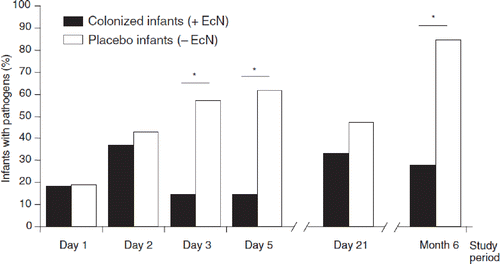
Figure 7. Antagonistic activity of E. coli Nissle 1917 (EcN) in humans, as shown by the effect of preventive administration of EcN on the colonization of the newborn's gut with true and potential microbial pathogens (Citation96). Using a double-blind study design, 54 full-term newborns were randomly assigned to two treatment groups and received orally either 1 ml of an EcN suspension (108 cfu, black bars) or 1 ml of a placebo suspension (open bars) once a day for the first 5 days of life. During the hospital stay (on days 1, 2, 3, and 5) and thereafter (on day 21 and during the 6th month), colonization of the gut with true and potential microbial pathogens was determined in fecal samples and is presented as the percentage of children carrying pathogenic and potentially pathogenic microorganisms. Regarding the colonization with pathogens, differences between the EcN group and the placebo group were recognized first on day 2 and were significant on day 3 (15% vs 57%, p < 0.003), day 5 (15% vs 62%, p < 0.001), and after 6 months (28% vs 85%, p < 0.002). On day 21, the difference amounted to 33% vs 47%, but this was not significant. *Significantly different colonization between EcN and placebo groups.
In patients with ulcerative colitis in remission, mucosal biopsies have been obtained during sigmoidoscopies, both before and after a 6-week oral treatment with EcN. The samples were analyzed for their content of mucosa-associated aerobic and anaerobic intestinal bacteria using classic microbiological cultural methods and quantitative PCR. Treatment with EcN resulted in a significant decrease of bacterial counts of aerobic and anaerobic mucosa-associated bacteria in a majority of the patients (Citation98).
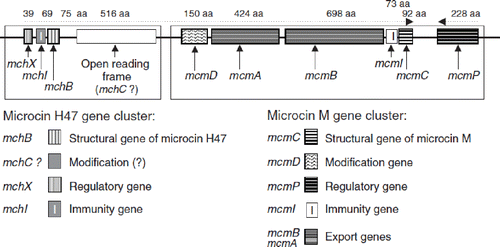
As stated above, the antagonistic actions of EcN are mainly due to the formation of microcins (micro-cin H47, microcin M). The genetic organization of the two chromosomally localized gene clusters responsible for synthesis, transport, and secretion of both microcins discovered to date in EcN as well as for the immunity of the strain against these antimicrobial substances, has been examined in detail (Citation79). The DNA sequences of the microcin genes have been determined (Citation79) (). Like colicin V, both microcins of EcN are secreted with the help of a protein export machinery of the RND type containing the membrane protein TolC.
Figure 8. Structural organization of the chromosomal gene clusters located on GEI I, encoding the components necessary for synthesis, export, and regulation of microcins H47 and M of E. coli Nissle 1917 (EcN) (Citation79). The number of amino acids (aa) of the individual gene products of the corresponding genes are given on top of the figure. The microcin M gene cluster (‘M’ from Mutaflor) was discovered for the first time in the EcN strain.
Inhibition of the invasion of epithelial cells by enteroinvasive bacteria
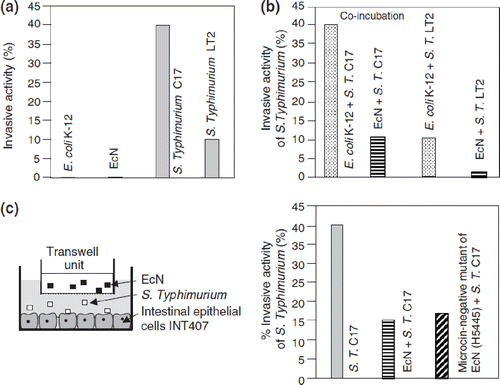
In cell culture experiments using the human embryonic intestinal cell line INT407, EcN inhibits the invasion of epithelial cells by different pathogenic invasive bacteria, such as Salmonella, Shigella, entero-invasive E. coli, Listeria, Yersinia, and Legionella strains (Citation99,Citation100), as well as adherent-invasive E. coli strains from patients with Crohn's disease (Citation101). Interestingly, this inhibitory effect is not due to the production of microcins, since the isogenic, microcin-negative EcN mutant H5445 in this test system exhibits nearly the same inhibitory efficacy as the original strain (). In addition, it has been shown that no physical contact is necessary for the anti-invasive effect, neither between EcN and the epithelial cells nor between EcN and the invasive bacterial pathogens. Moreover, the anti-invasive activity of EcN is not plasmid-coded and is not dependent on the presence of adhesins (F1A fimbriae, F1C fimbriae), since isogenic, Fim- and Foc-negative deletion mutants of EcN have been shown to be as effective as the original strain. With the help of a so-called ‘transwell’ filter system introduced into the cell culture, by which EcN can be separated from both the epithelial cells and the invasive bacteria, it has been demonstrated that the invasion-inhibiting principle is diffusible and is secreted by EcN (Citation99,Citation100). It might be that this soluble factor inhibits the manipulation of the host cell cytoskeleton by the pathogenic microorganisms.
Figure 9. Inhibition of Salmonella typhimurium (S.T.) invasion into INT407 intestinal epithelial cells by E. coli Nissle 1917 (EcN) (Citation99,Citation100). (a) Cell cultures with INT407 cells were separately incubated with either E. coli K-12 or EcN, or with the S. typhimurium strains C17 or LT2. While both non-pathogenic E. coli strains were also non-invasive, the Salmonella typhimurium strains showed differently strong invasive activity. (b) Co-incubation of INT407 cells with E. coli K-12 and S. typhimurium C17, EcN and S. typhimurium C17, E. coli K-12 and S. typhimurium LT2, and EcN and S. typhimurium LT2. While co-incubation with E. coli K-12 had no effect on the invasive activity of the S. typhimurium strains, co-incubation with EcN markedly inhibited the invasive activity of both S. typhimurium strains. (c) As shown by cell culture experiments using a transwell system (left), the anti-invasive activity of EcN is not due to direct interaction between EcN and S. typhimurium nor to occupation of receptor sites by EcN at the epithelial surface, since the effect was still present after separating EcN from S. typhimurium and from the intestinal cells as well (right).
Immunomodulatory and anti-inflammatory effects
In vitro and in vivo experiments have demonstrated EcN to possess remarkable immunomodulatory activities, which address both the innate and the adaptive immune system. With respect to adaptive immunity, EcN affects the humoral as well as the cellular branch of the immune system. However, in vivo significant immunomodulating activities are only reliably detected in gnotobiotic experimental animals, in human newborns, or in diseased animals and patients. In healthy human adults and animals, the immunomodulating activities of EcN are only very weakly expressed or not detectable at all (Citation102,Citation103) (R. Gruber, unpublished results).
In vitro experiments. A significant dose-dependent improvement of secretory performance of mouse macrophages after direct contact with formalin-killed cells of EcN has been observed in vitro (Citation104). The production of interleukin-6 (IL-6), tumor necrosis factor (TNF), and oxygen radicals increased significantly. Further experiments revealed an augmentation of tumor cell lysis and parasite lysis (leishmaniae) by macrophages prestimulated with EcN. On the other hand, the efficiency of phagocytosis of the macrophages was raised only slightly. However, these in vitro effects could only partially be confirmed in vivo, following oral administration of EcN to conventionally kept mice. In vivo, for instance, no induction of TNF synthesis has been observed.
Experiments with epithelial cell lines and isolated lymphocytes have shown EcN to exhibit different immunomodulating activities, and these strongly depend on the cell types/cell lines employed and the parameters and read-out systems used (Citation105–115). One example is the dose-dependent inhibition of the TNF-α-induced IL-8 secretion by EcN in HCT-15 intestinal epithelial cells (Citation108,Citation116). The inhibition is reflected by the reduced expression level of IL-8 mRNA in the epithelial cells, as shown by real-time PCR. The inhibition of IL-8 synthesis is caused by secreted soluble factors that have not been identified so far. Interestingly, the EcN-mediated inhibition of TNF-α-induced IL-8 transcription is independent of the AP-1- and nuclear factor κB (NF-κB)-regulated gene expression. The latter, however, is important for the induction of human β-defensin-2 (HBD-2) synthesis in gut epithelial cells (Caco-2 cells) by EcN (Citation114).
In the mouse monocyte/macrophage cell line J774A.1, addition of EcN resulted in an increase of IL-12, TNF-α, and IL-10 secretion (Citation105). By contrast, IL-18 and TGF-β synthesis were not affected. Compared with the effects of viable EcN cells, addition of killed bacterial cells resulted in a much less pronounced induction of cytokine synthesis. In confluent Caco-2 epithelial cells, contact with EcN resulted in an up-regulation of gene expression of monocyte chemoattractant protein-I ligand 2 (MCP-I) and an increase of MCP-I secretion (Citation113). MCP-I is thought to be involved in the inflammatory immune reaction. On the other hand, Otte et al. (Citation117) found that contact of intestinal epithelial cells (Colo320 and SW480 cells) with EcN led to inhibition of the inflammatory response by down-regulation of TNF-α- or gastrin-induced cyclooxygenase-2 (COX-2) activity. This was followed by reduced secretion of proinflammatory prostaglandin E2 (PGE2).
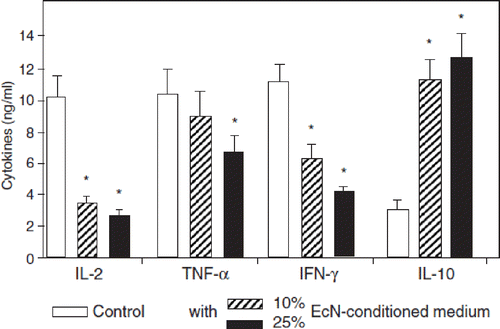
The immunomodulating action of EcN on peripheral as well as on gut mucosal lamina propria T lymphocytes has also been studied in vitro (Citation112). Anti-CD3-stimulated peripheral and lamina propria T cells have been exposed to heat-inactivated cells of EcN and to EcN-conditioned medium (spent culture supernatants), and the effects of these treatments on lymphocyte cell cycle and on markers of apoptosis have been examined on the molecular level. EcN-conditioned medium dose-dependently inhibited cell cycle progression and T-cell expansion of lymphocytes from peripheral blood, but not of lymphocytes from the gut mucosa. This selective action on T cells from peripheral blood could also be shown by using bacterial lipoproteins. In contrast to this, heat-inactivated cells of EcN, its purified LPS, or CpG-DNA had no effect. In peripheral T cells, addition of EcN-conditioned medium reduced expression of cyclin D2, B1, and retinoblastoma protein, which are involved in T-cell proliferation. Interaction of EcN-conditioned medium with peripheral T cells significantly inhibited the expression of IL-2, TNF-α, and IFN-γ, whereas the production of IL-10 was enhanced (). The differential effects of EcN on circulating and intestinal T cells is supposed to be an important aspect of its efficacy in inflammatory bowel diseases (Citation112,Citation118). By down-regulation of the expansion of newly recruited T cells into the gut mucosa, intestinal inflammation will be limited. Compared with this, activated T cells already residing in the gut wall are not influenced by EcN and thus are available for the continuous fight against pathogenic microorganisms (Citation112). In the T-cell subgroup of γδ T cells, EcN increased activation, cell cycling, and cell expansion, but thereafter induced apoptosis via caspase- and FasL-dependent signaling pathways (Citation119). These effects of EcN were target-specific, since they were only seen with γδ T cells, but not with αβ T cells.
Figure 10. Anti-inflammatory effects of E. coli Nissle 1917 (EcN) on human peripheral blood T cells (PBT) in vitro, as shown by cytokine expression profiles induced by EcN-conditioned medium (EcN-CM) (Citation112). EcN-CM was obtained after 2 h incubation of EcN bacteria in T-cell medium (RPMI with 10% FCS, 1.5% HEPES) at 37°C and 5% CO2. Thereafter, bacteria were removed by centrifugation. The resulting supernatant was sterile-filtered through a filter of 0.22 μm pore size. The sterile-filtered supernatant (EcN-CM) was added to freshly isolated PBT (105 cells) in different concentrations (open bars, controls = no addition of EcN-CM; hatched bars, 10% v/v EcN-CM; black bars, 25% v/v EcN-CM). T cells were then stimulated by adding anti-CD3-mAb and cultured for 3 days in RPMI. Culture supernatants were collected and assayed for cytokines. EcN-CM dose-dependently and significantly inhibited IL-2, TNF-α, and IFN-γ synthesis, while IL-10 production was markedly up-regulated. *p < 0.05 vs CD3 controls.
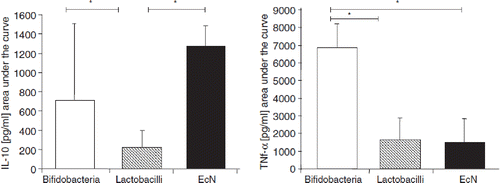
Using human peripheral blood mononuclear cells, Helwig et al. (Citation107) compared the immunomodulating activities of gram-positive Lactobacillus and Bifidobacterium strains with those of the gram-negative EcN strain. These experiments revealed that gram-positive and gram-negative bacteria exerted partly different, but also partly similar immunomod-ulatory activities on the blood mononuclear cells (). With respect to some special immune parameters, different responses were seen also between the bifidobacteria and the lactobacilli, leading to the assumption that the immunomodulatory action of probiotics might be rather strain-specific and not genus- or species-specific. Interestingly, in this test system the EcN strain provoked a high output of the anti-inflammatory signal molecule IL-10. Fink and Frokiaer (Citation106) could show that interaction of different bacteria including EcN resulted in different maturation responses of dendritic cells from different locations of the body (Peyer's patches, mesenteric lymph nodes, and spleen).
Figure 11. Immunomodulatory activities of gram-positive (bifidobacteria, lactobacilli) and gram-negative (E. coli Nissle 1917, EcN) bacteria on human peripheral blood mononuclear cells (PBMNCs) in vitro (Citation107). Supernatant concentrations of the cytokines IL-10 and TNF-α after co-incubation of PBMNCs with cell debris of bacteria from different genera and species are shown. With regard to bifidobacteria (open bars), results are pooled from the data obtained by testing B. breve, B. infantis, and B. longum. With regard to lactobacilli (hatched bars), results are pooled from the data obtained by testing L. acidophilus, L. bulgaricus, L. casei, L. plantarum, and L. rhamnosus strain GG (LGG). Data from experiments with E. coli Nissle 1917 (EcN) are presented by the black bars. Concentrations of IL-10 and TNF-α are shown in pg/ml as area under the curve (AUC, mean ± SE). *Significantly different, p < 0.05.
Recently, in vitro experiments with isolated blood lymphocytes from healthy and allergic persons (Citation111) have shown that the EcN strain induced strong anti-allergic responses. These are interesting new findings that may lead to new indications for EcN in the future. The investigations with mononuclear cells from peripheral blood, obtained from allergic persons, have shown that contact of the lymphocytes with EcN induced a shift from a T-helper-2 (Th2)-dominated, allergy-specific immune response to a Th1-dominated, non-allergic response (Citation111).
In vivo experiments. In young gnotobiotic piglets, colonization with EcN resulted in a rapid development of the gut-associated immune system. This was reflected by a significant increase in the numbers of IgA, IgM, and IgG antibody-producing lymphocytes in the lamina propria of intestinal villi and in the intestinal lymph follicles as well as by an increase in SLA-D+(MHC II+) cells and CD4-positive cells in all mucosal and submucosal tissues, without any signs of an inflammatory reaction (infiltration of granulocytes) (Citation93,Citation120).
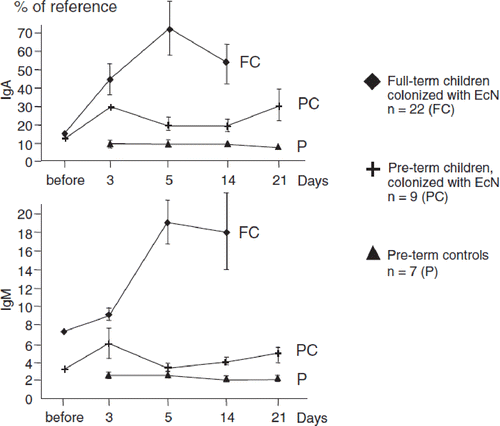
Investigations on human newborns have revealed that both full-term neonates and premature infants show a significant rise in the levels of immunoglobulins (IgA and IgM) in blood serum and of secretory IgA and IgM in stool filtrates within a few days after intentional colonization of the gut with EcN (Citation121) (). On the other hand, there was no influ-ence on the IgG level in blood serum. Besides these immunomodulatory effects on the development of the humoral branch of the immune system, stimulation of cell-mediated immune responses and of non-specific natural immunity after oral administration of EcN has also been detected in a controlled clinical study with preterm infants (Citation122).
Figure 12. Effect of intentional colonization of the gut of newborns by E. coli Nissle 1917 (EcN) on the levels of secretory IgA and IgM (Citation121). One ml of EcN suspension (108 cfu/ml) was administered p.o. once a day for 5 days, starting from the first day of life. IgA and IgM levels against EcN antigen were measured in stool filtrates after colonization of full-term and preterm infants with EcN and compared to the IgA and IgM levels in stool filtrates of non-colonized preterm infants (controls). Antibody levels are expressed as percentage of the reference sample (mean ± SE). In stool filtrates of full-term infants IgA and IgM levels were significantly higher (from day 3 onwards) than the levels detected before colonization (p < 0.05). IgA levels were significantly higher in full-term than in preterm infants on day 5 and day 14 (p < 0.01), and IgM levels were higher on all days after colonization (p < 0.05 ... p < 0.01). Preterm colonized infants had higher fecal IgA and IgM titers than non-colonized preterm infants (controls) on all days after colonization (p < 0.05 ... p < 0.01).
In established animal models of inflammatory bowel diseases (IBD), such as the IL-2 knockout mouse, EcN shows no proinflammatory effects (Citation123,Citation124). On the contrary, using different experimental animal models for IBD (T-lymphocyte transfer model in SCID mice, IL-10 knockout mice, dextran sodium sulfate (DSS)-induced or trinitrobenzenesulfonic acid (TNBS)-induced colitis), anti-inflammatory effects of EcN have been consistently observed (Citation118,Citation125–130). In one model of acute intestinal inflammation, oral administration of EcN to mice pretreated with 2% DSS led to a decrease of the proinflammatory cytokines IFN-γ and IL-6, without showing a significant effect on mucosal inflammation (Citation129). In another model of acute gut inflammation, oral application of EcN to mice pretreated with 1.3% DSS significantly ameliorated body weight loss, disease activity index, as well as macroscopic and microscopic damage of the intestinal mucosa (Citation127). Interestingly, administration of heat-killed EcN as well as of its genomic DNA also showed some anti-inflammatory actions in DSS colitis mice (reduced colon weight, lower histological score), although the effects on the disease activity index (DAI) were not as marked as those achieved by viable bacterial cells (Citation127) (). In IL-10 knockout mice (IL-10−/− mice), an animal model of chronic intestinal inflammation, per os administration of EcN led to a decrease of tissue lesions and a decrease of the infiltrations of the colonic mucosa by mononuclear cells and polymorphonuclear cells, as judged by macroscopic and microscopic examinations (Citation127). Moreover, EcN treatment sig-nificantly reduced the number of mice with rectal prolapse (a characteristic symptom of severe rectal inflammation), lowered the concentrations of the inflammatory marker protein serum amyloid A (SAA) in blood serum, and reduced the expression of proinflammatory cytokines and chemokines in colonic Lamina propria mononuclear cells (LPMCs) of colitis mice (significant for IFN-γ and MIP-2).
Table V. Effects of live and heat-killed E. coli Nissle 1917 (EcN) and of EcN-DNA on DSS-induced colitis in specific pathogen-free C57BL/6 mice.
In the immunologically mediated chronic colitis model of T-lymphocyte transfer in SCID mice, oral application of EcN significantly ameliorated intestinal inflammation, as judged by histologic examinations, and reduced secretion of proinflammatory cytokines IFN-γ, IL-5, and IL-6, without affecting the level of the anti-inflammatory cytokine IL-10 (Citation129) ().
Figure 13. Anti-inflammatory action of E. coli Nissle 1917 (EcN) in immunodeficient C.B.-17 SCID mice with chronic colitis (Citation129). Chronic colitis was induced by adoptive T-cell transfer, using purified CD4+ CD62L+ splenic T lymphocytes from healthy BALB/c mice, which were introduced intraperitoneally into recipient SCID mice. After T-cell transfer, mice were divided into two groups. The first group received 200 μl of EcN suspension (5 × 1010 cfu/ml) by gastric gavage twice per week from week 1 post-transfer to week 8 at the end of the experiment (= T-cell transfer mice + EcN, black bars). The second group of SCID mice was treated in the same way and for the same period of time, but received plain water by gastric gavage instead of EcN (= T-cell transfer mice - EcN, open bars). This second group served as positive control (colitis mice). Healthy BALB/c mice were treated intraperitoneally with PBS (phosphate-buffered saline) instead of splenic T cells, and thereafter received EcN by the oral route (= PBS-treated healthy mice + EcN, gray bars). These mice served as negative controls. (a) Clinical score of CD4+ CD62L+ SCID mice treated or not treated with EcN p.o., in comparison to the score of the healthy control group injected i.p. with PBS and treated with EcN p.o. *p < 0.001 vs CD62L. (b) Histological score of the colonic inflammation in mice following the induction of colitis by transfer of naïve CD4+ CD62L+ T lymphocytes and p.o. administration of EcN, compared to that of colitis mice not treated with EcN, and to the healthy control group which received an i.p. injection of PBS and was treated with EcN p.o. *p < 0.02 vs CD62L. (c) Evidence of bacterial translocation into mesenteric lymph nodes (MLNs). Total concentration of E. coli-like colonies in MLNs of mice from the transfer model following oral administration of EcN for 8 weeks compared to that of colitis mice not treated with EcN. EcN was identified by REP-PCR. *p < 0.0001 vs CD62L. (d, e, f) Proinflammatory cytokine secretion from MLNs following the induction of colitis in SCID mice by transfer of naïve CD4+ CD62L+ T lymphocytes. Anti-inflammatory effect of the p.o. administration of EcN, compared to the results obtained in colitis mice not treated with EcN, and to the healthy control group which received an i.p. injection of PBS and was treated with EcN p.o. Values given are means ± SEM (pg/ml). (d) IFN-γ, *p < 0.03 vs CD62L; (e) IL-6, *p = 0.02 vs CD62L; (f) IL-5, *p = 0.02 vs CD62L.
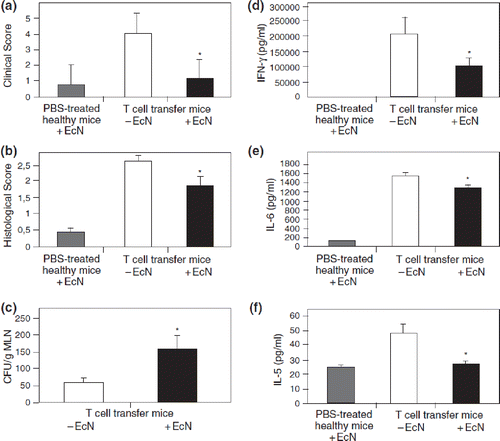
In both a rat model of TNBS-induced colitis and a mouse model of LPS-induced sepsis, orally administered EcN exerted local as well as systemic anti-inflammatory effects (Citation125). TNF-α level was reduced in the gut of colitic rats, while TNF-α and T-cell cytokines were reduced in blood plasma and lungs of sepsis mice, and the IgG release from splenocyte-derived B cells was down-regulated.
Using Toll-like receptor 2 (TLR-2) and Toll-like receptor 4 (TLR-4) knockout mice, it has been demonstrated that the inhibition of T-cell proliferation and the amelioration of DSS-induced colitis by EcN is mediated via TLR-2- and TLR-4-dependent pathways (Citation112,Citation118).
In an experimental allergy study conducted by Bickert and colleagues (Citation131), it was found that EcN inhibits the allergen-induced Th2-dominated immune response in the airways of C57BL/6 mice that had been hypersensitized by i.p. and i.v. administration of ovalbumin. However, this anti-allergic effect could only be demonstrated, if EcN was given together with the allergen.
Infection-preventing effects
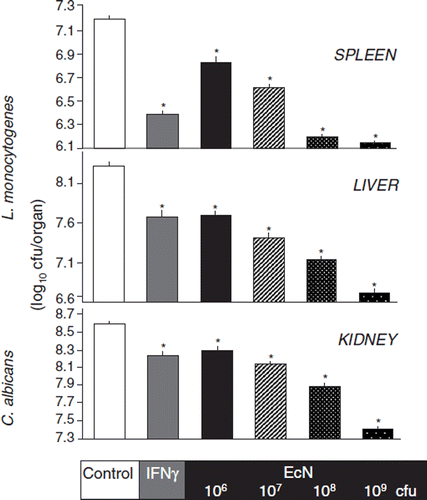
In in vivo experiments using conventionally housed C3H/HeN mice, single oral doses of EcN inhibited proliferation of subsequently intravenously inoculated Candida albicans and Listeria monocytogenes infections in a dose-dependent manner (Citation132) (). In a placebo-controlled veterinary clinical trial on 335 newborn calves it was shown that administration of EcN significantly (p < 0.001) reduced the incidence of neonatal calf diarrhea from 63% (placebo group) to 12% (EcN group) (Citation133).
Figure 14. Augmentation of host defense against systemic bacterial and fungal infections in mice by oral pretreatment with E. coli Nissle 1917 (EcN) (Citation132). Four groups of mice were pretreated once with 106, 107, 108, or 109 viable cells of EcN by oral administration, before they were challenged 24 h later by intravenous infection with Listeria monocytogenes (6 × 103 cfu) or Candida albicans (5 × 105 cfu). For each infection model, further groups of mice served as controls. These were either pretreated with placebo (negative controls, open bars) or with murine IFN-γ (positive controls, gray bars). Three days after infection with L. monocytogenes and 1 day after infection with C. albicans, mice were sacrificed and the parasite burden of the respective main target organs was determined. Compared with placebo, the pretreatment with IFN-γ resulted in a significant decrease of parasite load in spleen and liver of mice infected with L. monocytogenes, and a significant decrease of C. albicans counts in the kidneys. Likewise, pretreatment with EcN via the oral route significantly and dose-dependently reduced the parasite burden in spleen, liver, and kidneys. *p < 0.05 vs negative controls.
Communication with intestinal epithelial cells
In the last few years, it has become obvious that many probiotic effects of orally administered microbial preparations are due to interactions between the bacteria, the gut epithelial cells, and the gut immune system (Citation115,Citation130,Citation134,Citation135). In particular, the communication with the epithelial cell lining seems to be important, leading to a strengthening of the epithelial barrier function (Citation115,Citation130,Citation136).
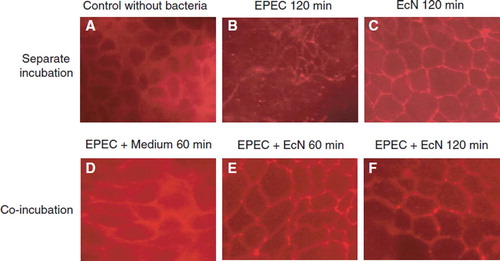
Thus, co-incubation of EcN with human intestinal epithelial cells (polarized T84 cells) resulted in a significant modulation (up- and down-regulation) of the activity of about 300 different genes in the epithelial cells, as demonstrated by transcriptome analysis using the microarray technique with DNA chips (Affimetrix) (Citation115,Citation137). Moreover, after infection of T84 cells with an enteropathogenic E. coli strain (EPEC E2348/69), treatment with EcN eliminated the negative impact of EPEC infection on the epithelial barrier function, i.e. EPEC-induced disruption of epithelial cell tight junctions (). This action of EcN seems to be mediated via the protein kinase C signaling pathway in the epithelial cells.
Figure 15. Effects of the non-pathogenic E. coli strain Nissle 1917 (EcN) and the enteropathogenic E. coli (EPEC) strain E2348/69 on the distribution of the tight junction protein zonula occludens-2 (ZO-2) in T84 epithelial cells (Citation115). T84 monolayers were incubated with bacteria for different periods of time and stained for ZO-2 using a fluorescent anti-ZO-2 antibody. (A) Control, T84 epithelial cells without bacteria. (B) T84 cells incubated with EPEC for 120 min. (C) T84 cells incubated with EcN for 120 min. (D) T84 cells incubated with EPEC for 60 min, then washed and further incubated with regular medium for another 60 min. (E) T84 cells incubated with EPEC for 60 min, then washed and further incubated with EcN for another 60 min. (F) T84 cells co-incubated with EcN plus EPEC for 120 min. Bacteria were added in a 1:1 ratio. The EcN strain had no negative influence on the distribution of the tight junction protein ZO-2, but abolished the negative impact of EPEC on ZO-2 distribution.
In another cell culture study, it has been observed that co-incubation of EcN with human epithelial cell lines T84 and HT-29 resulted in increased IL-8 secretion. Interestingly, the presence of EcN prevented cell death of the epithelial cells, induced by exposure to Salmonella dublin (Citation110).
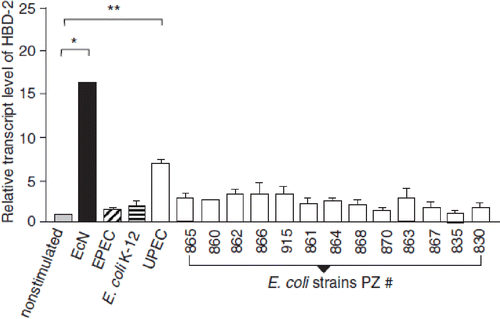
Examinations of cell cultures of human intestinal epithelial cells (Caco-2 cells, HT-29 cells) have demonstrated EcN to stimulate the synthesis of inducible antimicrobially acting defensins (HBD-2, HBD-3) (Citation114,Citation138) (). Induction of HBD-2 gene expression by EcN has been shown to exceed several times the extent of induction mediated by 40 other E. coli strains tested. By contrast, gene expression of constitutively synthesized HBD-1 defensin was not influenced by EcN. Stimulation of HBD-2 synthesis by EcN is mediated in the epithelial cell nucleus by transcription factors NF-κB and AP-1 (Citation114). The factor responsible for HBD-2 synthesis induction by EcN is the structural flagellum component flagellin (Citation139). The flagellin signal might be transmitted by TLR-5, which is present in Caco-2 epithelial cells, but other signaling pathways might also be involved (Citation139). Here, further investigations are required.
Figure 16. Stimulation of human β-defensin-2 (HBD-2) gene transcription in Caco-2 intestinal epithelial cells by pathogenic (EPEC E2348/69, UPEC 536) and non-pathogenic E. coli strains (E. coli K-12 DSM 498, EcN), and by E. coli strains with unknown pathogenicity (fecal isolates from healthy persons (PZ 860-915) and colitis ulcerosa patients (PZ 830, 835) (Citation114,Citation138). Caco-2 cells were stimulated for 4.5 h with 3 × 108 heat-inactivated bacteria/ml. Transcription of the HBD-2 gene was analyzed by real-time PCR. HBD-2 gene expression is shown after stimulation by the different E. coli strains, including probiotic E. coli Nissle 1917 (EcN). Data represent the means ± SEM normalized to the basal expression of controls (set at 1) from one to six separate experiments run in triplicate. *p = 0.0006; **p < 0.0001.
In mice with DSS-induced colitis, the interaction between EcN and the intestinal mucosa resulted in the restoration of the impaired permeability barrier (‘leaky gut’) () (Citation130). The contact of EcN with the intestinal epithelial cells induced the de novo synthesis and the redistribution of tight junction proteins, such as zonula occludens protein-1 (ZO-1), from the cytosol to the cell membrane. This effect is important for the cohesiveness of the epithelial cell layer. Concomitantly, DSS-induced disturbances of epithelial transport functions, such as the strongly decreased activity of the membrane-bound sodium pump, were restored (Citation130). For bacterial–epithelial interactions, the presence of the K5 capsule in the EcN strain also seems to be important (Citation140).
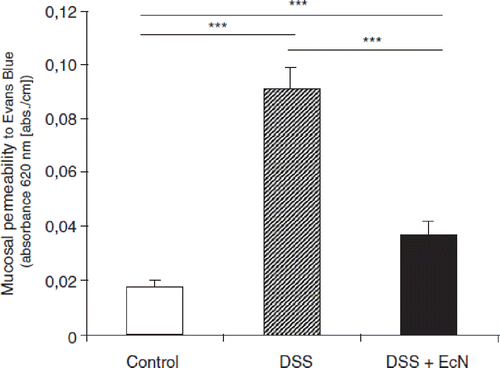
Figure 17. Inhibition of ‘leaky gut’ phenomena by oral administration of E. coli Nissle 1917 (EcN) to mice with dextran sodium sulfate (DSS)-induced colitis (Citation130). Intestinal permeability to Evans Blue was determined in healthy mice (control, open bar), mice with DSS-induced colitis (hatched bar), and mice treated with DSS plus EcN (black bar). Compared with healthy control mice, a significant increase in the uptake of Evans Blue by the colonic mucosa of DSS-treated mice was observed. This increase was strongly reduced in the group of mice treated with DSS plus EcN to almost normal values. ***p < 0.001.
Similar mechanisms of action of EcN are responsible for the recently detected prevention of the development of gastric mucosal lesions in mice, induced by acute stress (Citation141). The authors showed EcN to exhibit anti-inflammatory and blood flow-stimulating activities. Concomitantly, in the epithelial cells an increased synthesis of stress-protective proteins, such as heat-shock protein 70 (Hsp 70), could be demonstrated. The stress-induced enhanced gene expression of mucosal inflammatory mediators and the activation of transcription factor NF-κB was down-regulated by EcN.
Calprotectin is a calcium- and zinc-binding protein, the synthesis of which is stimulated by LPS (Citation142,Citation143). Calprotectin exerts antimicrobial activity by binding zinc and inhibiting the adhesion of bacteria to epithelial cells of the gut mucosa (Citation144). In 8-day-old germ-free piglets, oral administration of EcN led to an increase of calprotectin synthesis in the small intestine (Citation145). Another non-pathogenic E. coli strain (E. coli O86) and an enteropathogenic isolate (E. coli O55) did not show this effect in the gut. In contrast to EcN and to E. coli O86, oral administration of the enteropathogenic O55 strain provoked an increase of calprotectin level in blood plasma and, in addition, a pronounced increase of calprotectin concentration in bronchoalveolar lavage that was coincident with the development of septicemia.
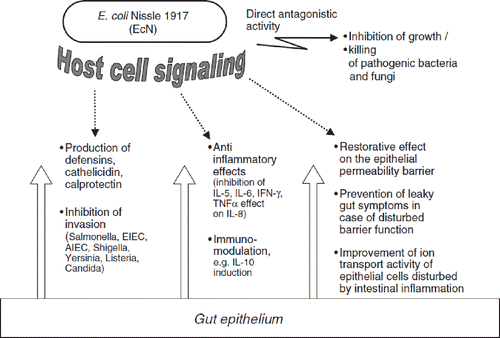
All these favorable effects induced by EcN are thought to be due to communication between the probiotic and the epithelial cells by so-called bacterial–epithelial crosstalk (Citation19,Citation146–149), mediated via specific signaling molecules, most of them being unknown up to now ().
Effects on visceral hypersensitivity and constipation
The irritable bowel syndrome (IBS) belongs to those functional bowel diseases that in about 25% of patients have developed some time after an episode of acute infectious diarrhea (Citation150,Citation151). This post-infectious IBS is characterized by a hyper-sensitivity of the visceral sensory system of the gut (so-called visceral hyperalgesia). In an established rat model of TNBS-induced visceral hyperalgesia, oral administration of EcN led to a significant reduction of the visceromotor reflexes (VMR) of the gut muscles, which were enhanced before as a consequence of the short-term inflammation induced by TNBS (Citation152).
Another functional bowel disease is chronic constipation, where colonic motility is reduced and, accordingly, intestinal transit slows down. In this context, it is important that cell-free spent supernatants of EcN cultures positively modulated colonic motility in an in vitro organ bath study with human colonic circular smooth muscle strips (Citation153). This effect may be due, at least in part, to EcN's ability to produce acetic acid, the main metabolic end product of its carbohydrate metabolism.
Antimutagenic activity
Microsatellite instability (MSI) in chronically inflamed colonic tissue has been suggested to be associated with the increased risk of ulcerative colitis patients developing colorectal carcinoma. Substances with anti-inflammatory activities, such as mesalazine (5-aminosalicylic acid) or EcN, might improve MSI. Therefore, Goel and co-workers (Citation154) performed an ex vivo analysis of MSI in 156 biopsies from 39 colitis ulcerosa patients who had been treated with either mesalazine or EcN in a randomized, double-blind clinical trial (Citation155). Overall, 20% (31/156) of biopsies displayed MSI. After 1 year on medication, 6/20 patients showed MSI improvement (change to microsatellite stability (MSS): 3 on mesalazine and 3 on EcN). Worsening of MSI (change from MSS to MSI) was also observed, but only in patients treated with mesalazine (6/20).
Using standard mutagenicity tests (Ames-test, Comet-assay) (Citation156,Citation157), it has been shown recently that EcN also exhibits antimutagenic activity against some well-known mutagens, such as 4-nitroquinoline 1-oxide (NQO), benzo(a)pyrene, and H2O2 (Citation158).
Colonization capability
Following oral administration of E. coli Nissle 1917, the strain exhibits good colonization capability in the gut of gnotobiotic rats (Citation92) and piglets (Citation93). In general, oral administration of EcN to different conventionally kept mice and rats resulted in a longer-term persistence or even a true colonization of the gut. This has been shown for the original strain and for recombinant and transformed EcN derivatives as well (Citation63,Citation95,Citation159,Citation160). In NMRI mice, the good colonization capability of EcN is dependent on the presence of the regulatory protein RfaH, as has been shown by colonization experiments comparing the original EcN strain with a RfaH-negative mutant (Citation161). In this context, RfaH does not seem to be important for bacterial adhesion to epithelial cells, but seems to enhance resistance of the EcN strain to bile acids. In vivo, the higher resistance to bile provides better chances of survival during transit of EcN through the gastrointestinal tract (Citation161).
Colonization is thought to be facilitated by the capability of EcN to produce and secrete cellulose, a feature important for biofilm formation (Citation162). Interestingly, EcN is able to form biofilms at 37°C (). In this respect the strain is different from other E. coli strains, which are only able to produce biofilms at lower temperatures (≤30°C). It is thought that this characteristic of EcN might also be important for its persistence in the gut after oral inoculation (Citation63,Citation162). Curli fimbriae of E. coli are important factors mediating the formation of microcolonies, which represent the first step in biofilm formation (Citation163). Besides curli fimbriae, EcN expresses F1A and F1C fimbriae (Citation54,Citation64). The corresponding gene clusters for the individual fimbriae types of EcN are localized on the bacterial chromosome. Their existence on the chromosomal DNA has been shown by using specific DNA probes (Citation164–167). The structural genes coding for the F1A fimbriae (fimA) and the F1C fimbriae (focA) have been sequenced and partially differ from corresponding fimbrial DNA sequences found in other E. coli strains (Citation53).
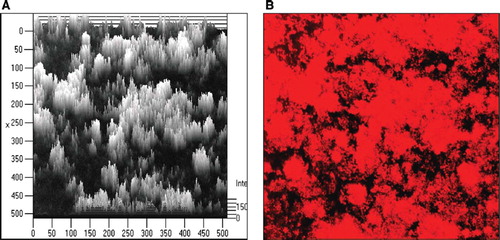
Figure 19. Biofilm formation by E. coli Nissle 1917 (EcN) in vitro as demonstrated by laser scanning microscopy. (A) Computer-assisted height measurement; (B) density measurement. Pictures taken from: J. Schulze, M. Schiemann, U. Sonnenborn. 120 years of E. coli – its importance in research and medicine. Alfred-Nissle-Gesellschaft, editor. Hagen, p 33, 2006; with kind permission. Scanning micrographs courtesy of U. Dobrindt and H. Merkert, University of Wuerzburg, Germany.
The parallel existence of multiple iron acquisition systems (siderophores) is peculiar to the EcN strain. They are thought to be ‘fitness factors’, which probably are necessary for competition with other microorganisms in the gut (Citation69,Citation84,Citation168). It might also be one of the reasons for its ability to colonize or at least to persist for some time in the intestine (Citation79).
Also in humans, long-term intestinal colonization by EcN could be achieved following oral administration of the strain, but only under certain specific conditions (Citation96,Citation97,Citation121,Citation169) (see also Colonization prophylaxis, below). In clinical trials with newborn babies who received daily oral doses of 108 cfu of EcN, starting immediately after birth, for the first 5 days of life, the applied strain could be recovered from stool samples for a period of several months (Citation96,Citation97). In adult volunteers, intentional disturbance of the intestinal microbiota was achieved by treatment with ciprofloxacin and metronidazole, followed by an orthograde gastrointestinal lavage. Thereafter, EcN was administered orally for 14 days, and the strain could be detected in stool samples after the end of medication for individually different periods of time (up to 129 days in one volunteer) (Citation169). Accordingly, in a more recent study, after administration of the EcN strain for 1 week long-term persistence of EcN in the gut of healthy adults was observed only in about 50% of the volunteers after 2 weeks and in less than 10% after 48 weeks (Citation170).
Taken together, the results obtained in gnotobiotic and conventional animals, in human newborns, and in adults treated with antibiotics and intestinal lavage clearly show that in principle EcN is able to colonize the gut, even in non-human species. However, these are special circumstances: in gnoto-biotic animals and human neonates no interfering intestinal microbiota is present, which could prevent colonization by the ‘intruder’ EcN. In adults pre-treated with antibiotics plus lavage, the endogenous microbiota is heavily disturbed, facilitating the settlement of the newly introduced probiotic strain.
In contrast to this, in most cases permanent colonization of the intestine by EcN cannot be achieved in healthy adults after oral administration, since the present microbiota will try to prevent integration of EcN into the existing microbial consortium. This is also true for other probiotic strains (Citation171). The indigenous intestinal microbiota does not differentiate between microbial intruders that might be harmful to the host (pathogens) and other microorganisms that are useful (probiotics) (Citation19). For the established microbial consortium in the gut all foreign microorganisms entering the ecosystem are competitors for the locally available substrates and ecological niches.
Toxicological potential and safety aspects (biosafety)
Investigations on possible adverse or even toxic effects of probiotics are becoming more and more important in the relevant literature (Citation172–179). This holds true for probiotics containing new lactic acid bacteria that have no traditional use in humans, but even more for probiotics with strains from bacterial species that also contain pathogenic variants, such as, for example, E. coli.
Since the species E. coli includes enteropathogenic and extraintestinal pathogenic strains (Citation54,Citation68,Citation180–184), particular attention has been paid to the toxicological and biosafety aspects of the Nissle strain (). As a general rule for probiotics from Enterobacteriaceae, selected probiotic candidate strains should not exhibit pathogenic adhesion factors and should not be able to form enterotoxins and cytotoxins or to invade epithelial cells. Moreover, such strains should not carry virulence factors that are typical for extraintestinal pathogenic E. coli, such as serum resistance or hemolysin production, and should not show uropathogenicity or provoke immunotoxic effects (Citation19).
Table VI. Biosafety aspects of E. coli strain Nissle 1917 (EcN) important for preventive and therapeutic use in human and veterinary medicine.
Toxin production
The EcN strain does not produce any of the toxins associated with pathogenic E. coli strains, such as the heat-labile enterotoxins (H-LT), heat-stable entero-toxins (H-ST), cytotoxins (e.g. cytotoxic necrotizing factor, CNF 1) or shiga-like toxins (SLT I, SLT II) (). Toxin production was tested in vitro and in vivo, using various assay systems (cell culture methods with Y1 adrenal cells and Vero cells, specific GM1-ELISA, baby mouse test). Examinations of general cytotoxic activity and of the presence of SLT I and SLT II were done according to the method of Karch and Meyer (Citation185). Testing for CNF 1 activity was performed as described by Blum et al. (Citation54,Citation180). In contrast to some ExPEC strains, EcN does not produce any hemolysin (Citation54,Citation109). These findings are due to the fact that the EcN strain does not possess any genetic information for the production of the above-mentioned toxins, as demonstrated by PCR techniques using specific DNA probes (Citation54,Citation164,Citation180,Citation185).
Using standard toxicological test methods (according to EC Guideline L 383 A: B1) for the determination of acute toxicity in rats and mice, EcN shows no toxic effects after oral administration of up to 50 ml/kg body weight of a suspension containing 2 × 1011 viable bacteria/ml (J. Leuschner, unpublished results). For that reason, an LD50 value could not be determined. Also, in young germ-free and colostrum-deprived piglets, colonization with EcN did not lead to any toxic effects (Citation93). This finding is particularly remarkable, because these animals represent an infection model of a completely immunodeficient host organism (Citation186).
Invasiveness and translocation
The EcN strain does not have invasive properties. In vivo testing was done according to the classic method of Sereny (Citation187) by following the development of keratoconjunctivitis after inoculation of bacteria into guinea pig eyes. Contrary to known invasive E. coli strains, EcN did not lead to keratoconjunctivitis (G. Böhme, unpublished results). In vitro testing was performed by using different epithelial cell lines (Citation99–101,Citation188,Citation189). Here, EcN also did not show any invasive activity, which can be explained by the fact that the strain does not carry the genes coding for the invasion machinery of enteroinvasive E. coli (EIEC) or Shigella strains (Citation56). In in vitro experiments with HT-29/B6 cells stressed by treatment with proinflammatory cytokines, EcN did not show translocation across the colonic monolayers, in contrast to a uropathogenic E. coli O4 strain (Citation190).
Serum resistance
A common feature of ExPEC strains producing sepsis and meningitis is their ability to resist the bactericidal activity of blood serum (Citation59). This characteristic is called serum resistance. The EcN strain does not show serum resistance when inoculated into human, bovine or pig serum. This finding can be explained by the presence of its modified (semi-rough) O6 surface antigen (Citation55,Citation191). Serum resistance testing was done by employing the classic in vitro assay of Hughes et al. (Citation60).
Pathogen-specific adhesion factors
Mannose-resistant hemagglutination is a feature that is common in pathogenic E. coli strains. EcN shows no mannose-resistant hemagglutination and forms no CFA I/II fimbriae, which are closely associated with the enteropathogenicity of certain E. coli strains (Citation181,Citation192). In the absence of mannose, EcN is capable of agglutinating erythrocytes, but not in the presence of mannose. This behavior is typical of E. coli strains possessing the so-called common type I fimbriae (F1A-fimbriae) (Citation54,Citation193). The test for the presence of CFA I or CFA II fimbriae was carried out by slide agglutination using specific anti-CFA I and anti-CFA II antisera (Citation181,Citation194,Citation195). Moreover, EcN does not express P, M or S fimbriae, which are associated with the virulence of certain ExPEC strains (Citation196) and does not possess the respective gene loci for these adherence factors (Citation56).
Uropathogenicity
The genome of the Nissle strain exhibits high homology to that of the UPEC strain CFT073 (Citation56), although genes for classic uropathogenicity traits are lacking. This has led some researchers to speculate whether the EcN strain might have some virulence in the urogenital system (Citation83). However, EcN shows none of the uropathogenicity traits typical of UPEC strains (Citation196,Citation197) (). Compared with uropathogenic and other tested E. coli strains, it possesses only a very limited potential for colonization of the urinary tract, as was demonstrated by using the transurethrally infected rat model (Citation197). In this respect, the colonizing ability of EcN is comparable to that of the avirulent laboratory strain E. coli K-12. Following transurethral infection of 20 rats with 5 × 107 viable cells of EcN, no lethal effects could be observed in any case, in contrast to transurethral infection with uropathogenic E. coli strains ().
Table VII. Comparison of E. coli strain Nissle 1917 (EcN) with non-pathogenic and uropathogenic E. coli (UPEC) strains in vitro and in a rat transurethral infection model.
According to its DNA fragment profile, as revealed by pulsed-field gel electrophoresis (PFGE) following restriction of its genomic DNA, EcN belongs to a non-uropathogenic O6:K5 clone, if compared with DNA profiles of serologically related uropathogenic and non-uropathogenic E. coli strains of the O6 lineage (J. Hacker, unpublished results; K. Eiteljörge, unpublished results). Macrorestriction analyses were done according to the methods of Ott et al. (Citation58) and Zingler et al. (Citation166,Citation167).
Resistance to antibiotics
As an isolate from the pre-antibiotic era, EcN is not resistant to commonly used antibiotics that are effective against gram-negative enterobacteria (tests according to NCCLS) (Citation198). Furthermore, the strain does not possess any antibiotic resistance plasmid. The two small cryptic plasmids characteristic of EcN (pMUT1 and pMUT2) do not carry any genetic information for antibiotic resistances (Citation53). Besides this, EcN – like all other E. coli strains – shows natural resistance to antibiotics that are mainly directed against gram-positive bacteria, such as clindamycin, erythromycin, metronidazole, penicillin G, rifampin, and vancomycin ().
Table VIII. Sensitivity and resistance of E. coli Nissle 1917 (EcN) against different antibiotics*.
Presence of large plasmids
EcN does not possess plasmids of high-molecular mass that are known to carry genes coding for antibiotic resistance and/or pathogenicity traits (Citation183). For example, the genes coding for invasiveness of enteroinvasive E. coli (EIEC) and the genes coding for many other pathogenicity factors are localized on high-molecular mass plasmids (Citation183).
Immunotoxicology
To determine the immunogenic and immunotoxic potential of EcN, the original EcN strain as well as a recombinant clone of EcN expressing the HA110-120 peptide of influenza A virus on its cell surface have been used (Citation160). For this purpose, the bacteria have been administered orally to different conventionally kept mouse strains (BALB/c, RAG-1−/−, TCR-HA-transgenic mice, and double transgenic mice expressing the influenza HA peptide under the control of the rat insulin promoter [INS-HA × TCR-HA]), and the immunological effects of EcN on the respective HA110-120-specific CD4+ T cells in the gut and in the peripheral lymphatic tissues have been analyzed. In addition, the original and the recombinant EcN strain have been tested for colonization capability and for effects on the gut mucosa of mice, under normal conditions as well as after genetically induced diabetes and after DSS-induced colitis, respectively. Although both strains, the original EcN and its recombinant derivative, exhibited good colonizing ability in the gut, there were no effects on migration, clonal expansion, or state of activation of specific CD4+ T cells. This was true for healthy as well as for diseased mice. In the double transgenic mouse strain, which is considered to be a highly susceptible model for autoimmune diseases (genetically induced diabetes) (Citation160), oral administration of recombinant EcN had no effect on induction or breakdown of T-cell tolerance.
Further experiments on gnotobiotic IL-2−/− mice mono-colonized with the EcN strain revealed that EcN did not induce colitis, in contrast to colonization with the colitogenic E. coli strain mpk (Citation199).
However, contrary to the well-known but ‘degenerated’ E. coli K-12 laboratory strain, EcN is an E. coli strain that is thought to have retained some wild-type characteristics (Citation56). Therefore, the sensitivity to EcN in mice is dependent on the genetic background of the animals (severity of immune dysfunction) as well as on the microbial environment (colonized vs germ-free state). This has been shown in a recent immunotoxicological study in genetically engineered mouse strains (knockout mice) with different immunological handicaps. EcN did not exhibit toxicity after a 1-week course of oral administration to germ-free and conventionally kept animals (Citation200). The only exceptions were the double-mutant C3H/ HeJZtm mice, which have defects in the gene cluster coding TLR-4. TLR-4, which is present on gut epithelial cells as well as on different types of lymphocytes, is responsible for the recognition of the immunologically highly active LPS of gram-negative bacteria, such as E. coli. Germ-free mutant mice experienced enhanced translocation of EcN from the gut into the bloodstream, leading to septicemia and infection of several organs. It is not known whether EcN might show serum resistance in blood serum of these mice. Interestingly, this infection of gut origin could only be induced in germ-free C3H/HeJZtm mice, not in conventionally housed mutant mice.
In healthy young pigs housed under conventional conditions, oral application of EcN did not induce significant changes of immunological parameters in the gut-associated lymphoid tissue (Citation102,Citation103).
Genetic stability
EcN is genetically stable, both in vitro following 100 sequential cultivation cycles (K. Eiteljörge, unpublished results), and in vivo following implantation and passage through the intestinal tract of newborns, which has been shown with EcN re-isolates obtained from stool samples of 14 children, 24 months after administration of EcN suspension (K. Eiteljörge, U. Sonnenborn, unpublished results). Evidence of genetic stability has been provided by means of standard comparative macrorestriction analysis using PFGE of chromosomal DNA fragments obtained from the original strain and from re-isolates as well as by means of specific REP-PCR (Citation166,Citation167,Citation201,Citation202).
EcN is a poor recipient for conjugative foreign DNA. Testing of EcN for the acceptance of foreign plasmid DNA was done by determination of conjugation frequencies following standard conjugation techniques with various plasmid groups (incompatibility groups, Inc) (Citation203). Conjugation frequencies of EcN were compared with those of the E. coli K-12 laboratory strain, which served as a positive (100%) control (, G. Böhme, unpublished results). The fact that EcN does not take up plasmids of the IncFI and IncFII types (conjugation frequency 0%) is of special importance, because in pathogenic E. coli strains these Inc groups often contain virulence plasmids.
Table IX. Test of the ability of E. coli Nissle 1917 (EcN) to serve as a recipient for foreign plasmid DNA.
EcN cannot be transformed by the tox-phages of enterohemorrhagic E. coli strains (EHEC), which otherwise are capable of transfering the slt genes for the production of shiga-like toxins to other recipient strains (Citation204). Attempts to introduce the slt genes into EcN by means of electroporation have also not been successful (Citation204). Phage-encoded genetic information for the production of EHEC-specific shiga-like toxins (Citation205–207) is not taken up by EcN.
Risk group assessment
EcN has been classified as a risk group I organism (lowest risk group for microorganisms) by the official German Central Commission for Biological Safety (ZKBS, Zentrale Kommission für Biologische Sicherheit), due to the lack of toxicity traits and its extensive molecular genetic and functional characterization.
Clinical efficacy and safety of E. coli Nissle 1917 in human medicine
The licensed drug Mutaflor® is available either in capsule form containing lyophilized powder with viable E. coli Nissle 1917 (2.5–25 × 109 cfu/capsule) or as a bacterial suspension (108 cfu/ml). The capsules are enteric coated in such a way that viable EcN bacteria are released no earlier than in the terminal ileum. This galenic formulation is necessary to protect EcN against gastric acidity and the antimicrobial action of bile and digestive enzymes in the small intestine. The suspension contains viable EcN bacteria in an electrolyte solution free of carbohydrates and nitrogen sources. This preparation has been developed especially for babies and toddlers, but may also be used by adults with difficulties in swallowing capsules. With respect to the clinical use of Mutaflor®, about nine decades of experience exist. The spectrum of indications for which EcN has been successfully used is relatively broad, which was demonstrated in a recently conducted prospective post-marketing surveillance study (Citation51). Controlled clinical trials that are able to prove efficacy are available for the indications ulcerative colitis, chronic habitual constipation, and acute and protracted (prolonged) diarrhea in young children. This will be discussed in more detail below. In addition, several pilot studies useful for the generation of hypotheses have been executed so far and gave hints for possible effectiveness of this probiotic for other indications. This concerns IBS (Citation208–210), collagenous colitis (Citation211), pseudomembraneous colitis (Citation212,Citation213), proctitis (Citation214), Crohn's disease (Citation215), diverticulosis (Citation216), pouchitis (Citation217), halitosis (Citation218), liver cirrhosis (Citation219), and interestingly, also in a dermatological allergic disease (polymorphic light eruptions, ‘sun allergy’) (Citation220).
Inflammatory bowel diseases
Inflammatory bowel diseases (IBD) are severe diseases of the gastrointestinal tract connected with enormous suffering for the respective patients. Ulcerative colitis and Crohn's disease are the most common forms of IBD (Citation221-223). While the etiological factors for the onset of IBD are unknown in most cases, there is intensive knowledge today with respect to the pathogenesis of these diseases, supporting the hypotheses that an over-reactive immune system on the basis of a genetical predisposition causes inflammation of the intestinal mucosa (ulcerative colitis) or of the entire gut wall (Crohn's disease) (Citation12,Citation13,Citation23,Citation221). This process is triggered, sustained, and intensified by different environmental factors (Citation224,Citation225). Since the recognition of the importance of the environmental factor ‘intestinal microbiota’ for the pathogenesis of IBD, the application of preparations containing viable microorganisms (probiotics) to IBD patients is gaining more and more interest and acceptance in medical, and especially in gastroenterological practice (Citation12,Citation21,Citation23,Citation44,Citation47,Citation226–240).
In three randomized controlled clinical trials (RCTs) EcN has been used for the maintenance of remission in patients with ulcerative colitis and compared to the gold standard mesalazine. The first study was a controlled clinical pilot trial with double-dummy design (Citation241); 103 patients in remission were enrolled. Fifty-three patients received 6 × 250 mg mesalazine per day (control group), while 50 patients received 2 capsules with EcN per day. The aim of the study was the maintenance of remission during a 3-month period. This was controlled by following the development of the clinical disease activity index. Life-table analysis revealed no difference between groups with respect to the length of time free of relapses (103 ± 4 days for the group taking mesalazine vs 106 ± 5 days for the group taking EcN). These results were confirmed by histological examinations and by the personal judgment of the patients with respect to the intensity of their disease at the end of the trial.
This first study on the usefulness of a probiotic in ulcerative colitis was followed by another randomized controlled clinical trial, which was conducted for a 12-month period, including patients with active ulcerative colitis (Citation242). This trial was based on the experience that acute relapses in IBD patients could be initiated and intensified by strongly adherent E. coli bacteria (Citation243). The question was whether EcN was able to maintain remission as effectively as mesalazine after initial treatment with antibiotics and corticosteroids. In all, 116 patients with active ulcerative colitis were enrolled and were randomly assigned to 2 groups in a double-blind, double-dummy fashion to receive either 3 × 800 mg mesalazine or 2 × 2 capsules with EcN. All patients received predniso-lone to stop the intestinal inflammation and, in addition, a 1-week course of gentamicin. Those patients who were not in remission after 12 weeks on prednisolone were excluded from the further trial. Patients in remission (44 on mesalazine, 39 on EcN) then received per day either 3 × 400 mg mesalazine or 1 × 2 capsules with EcN for the following 12 months. The efficacy of both forms of therapy was calculated with respect to the numbers of relapses occurring during the study period of 1 year. The result was that no differences could be detected between the two treatment groups: 73% of the patients receiving mesalazine and 76% of the patients receiving EcN had a relapse during this time (p = 0.0059, one-sided test for proof of non-inferiority). Also the duration of the remission phase was not significantly different between the two treatment groups (mesalazine, 206 days; EcN, 221 days), which is also reflected by the Kaplan-Meier plot of patients in remission in the course of the study ().
Figure 20. Comparison of mesalazine (5-aminosalicylic acid, 5-ASA) vs E. coli Nissle 1917 (EcN) for remission maintenance in patients with ulcerative colitis (Citation242). A total of 116 patients with active ulcerative colitis were randomly assigned to one of two treatment groups in a double-blind clinical trial with double-dummy design. Patients who had reached remission by standard corticosteroid therapy within 12 weeks were then given either mesalazine or EcN for 1 year. The Kaplan–Meier plot shows the percentage of patients in remission during the course of the study (straight line, EcN; dotted line, mesalazine). With respect to clinical efficacy and time course of remission, there were no statistically significant differences between the two study groups.
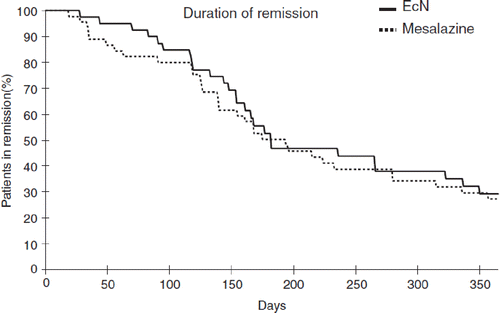
To confirm these results, another RCT with double-dummy design was initiated to prove that EcN is not inferior to mesalazine for maintenance of remission in patients with quiescent ulcerative colitis (Citation155). This large double-blind study was conducted in 10 European countries and included 327 patients, thus being suitable for equivalence testing. Patients were randomly assigned to two study groups and received either 3 × 500 mg mesalazine or two capsules with EcN for a period of 1 year. The results of all patients recruited for the study (intention-to-treat analysis) as well as the results of those patients fulfilling all criteria of the study protocol (per-protocol analysis, n = 222) revealed that treatment with EcN was equivalent to treatment with mesalazine. In the EcN group 36.4% of the patients experienced a relapse during the course of 1 year; in the mesalazine group 33.9% relapsed (). The one-sided equivalence test showed a p value of 0.003. This study proved that EcN represents an effective alternative to mesalazine for maintaining remission in patients with ulcerative colitis.
Figure 21. Clinical equivalence study between mesalazine (5-aminosalicylic acid, 5-ASA) and E. coli Nissle 1917 (EcN) for maintaining remission of ulcerative colitis (Citation155). A total of 327 patients with quiescent ulcerative colitis were recruited and randomly assigned to a double-blind clinical multicenter trial with double-dummy design that was performed in 10 European countries. Patients received either mesalazine or EcN for 1 year. At the end of the study, 222 patients could be included in the per-protocol analysis. Of these, 40/110 (36.4%) in the EcN group and 38/112 (33.9%) in the mesalazine group showed a relapse during the 1-year period of treatment. The data show significant equivalence between both treatment groups with respect to clinical efficacy (maintenance of remission) (p = 0.003).
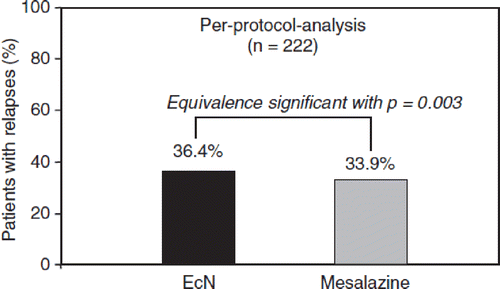
On the basis of these study results, EcN has been included as an effective therapeutic agent for the maintenance of remission in patients with ulcerative colitis into the treatment guidelines of the German and the Austrian gastroenterological associations as well as in the guidelines of the European Crohn and Colitis Organisation (ECCO) (Citation244–247).
Recently, similar results were obtained in an open controlled pilot study in children and teenagers with ulcerative colitis. In this study, 24 patients (mean age 14.6 years) received two capsules with EcN per day for a 1-year period and 10 patients (mean age 14.0 years) received 1.2 g mesalazine per day for the same time course. After 1 year, 25% of patients in the EcN group and 30% of patients in the mesalazine group experienced a relapse (Citation248).
Jöres-Nguyen-Xuan et al. (Citation170) have shown recently in healthy volunteers that mesalazine and EcN do not interfere when administered together, opening the way for clinical trials on the usefulness of a combination therapy for IBD.
Chronic constipation
The effectiveness of EcN to induce a normalization of intestinal functions could also be shown in patients with chronic constipation. Using a parallel group comparison, constipated patients (after stopping the use of laxatives) received either 2 × 15 ml lactulose syrup per day (n = 52) or 3 × 1 capsule with EcN per day (n = 56) (Citation249). After 12 weeks of treatment, in the EcN group the number of bowel movements increased to 6.3 per week. In the lactulose group the number of bowel movements increased to 5.5 per week. The ease of evacuation improved significantly in those patients taking EcN compared with those in the lactulose group (p < 0.001). Adverse events (mainly flatulence and abdominal cramps) did occur sometimes, but were seen more often in the lactulose group.
In a further study (Citation250) chronically constipated patients received in a randomized double-blind study either capsules with EcN or placebo capsules over a period of 8 weeks. Initially, in a ‘run-in-phase’ all patients received placebo for 1 week. Only those patients who did not have more than two bowel movements during the run-in-phase were thereafter randomly attributed to verum or placebo group. The EcN and the placebo capsules were taken at breakfast each day. The following doses were applied: 3 × 2 capsules per day on days 1 and 2, 1 × 4 capsules per day from day 3 onwards for a total of 8 weeks. Within 4 weeks the number of bowel movements in the EcN group increased significantly (4.9/ week, p < 0.001) compared with the placebo group (2.6/week) (). While patients in the EcN group experienced a further increase in bowel movements during the next 4 weeks (up to 6.0/week), the number of bowel movements of patients in the placebo group decreased to 1.9/week. The study design included a change-over to the other kind of treatment for patients who did not experience an effect of either medication (less than three bowel movements per week) during the first 4 weeks. With respect to this, 13 placebo patients changed to EcN treatment, which resulted in an increase of bowel movements during the next 4 weeks up to 5.15/week. On the other hand, two patients changed from EcN to placebo, but did not experience any increase of bowel movements (2.5 per week).
Figure 22. Double-blind placebo-controlled randomized clinical trial with E. coli Nissle 1917 (EcN) for the treatment of chronic habitual constipation (Citation250). Initially, all constipated patients (n = 134, two bowel movements per week or less) in a blinded manner received a placebo medication for 1 week which, however, was handed over to them by the principal investigator as a ‘new biological drug against constipation’. After the 1-week course on placebo, only those patients who did not respond to this treatment (n = 70) were allowed to enter the real double-blind study and were randomly assigned to receive either EcN or placebo. The figure shows the development of the main target criterion (number of bowel movements per week) during the 8-week course of the study. EcN was significantly better than placebo in increasing the weekly frequency of bowel movements after 4 and after 8 weeks on medication (p < 0.001 for EcN vs placebo).
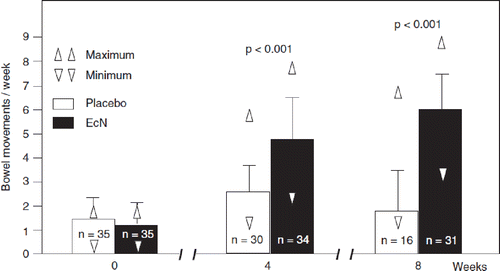
The therapeutic efficacy of EcN in chronically constipated patients might rather reflect the probiotics’ ability to produce gut motility-enhancing short-chain fatty acids, such as acetic acid (Citation153) (discussed earlier), but not the production of gases (CO2, H2), since in a study with healthy volunteers EcN has been shown to exert no effect on intestinal gas dynamics and abdominal sensation (Citation251).
Diarrhea in young children
Recently, efficacy and safety of EcN for the treatment of acute diarrhea of babies and toddlers were studied in a randomized placebo-controlled double-blind clinical multicenter trial (Citation252). Acute diarrhea was defined as showing three non-bloody evacuations per day with reduced consistency (liquid or almost liquid). Acute diarrhea should not last longer than 3 consecutive days. A total number of 113 babies and toddlers, aged 2–46 months (median 23 months), were recruited for the study and randomly assigned to the EcN group (n = 55) or to the placebo group (n = 58). Patients received either EcN suspension (108 cfu/ml) or an indistinguishable placebo from day 0 until day 9 in a dosage dependent on age: babies (<1 year) 1 × 1 ml suspension/day, toddlers (≥ 1 to ≤3 years) 2 × 1 ml/day, and older children (> 3 to <4 years) 3 × 1 ml/day. Before enrollment, the mean duration of diarrhea was 1.4 days for the infants in the EcN group and 1.6 days for the infants in the placebo group. None of the children was dehydrated when included in the study. In both treatment groups diarrhea was partly due to bacterial or viral infections as well as other unspecific causes that were not further elucidated. The primary goal of the trial, to reduce the number of bowel movements (less than or equal to three liquid or almost liquid evacuations per day on at least 2 consecutive days), was reached by 52/55 patients (94.5%) in the EcN group and 39/58 patients (67.2%) in the placebo group. By analysis according to Kaplan–Meier the mean duration until reaching this goal was 2.5 days in the verum and 4.8 days in the placebo group (). Treatment with EcN thus reduced the mean duration of diarrhea by 2.3 days. This difference between verum and placebo is statistically significant (p = 0.007).
Figure 23. Two double-blind placebo-controlled randomized clinical trials have been performed with E. coli Nissle 1917 (EcN) suspension (108 cfu/ml) for the treatment of acute diarrhea (A) or prolonged diarrhea (B) in young children (Citation252,Citation253) (see text for details). In the study on acute diarrhea (A), EcN treatment shortened the duration of diarrhea by 2.3 days compared with placebo (p < 0.0007). In the study on prolonged diarrhea (B), EcN treatment shortened the duration of diarrhea by 3.3 days (p < 0.0001). These results demonstrate that EcN suspension is an effective treatment option for diarrheal diseases in infants.
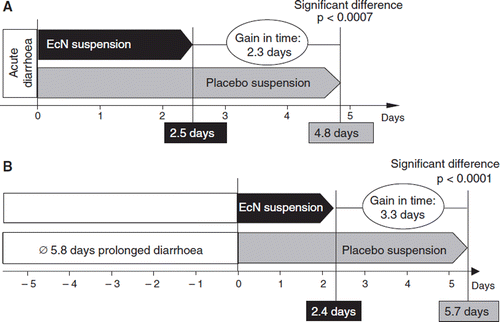
In a second randomized placebo-controlled double-blind multicenter study the efficacy of EcN for the treatment of unspecific protracted diarrhea in babies and toddlers was examined (Citation253). Protracted diarrhea was defined as follows: more than three non-bloody liquid to almost liquid evacuations per day. The diarrhea should have been present for at least 4 days and not longer than 2 weeks. A total of 151 babies and toddlers, aged 1–47 months, who experienced a prolonged diarrhea as defined above were recruited for the study. They were randomly assigned to the EcN group (n = 75) or to the placebo group (n = 76). At the beginning of the study the mean duration of diarrhea was 5.8 days for both groups. The number of bowel evacuations during the last 24 h before the onset of the study was four to six, mostly of liquid consistency and with mucus shedding. Patients received either the verum or the placebo suspension from day 0 until day 20, applying the same age-dependent dosage scheme as in the study on acute diarrhea described above. To prevent dehydration because of loss of liquid and electrolytes, all patients initially received an oral rehydration therapy (ORT) for 4 h according to the WHO Guideline ‘Treatment of diarrhoea (2001)’ in an outpatient setting.
The main goal of this study was to achieve a decrease of bowel movements (three or less liquid or almost liquid evacuations per day for at least 4 consecutive days). Treatment with EcN should have demonstrated superiority compared with placebo on days 7 and 14. The primary aim of the study was reached by 59/75 (78.7%) of patients in the EcN group and by 45/76 (59.2%) of patients in the placebo group on day 7. After 14 days this goal was reached by 70/75 (93.3%) of the patients on EcN and by 50/76 (65.8%) of the placebo patients (). Therapy was stopped for 13 patients because of ineffectiveness (2 patients on EcN, 11 patients on placebo). These patients were treated thereafter with other drugs. Using sequential group analysis it could be shown that already on day 7 there was a strong tendency for superiority of EcN therapy over placebo (p = 0.0758). The difference between the two groups increased in the course of the study and was significant on day 14 (p = 0.0017). Using Kaplan-Meier analysis, the time to reach the main goal of the study was calculated to be 2.4 days in the EcN and 5.7 days in the placebo group. Treatment with EcN reduced the mean duration of protracted diarrhea for about 3.3 days.
In summary, probiotic therapy with the Nissle strain showed statistically significant superiority compared with placebo for acute as well as for prolonged (protracted) diarrhea in babies and toddlers. Interestingly, with respect to the time-sparing effect of EcN, a comparison of the efficacy of EcN to that of other probiotics (Citation19) also suggested superiority of EcN over several LAB preparations, although a true comparison between the preparations used is diffi-cult because of very different study designs.
Colonization prophylaxis
Studies concerning colonization prophylaxis in pre-term and full-term neonates have shown that the oral application of EcN during the first days of life resulted in a long-standing colonization of the gut with this probiotic, followed by a reduced colonization with true and potential pathogens (Citation96,Citation97). In addition, this intentional colonization resulted in a significant increase of specific immunoglobulins IgA and IgM (Citation121) as well as in an early induction of natural immune defenses (Citation122). This points to the development of the gut immune system in the direction towards an infection-preventing state, which is reflected by an anti-allergic Th1-dominated immune situation (Citation111). Since children of allergic parents have a high risk of developing allergies early in life, intentional colonization of the gut with EcN might turn out to be an effective alternative for allergy prevention.
Safety aspects in clinical use
EcN-treatment is generally well tolerated. No serious adverse drug reactions have been reported from the explorative and confirmatory clinical trials conducted to date to demonstrate efficacy and tolerability of EcN for different indications. Flatulence, which is occasionally observed, especially at the beginning of the probiotic therapy, is suspected to be due to the metabolic activity of EcN in the gut. This ailment probably represents individual overdosing and does not require a cessation of medication nor specific treatment, since in general it vanishes after reducing the dose. In clinical investigations on the tolerability of EcN in healthy volunteers, up to nine capsules per day (about 1011 cfu/day) were tolerated without any problems (E. Zieseniss, unpublished results).
High-risk patients, such as preterm infants and also full-term babies, in whom the intestinal mucosal barriers are not yet fully developed, are nevertheless tolerant to a daily oral application of 1 ml EcN suspension (108 cfu/day). This has been shown repeatedly during the course of several clinical trials on neonates (Citation96,Citation97,Citation121,Citation122). Although the application of EcN suspension to newborn infants directly after birth (1 ml once a day for 5 days) resulted in an early induction of the development of both the gut and the systemic immune system (Citation121,Citation122), in a study on healthy adult male volunteers the application of two capsules with EcN once a day for a total of 3 days did not reveal significant changes in any of the immune parameters tested (cytokines, receptor molecules) (R. Gruber, unpublished results). Also, neither induction of autoimmune phenomena nor immunotoxic effects have been observed. These findings underline the clinical safety of the EcN strain.
Clinical efficacy of E. coli Nissle 1917 in veterinary medicine
Until the redefinition of the term probiotic by Fuller in 1989 (Citation30), viable microorganisms (LAB) were used as feed additives or medical remedies mainly in animals, although human experiences have existed for several thousand years by the traditional consumption of fermented foods. In animal breeding the addition of viable bacteria to the feed was thought to stimulate the growth of the animals, similar to the prophylactic use of low-dose antibiotics, in order to increase the efficacy of feed exploitation followed by the enhanced production of meat, milk, and eggs and to improve the general health condition of the animals (Citation254). At that time, there was more speculation than proof as regards the scientific basis of clinical efficacy and the modes of action of probiotics. Since the scientific interest has concentrated in humans on the elucidation of the modes of action of such preparations, the number of studies on the use of probiotics in domestic animals has decreased. Nevertheless, infectious diarrhea is the leading cause of morbidity and mortality in young piglets and calves, induced by shortening of the lactation period, industrialized maintenance conditions, and inadequate feeding. Often more than 50% of newborn calves are affected by diarrhea between the seventh and the tenth day of life, which reduces the development of the animals and may be the cause of about 8% of all newborn deaths (Citation255).
The efficacy of a suspension with EcN for preventing diarrhea in newborn calves has been tested in a pilot study as well as in a confirmatory placebo-controlled blinded clinical trial (Citation133). In both studies the animals received 15 ml EcN suspension (108 cfu/ml) on the day of birth, before the first intake of colostrum, and then continuously once a day until day 10 or 12. The oral application of EcN suspension was executed each morning before conventional feeding was performed. The control group of animals received a similar placebo suspension (electrolyte solution) also before feeding in the morning. The newborn calves were fed colostrum on the first day of life. Colostrum was then replaced by the addition of pooled milk or commercially available milk replacer. In the pilot study 172 calves were included (placebo group, n = 89; EcN group, n = 83), and the confirmatory controlled study comprised 163 calves (placebo group, n = 81; EcN group, n = 82). In both studies the number of animals experiencing diarrhea were counted. Diarrhea was defined as loose or watery excrements on at least 2 following days. In the first study, 58 animals (65.2%) in the placebo group experienced diarrhea, but only 22 animals (26.5%) in the EcN group. In the confirmatory clinical trial, 51 animals (63%) in the placebo group, but only 10 animals (12.2%) in the EcN group showed diarrhea. This difference of 50.8% (p < 0.001) represents a decrease in diarrhea episodes in the animal population of more than 80% and proves the high efficacy of EcN in the prophylaxis and therapy of diarrhea in neonatal calves (Citation133).
The anti-diarrheic efficacy of EcN was also assessed in a pig model of intestinal bacterial infection (Citation256). Here, 1010 viable cells of porcine enterotoxigenic E. coli strain Abbotstown (EcA) were given via an orogastric tube to weaned piglets (n = 7) at day 21 postpartum. At 48 h after challenge electrophysiological parameters of isolated jejunal epithelium were assessed in Ussing chambers. In agreement with clinical signs of diarrhea, tissue of challenged animals showed an overshoot of secretory response after stimulation of the cAMP-mediated second messenger pathway by forskolin, indicating higher excitability of chloride secretory channels under the infection. These data were compared with the respective measurements of jejunal tissues from animals that received a daily dose of 1010 viable cells of EcN over 10 days before EcA challenge (n = 4), with a group on EcN only (n = 4), and with a group of untreated animals (controls, n = 6). EcN pretreatment completely abolished clinical signs of secretory diarrhea and the overshoot of secretory response of intestinal epithelium upon stimulation with forskolin, seen in mono-infected animals. These results demonstrate clinical efficacy of EcN in preventing gut infection and secretory diarrhea induced by enterotoxigenic E. coli in pigs.
Concluding remarks
Until recently, the use of living microorganisms (probiotics) for therapeutic purposes in general has been considered some kind of alternative or complementary medicine (Citation257). Fortunately, this view has changed considerably in the last couple of years, which is reflected by the growing interest of basic biomedical sciences and clinical medicine with respect to this group of ‘active substances’. The increasing interest is reflected by the exponentially growing numbers of scientific papers and published RCTs dealing with probiotics (see ).
Although most probiotic preparations traditionally contain LAB, as suggested by Metchnikoff in the old days, E. coli strain Nissle 1917 (EcN) has many features in common with the probiotic LAB. As described in detail above, the reported mechanisms of action, safety aspects, and clinical efficacy for defined indications allow EcN to be described as a probiotic microorganism. We could review several valid RCTs that were performed using this non-LAB organism as a probiotic. In these clinical studies, the therapeutic efficacy of EcN has been demonstrated for different gastrointestinal indications. Because of the numerous experimental studies elucidating the modes of action, the questions of biosafety, including the responsible genetic background on the basis of a differential genome analysis, EcN is now one of the best examined probiotic strains. As a logical consequence, EcN is often used as a reference strain and a model organism for different scientific purposes (Citation258–263). Owing to the excellent tolerability of EcN and its capability for colonization of or persistence in the human as well as the animal intestinal tract, EcN is very well suited to take on functions as a carrier for bioactive molecules, e.g. novel adhesins, insulinotropic proteins, signaling molecules or anti-HIV microbicides (Citation264–267). This also concerns further recombinant manipulations of the EcN genome, e.g. by constructing tripartite vector systems for live oral vaccine development (Citation268), no matter whether an application for therapeutic purposes will be possible in the near future.
Acknowledgments
The authors gratefully acknowledge the valuable assistance of Martina Schiemann, Doris Schrott, and Monika Wienbeck, Herdecke, in producing this review. Many thanks also to Tobias Ölschläger, Würzburg, for critical reading of the manuscript and helpful discussions. We are grateful to K. Eiteljörge, Herdecke, M.A. Schmidt and C. Cichon, Münster, and the Alfred-Nissle-Gesellschaft, Hagen, for the kind supply of photos.
Declaration of interest: Ulrich Sonnenborn is currently head of the Department of Biological Research at Ardeypharm GmbH, Herdecke, Germany. Jürgen Schulze was head of Ardeypharm's Department of Medicine until his retirement in January 2006. Ardeypharm GmbH produces and distributes the licensed microbial drug Mutaflor®, containing E. coli strain Nissle 1917 (EcN) as the active principle, in Germany and some other European countries. The authors alone are responsible for the content and writing of the paper.
References
- McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol 2001;3:1–11.
- Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol 2008;10:37–54.
- Guarner F, Malagelada JR. Gut flora in health and disease. Lancet 2003;360:512–19.
- Ouwehand AC, Vaughan EE. Gastrointestinal microbiology. New York: Taylor & Francis. 2006.
- Rambaud JC, Buts JP, Corthier G, Flourie B. Gut microflora – digestive physiology and pathology. Paris: John Libbey Eurotext. 2006.
- Stecher B, Hardt WD. The role of microbiota in infectious disease. Trends Microbiol 2008;16:107–14.
- Yan F, Polk DB. Commensal bacteria in the gut: learning who our friends are. Curr Opin Gastroenterol 2004;20:565–71.
- Chichlowski M, Hale LP. Bacterial–mucosal interactions in inflammatory bowel disease – an alliance gone bad. Am J Physiol Gastrointest Liver Physiol 2008;295:G1139–49.
- Haenel H, Bendig J. Intestinal flora in health and disease. Prog Food Nutr Sci 1975;1:21–64.
- Mai V, Draganov PV. Recent advances and remaining gaps in our knowledge of associations between gut microbiota and human health. World J Gastroenterol 2009;15:81–5.
- Savage DC. The ecological digestive system and its colonisation. Rev Sci Tech Off Int Epiz 1989;8:259–73.
- Schultz M, Schölmerich J, Rath HC. Rationale for probiotic and antibiotic treatment strategies in inflammatory bowel diseases. Dig Dis 2003;21:105–28.
- Schulze J, Sonnenborn U. The role of the gut flora in inflammatory bowel diseases. Shimoyama T, Axon A, Lee A, Podolsky DK, O’Morain C. Helicobacter meets inflammatory bowel disease. Tokyo: Medical Tribune Inc. 2002. 393–417.
- Arias CA, Murray BE. Antibiotic-resistant bugs in the 21st century – a clinical super-challenge. N Engl J Med 2009;360:439–43.
- Chadwick DJ, Goode J. Antibiotic resistance: origins, evolution, selection and spread. Ciba Foundation Symposium 207. Chichester: John Wiley & Sons. 1997.
- Davies J. Microbes have the last word. A drastic reevaluation of antimicrobial treatment is needed to overcome the threat of antibiotic-resistant bacteria. EMBO Rep 2007;8:616–21.
- Hawkey PM. Action against antibiotic resistance: no time to lose. Lancet 1998;351:1298–9.
- Witte W, Cuny C, Klare I, Nübel U, Strommenger B, Werner G. Emergence and spread of antibiotic-resistant gram-positive bacterial pathogens. Int J Med Microbiol 2008;298:365–77.
- Schulze J, Sonnenborn U, Ölschläger T, Kruis W. Probiotika - Mikroökologie, Mikrobiologie, Qualität, Sicherheit und gesundheitliche Effekte. Stuttgart: Hippokrates/Thieme. 2008.
- Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol 2009;44:26–46.
- Fric P. Probiotics in gastroenterology. Z Gastroenterol 2002;40:197–201.
- Montrose DC, Floch MH. Probiotics used in human studies. J Clin Gastroenterol 2005;39:469–84.
- Sartor RB. Probiotic therapy of intestinal inflammation and infections. Curr Opin Gastroenterol 2005;21:44–50.
- Sullivan A, Nord CE. Probiotics and gastrointestinal diseases. J Intern Med 2005;257:78–92.
- Tannock GW. Probiotics: time for a dose of realism. Curr Issues Intest Microbiol 2003;4:33–42.
- FAO/WHO. 2001. Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. Cordoba, Argentina, 1–4 October 2001.
- Vergin F. Anti- und Probiotika. Hippokrates 1954;25:116–9.
- Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science 1965;147:747–8.
- Parker RB. Probiotics, the other half of the antibiotic story. Anim Nutr Health 1974;29:4–8.
- Fuller R. Probiotics in man and animals. J Appl Bacteriol 1989;66:365–78.
- Guarner F, Schaafsma GJ. Probiotics. Int J Food Microbiol 1998;39:237–8.
- Elmer GW, Surawicz CM, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA 1996;275:870–6.
- McFarland LV, Bernasconi P. Saccharomyces boulardii: a review of an innovative biotherapeutic agent. Microb Ecol Health Dis 1993;6:157–71.
- Baumgart DC, Dignass AU. Current biological therapies for inflammatory bowel disease. Curr Pharm Des 2004;10:4127–47.
- Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology 2002;122:1592–608.
- Sandborn WJ, Faubion WA. Biologics in inflammatory bowel disease: how much progress have we made? Gut 2004;53:1366–73.
- Vermeire S, Rutgeerts P. Novel biological strategies in inflammatory bowel disease. Inflamm Bowel Dis 2004;10(Suppl 1):S44–51.
- Bansal T, Garg S. Probiotics: from functional foods to pharmaceutical products. Curr Pharm Biotechnol 2008;9:267–87.
- Bengmark S. Bioecologic control of the gastrointestinal tract: the role of flora and supplemented probiotics and synbiotics. Gastroenterol Clin North Am 2005;34:413–36.
- Callaway TR, Edrington TS, Anderson RC, Harvey RB, Genovese KJ, Kennedy CN, . Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev 2008;9:217–25.
- De Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol 2008;111:1–66.
- Marteau P. Living drugs for gastrointestinal diseases: the case for probiotics. Dig Dis 2006;24:137–47.
- Gill H, Prasad J. Probiotics, immunomodulation, and health benefits. Adv Exp Med Biol 2008;606:423–54.
- Hart AL, Stagg AJ, Kamm MA. Use of probiotics in the treatment of inflammatory bowel disease. J Clin Gastroenterol 2003;36:111–19.
- Heselmans M, Reid G, Akkermans LM, Savelkoul H, Timmerman H, Rombouts FM. Gut flora in health and disease: potential role of probiotics. Curr Issues Intest Microbiol 2005;6:1–7.
- Reid G, Devillard E. Probiotics for mother and child. J Clin Gastroenterol 2004;38(Suppl 2):S94–101.
- Schultz M. Clinical use of E. coli Nissle 1917 in inflamma-tory bowel disease. Inflamm Bowel Dis 2008;14:1012–18.
- Nissle A. Die antagonistische Behandlung chronischer Darmstörungen mit Colibakterien. Med Klin 1918;2:29–33.
- Nissle A. Weiteres über Grundlagen und Praxis der Mutaflorbehandlung. Dtsch Med Wochenschr 1925;44:1809–13.
- Hamilton-Miller JMT. A review of clinical trials of probiotics in the management of inflammatory bowel disease. Infect Dis Rev 2001;3:83–7.
- Krammer HJ, Kämper H, Von Bünau R, Zieseniß E, Stange C, Schlieger F, . Probiotische Arzneimitteltherapie mit E. coli Stamm Nissle 1917 (EcN): Ergebnisse einer prospektiven Datenerhebung mit 3807 Patienten. Z Gastroenterol 2006;44:651–6.
- Kruis W. Antibiotics and probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 2004;20(s4):75–8.
- Blum-Oehler G, Oswald S, Eiteljörge K, Sonnenborn U, Schulze J, Kruis W, . Development of strain-specific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in fecal samples. Res Microbiol 2003;154:59–66.
- Blum G, Marre R, Hacker J. Properties of Escherichia coli strains of serotype O6. Infection 1995;23:234–6.
- Grozdanov L, Zähringer U, Blum-Oehler G, Brade L, Henne A, Knirel YA, . A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 2002;184:5912–25.
- Grozdanov L, Raasch C, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, . Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J Bacteriol 2004;186:5432–41.
- Rietschel ET, Brade H, Holst O, Brade L, Müller-Loennies S, Mamat U, . Bacterial endotoxin: chemical constitution, biological recognition, host response, and immunological detoxification. Rietschel ET, Wagner H. Pathology of septic shock. Current Topics in Microbiology and Immunology, 216. Berlin: Springer-Verlag. 1996. 39–81.
- Ott M, Bender L, Blum G, Schmittroth M, Achtman M, Tschäpe H, . Virulence patterns and long-range genetic mapping of extraintestinal Escherichia coli K1, K5, and K100 isolates: use of pulsed-field gel electrophoresis. Infect Immun 1991;59:2664–72.
- Taylor PW. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev 1983;47:46–83.
- Hughes C, Phillips R, Roberts AP. Serum resistance among Escherichia coli strains causing urinary tract infection in relation to O type and the carriage of hemolysin, colicin, and antibiotic resistance determinants. Infect Immun 1982;35:270–5.
- Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut 2005;54:1182–93.
- Wang Q, Suzuki A, Mariconda S, Porwollik S, Harshey RM. Sensing wetness: a new role for the bacterial flagellum. EMBO J 2005;24:2034–42.
- Lasaro MA, Salinger N, Zhang J, Wang Y, Zhong Z, Goulian M, . F1C fimbriae play an important role in biofilm formation and intestinal colonization by the Escherichia coli commensal strain Nissle 1917. Appl Environ Microbiol 2009;75:246–51.
- Stentebjerg-Olesen B, Chakraborty T, Klemm P. Type 1 fimbriation and phase switching in a natural Escherichia coli fimB null strain, Nissle 1917. J Bacteriol 1999;181:7470–8.
- Hammar M, Arnqvist A, Bian Z, Olsen A, Normark S. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol 1995;18:661–70.
- Olsen A, Jonsson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature 1989;338:652–5.
- Ahmed N, Dobrindt U, Hacker J, Hasnain SE. Genomic fluidity and pathogenic bacteria: applications in diagnostics, epidemiology and intervention. Nat Rev Microbiol 2008;6:387–94.
- Hacker J, Kaper JB. Pathogenicity islands and the evolution of microbes. Annu Rev Microbiol 2000;54:641–79.
- Hacker J, Carniel E. Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep 2001;2:376–81.
- Hacker J, Hentschel U, Dobrindt U. Prokaryotic chromosomes and disease. Science 2003;301:790–3.
- Große C, Scherer J, Koch D, Otto M, Taudte N, Grass G. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol Microbiol 2006;62:120–31.
- Neilands JB. Mechanism and regulation of synthesis of aerobactin in Escherichia coli K12 (pColV-K30). Can J Micro-biol 1992;30:728–33.
- Schubert S, Rakin A, Karch H, Carniel E, Heesemann J. Prevalence of the “high-pathogenicity island” of Yersinia species among Escherichia coli strains that are pathogenic to humans. Infect Immun 1998;66:480–5.
- Schubert S, Rakin A, Fischer D, Sorsa J, Heesemann J. Characterization of the integration site of Yersinia high-pathogenicity island in Escherichia coli. FEMS Microbiol Lett 1999;179:409–14.
- Torres AG, Payne SM. Haem iron-transport system in enterohemorrhagic Escherichia coli O157:H7. Mol Micro-biol 1997;23:825–33.
- Azpiroz MF, Rodriguez E, Lavina M. The structure, function, and origin of the microcin H47 ATP- binding cassette exporter indicate its relatedness to that of colicin V. Antimicrob Agents Chemother 2001;45:969–72.
- Gaggero C, Moreno F, Lavina M. Genetic analysis of microcin H47 antibiotic system. J Bacteriol 1993;175:5420–7.
- Rodriguez E, Gaggero C, Lavina M. The structural gene for microcin H47 encodes a peptide precursor with antibiotic activity. Antimicrob Agents Chemother 1999;43:2176–82.
- Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 2003;149:2557–70.
- Homburg S, Oswald E, Hacker J, Dobrindt U. Expression analysis of the colibactin gene cluster coding for a novel polyketide in Escherichia coli. FEMS Microbiol Lett 2007;275:255–62.
- Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, . Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 2006;313:848–51.
- Zijlstra JB, Beukema J, Wolthers BG, Byrne BM, Groen A, Dankert J. Pretreatment methods prior to gas chromatographic analysis of volatile fatty acids from faecal samples. Clin Chim Acta 1977;78:243–50.
- Schmidt DS. Molekulare Analyse des probiotischen Stamms Escherichia coli Nissle 1917. Dissertation, Technische Universität Dresden, 2009.
- Dobrindt U. (Patho)-Genomics of Escherichia coli. Int J Med Microbiol 2005;295:357–71.
- Sun J, Gunzer F, Westendorf AM, Buer J, Scharfe M, Jarek M, . Genomic peculiarity of coding sequences and metabolic potential of probiotic Escherichia coli strain Nissle 1917 inferred from raw genome data. J Biotechnol 2005;117:147–61.
- Feldgarden M, Golden S, Wilson H, Riley MA. Can phage defence maintain colicin plasmids in Escherichia coli? Microbiology 1995;141:2977–84.
- Reissbrodt R, Hammes WP, Dal Bello F, Prager R, Fruth A, Hantke K, . Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol Lett 2009;290:62–9.
- Sonnenborn U, Greinwald R. 1991. Beziehungen zwischen Wirtsorganismus und Darmflora unter besonderer Berücksichtigung von Physiologie und Funktion der normalen Escherichia-coli-Flora. Stuttgart: Schattauer Verlag.
- Halbert SP. The antagonism of coliform bacteria against shigellae. J Immunol 1948;58:153–67.
- Mayr-Harting A, Hedges AJ, Berkeley RCW. Methods for studying bacterocins. Methods Microbiol 1972;7a: 315–422.
- Denou E, Rezzonico E, Panoff JM, Arigoni F, Brüssow H. A mesocosm of Lactobacillus johnsonii, Bifidobacterium longum, and Escherichia coli in the mouse gut. DNA Cell Biol 2009;28:413–22.
- Lorenz A, Schulze J. Establishment of E. coli Nissle 1917 and its interaction with Candida albicans in gnotobiotic rats. Microecol Ther 1996;24:45–51.
- Mandel L, Trebichavsky I, Splichal I, Schulze J. Stimulation of intestinal immune cells by E. coli in gnotobiotic piglets. Mestecky J, Russell MW, Jackson S, Michalek SM, Tlaskalova-Hogenova H, Sterzl J. Advances in mucosal immunology. New York: Plenum Press. 1995. 463–4.
- Schulze J, Lorenz A, Mandel L. Colonisation of Escherichia coli in different gnotobiotic animal models. Microb Ecol Health Dis 1992;5:iv–v.
- Leatham MP, Banerjee S, Autieri SM, Mercado-Lubo R, Conway T, Cohen PS. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect Immun 2009;77:2876–86.
- Lodinova-Zadnikova R, Sonnenborn U. Effect of preventive administration of a nonpathogenic Escherichia coli strain on the colonization of the intestine with microbial pathogens in newborn infants. Biol Neonate 1997;71:224–32.
- Schröder H. Entwicklung der aeroben Darmflora bei Neugeborenen nach Kolonisierung mit dem E. coli-Stamm Nissle 1917. Der Kinderarzt 1992;23:1619–25.
- Swidsinski A, Swidsinski S, Godzun A, Orthner M, Lochs H. Therapy with E. coli Nissle reduces concentrations of mucosa associated colonic flora in patients with ulcerative colitis. Gastroenterology 2000;118:A1138.
- Altenhoefer A, Oswald S, Sonnenborn U, Enders C, Schulze J, Hacker J, . The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol Med Microbiol 2004;40:223–9.
- Oelschlaeger TA, Altenhoefer A, Hacker J. Inhibition of Salmonella typhimurium invasion into intestinal cells by the probiotic E. coli strain Nissle 1917. Gastroenterology 2001; 120(5 Suppl 1):A-326.
- Boudeau J, Glasser AL, Julien S, Colombel JF, DarfeuilleMichaud A. Inhibitory effect of probiotic Escherichia coli strain Nissle 1917 on adhesion to and invasion of intestinal epithelial cells by adherent-invasive E. coli strains isolated from patients with Crohn's disease. Aliment Pharmacol Ther 2003;18:45–56.
- Duncker S. Auswirkung von oral verabreichtem Escherichia coli Nissle 1917 auf das Darm-assoziierte Immunsystem des Schweins. Dissertation, Tierärztl, Hochschule Hannover, 2005.
- Duncker SC, Lorentz A, Schroeder B, Breves G, Bischoff SC. Effect of orally administered probiotic E. coli strain Nissle 1917 on intestinal mucosal immune cells of healthy young pigs. Vet Immunol Immunopathol 2006;111:239–50.
- Hockertz S. Immunmodulierende Wirkung von abgetöteten apathogenen Escherichia coli Stamm Nissle 1917 auf das Makrophagensystem. Arzneim-Forsch/Drug Res 1991;41:1108–12.
- Cross ML, Ganner A, Teilab D, Fray LM. Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol Med Microbiol 2004;42:173–80.
- Fink LN, Frokiaer H. Dendritic cells from Peyer's patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scand J Immunol 2008;68:270–9.
- Helwig U, Lammers KM, Rizzello F, Brigidi P, Rohleder V, Caramelli E, . Lactobacilli, bifidobacteria and E. coli Nissle induce pro- and anti-inflammatory cytokines in peripheral blood mononuclear cells. World J Gastroenterol 2006;12:5978–86.
- Kamada N, Maeda K, Inoue N, Hisamatsu T, Okamoto S, Hong KS, . Non-pathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect Immun 2008;76:214–20.
- Krämer S, Sellge G, Lorentz A, Krueger D, Schemann M, Feilhauer K, . Selective activation of human intestinal mast cells by Escherichia coli hemolysin. J Immunol 2008;181:1438–45.
- Otte JM, Podolsky DK. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am J Physiol Gastrointest Liver Physiol 2004;286:G613–26.
- Rasche C, Wolfram C, Wahls M, Worm M. Differential immunomodulating effects of inactivated probiotic bacteria on the allergic immune response. Acta Derm Venereol 2007;87:305–11.
- Sturm A, Rilling K, Baumgart DC, Gargas K, AbouGhazale T, Raupach B, . Escherichia coli Nissle 1917 distinctively modulates T-cell cycling and expansion via toll-like receptor 2 signaling. Infect Immun 2005;73:1452–65.
- Ukena SN, Westendorf AM, Hansen W, Rohde M, Geffers R, Coldewey S, . The host response to the probiotic Escherichia coli strain Nissle 1917: specific up-regulation of the proinflammatory chemokine MCP-1. BMC Med Genet 2005;6:1–13.
- Wehkamp J, Harder J, Wehkamp K, Wehkamp-von Meissner B, Schlee M, Enders C, . NF-κB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun 2004;72:5750–8.
- Zyrek AA, Cichon C, Helms S, Enders C, Sonnenborn U, Schmidt MA. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCZeta redistribution resulting in tight junction and epithelial barrier repair. Cell Microbiol 2007;9:804–16.
- Maeda K, Inoue N, Kamada N, Hong KS, Ogata H, Ishii H, . Non-pathogenic Escherichia coli inhibits signal transduction pathway in intestinal epithelia cells: a novel mechanism of action. Gastroenterology 2004;126(4 Suppl 2): A-577.
- Otte JM, Mahjurian-Namari R, Brand S, Werner I, Schmidt WE, Schmitz F. Probiotics regulate the expression of COX-2 in intestinal epithelial cells. Nutr Cancer 2009;61:103–13.
- Grabig A, Paclik D, Dankof A, Baumgart DC, Erckenbrecht J, Raupach B, . Escherichia coli strain Nissle 1917 ameliorates experimental colitis via Toll-like receptor 2- and Tolllike receptor 4-dependent pathways. Infect Immun 2006;74:4075–82.
- Guzy C, Paclik D, Schirbel A, Sonnenborn U, Wiedenmann B, Sturm A. The probiotic Escherichia coli strain Nissle 1917 induces gammadelta T cell apoptosis via caspase- and FasL-dependent pathways. Int Immunol 2008;20:829–40.
- Trebichavsky I, Cukrowska B, Kozakova H, Schulze J, Rehakova Z, Sinkora J, . Association of germ-free piglets with two non-pathogenic E. coli strains: Nissle 1917 stimulates the immune system more than O86 strain. J Mol Med 1998;76:B-9.
- Lodinová-Zádníková R, Tlaskalová-Hogenová H, Sonnen-born U. Local and serum antibody response in fullterm and premature infants after artificial colonization of the intestine with E. coli strain Nissle 1917 (Mutaflor). Pediatr Allergy Immunol 1992;3:43–8.
- Cukrowska B, Lodinová-Zádníková R, Enders C, Sonnen-born U, Schulze J, Tlaskalová-Hogenová H. Specific proliferative and antibody responses of premature infants to intestinal colonization with nonpathogenic probiotic E. coli strain Nissle 1917. Scand J Immunol 2002;55:204–9.
- Bohn E, Bechtold O, Zahir N, Frick JS, Reimann J, Jilge B, . Host gene expression in the colon of gnotobiotic interleukin-2-deficient mice colonized with commensal colitogenic or noncolitogenic bacterial strains: common patterns and bacteria strain specific signatures. Inflamm Bowel Dis 2006;12:853–62.
- Waidmann M, Bechtold O, Frick JS, Lehr HA, Schubert S, Dobrindt U, . Bacteroides vulgatus protects against Escherichia coli-induced colitis in gnotobiotic interleukin-2-deficient mice. Gastroenterology 2003;125:162–77.
- Arribas B, Rodriguez-Cabezas ME, Camuesco D, Comalada M, Ballon E, Utrilla P, . A probiotic strain of Escherichia coli, Nissle 1917, given orally exerts local and systemic anti-inflammatory effects in lipopolysaccharide-induced sepsis in mice. Br J Pharmacol 2009;157:1024–33.
- Hudcovic T, Stepankova R, Kozakova H, Hrncir T, Tlaskalova-Hogenova H. Effects of monocolonization with Escherichia coli strains O6K13 and Nissle 1917 on the development of experimentally induced acute and chronic intestinal inflammation in germ-free immunocompetent and immunodeficient mice. Folia Microbiol 2007;52:618–26.
- Kamada N, Inoue N, Hisamatsu T, Okamoto S, Matsuoka K, Sato T, . Nonpathogenic Escherichia coli strain Nissle 1917 prevents murine acute and chronic colitis. Inflamm Bowel Dis 2005;11:455–63.
- Kokesova A, Frolova L, Kverka M, Sokol D, Rossmann P, Bartova J, . Oral administration of probiotic bacteria (E. coli Nissle, E. coli O83, Lactobacillus casei) influences the severity of dextran sodium sulfate-induced colitis in BALB/c mice. Folia Microbiol 2006;51:478–84.
- Schultz M, Strauch UG, Linde HJ, Watzl S, Obermeier F, Göttl C, . Preventive effects of Escherichia coli strain Nissle 1917 on acute and chronic intestinal inflammation in two different murine models of colitis. Clin Diagn Lab Immunol 2004;11:372–8.
- Ukena SN, Singh A, Dringenberg U, Engelhardt R, Seidler U, Hansen W, . Probiotic Escherichia coli Nissle 1917 inhibits leaky gut by enhancing mucosal integrity. PLoS One 2007;2:e1308.
- Bickert T, Trujillo-Vargas CM, Duechs M, Wohlleben G, Polte T, Hansen G, . Probiotic Escherichia coli Nissle 1917 suppresses allergen-induced Th2 responses in the airways. Int Arch Allergy Immunol 2009;149:219–30.
- Hockertz S. Augmentation of host defence against bacterial and fungal infections of mice pretreated with the non-pathogenic Escherichia coli strain Nissle 1917. Arzneim-Forsch/Drug Res 1997;47(I):793–6.
- Von Bünau R, Jaekel L, Schubotz E, Schwarz S, Stroff T, Krüger M. Escherichia coli strain Nissle 1917: significant reduction of neonatal calf diarrhea. J Dairy Sci 2005;88:317–23.
- Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol 2007;8:25–43.
- Zoumpopoulou G, Tsakalidou E, Dewulf J, Pot B, Grangette C. Differential crosstalk between epithelial cells, dendritic cells and bacteria in a co-culture model. Int J Food Microbiol 2009;131:40–51.
- Schultz M, Butt AG. Methodological aspects in probiotic research – a brief review with an emphasis on epithelial barrier function. Int J Probiotics Prebiotics 2007;2:85–96.
- Cichon C, Enders C, Sonnenborn U, Schmidt MA. DNA-microarray-based comparison of cellular responses in polarized T84 epithelial cells triggered by probiotics: E. coli Nissle 1917 (EcN) and Lactobacillus acidophilus PZ1041. Gastroenterology 2004;126(4 Suppl. 2):A-578–9.
- Wehkamp J, Harder J, Wehkamp-von Meissner B, Schwichtenberg L, Fellermann K, Herrlinger KR, . The probiotic E. coli Nissle 1917 (Mutaflor) induces defensins in intestinal epithelial cells: a novel mechanism of action. Gastroenterology 2002;122(4 Suppl):A75–6.
- Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin-2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun 2007;75:2399–407.
- Hafez M, Hayes K, Goldrick M, Warhurst G, Grencis R, Roberts IS. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect Immun 2009;77:2995–3003.
- Konturek PC, Brzozowski TM, Burnat G, Pajdo R, Hahn EG, Konturek SJ. Protective effects of probiotic Echerichia coli Nissle 1917 (EcN) bacterial strain against stress-induced acute gastric mucosal lesions. Gastroenterology 2007;132(4 Suppl S1):A-411.
- Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, . Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol 1997;50:113–23.
- Kido J, Kido R, Fagerhol MK, Nagata T. Calprotectin release from human neutrophils is induced by Porphyromonas gingivalis lipopolysacccharide via the CD14-Toll-like receptor-nuclear factor kappaB pathway. J Period Res 2003;38:557–63.
- Nisapakultorn K, Ross KF, Herzberg MC. Calprotectin expression inhibits bacterial binding to mucosal epithelial cells. Infect Immun 2001;69:3692–6.
- Splichal I, Fagerhol MK, Trebichavsky I, Splichalova A, Schulze J. The effect of intestinal colonization of germ-free pigs with Escherichia coli on calprotectin levels in plasma, intestinal and bronchoalveolar lavages. Immunobiology 2005;209:681–7.
- Hooper LV, Gordon JI. Commensal host–bacterial relationships in the gut. Science 2001;292:1115–18.
- Hord NG. Eukaryotic-microbiota crosstalk: potential mechanisms for health benefits of prebiotics and probiotics. Annu Rev Nutr 2008;28:215–31.
- Horswill AR, Nauseef WM. Host interception of bacterial communication signals. Cell Host Microbe 2008;4:507–9.
- Köhler H, McCormick BA, Walker WA. Bacterial–enterocyte crosstalk: cellular mechanisms in health and disease. J Pediatr Gastroenterol Nutr 2003;36:175–85.
- Spiller R, Campbell E. Post-infectious irritable bowel syndrome. Curr Opin Gastroenterol 2006;22:13–7.
- Spiller RC. Postinfective bowel dysfunction. Curr Opin Gastroenterol 1997;13:85–9.
- Liebregts T, Adam B, Bertel A, Jones S, Schulze J, Enders C, . Effect of E. coli Nissle 1917 on post-inflammatory visceral sensory function in a rat model. Neurogastroenterol Motil 2005;17:410–14.
- Bär F, von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, . Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil 2009;21:559–66.
- Goel A, Mittal A, Evstatiev R, Nemeth M, Kruis W, Stolte M, . In vivo effects of mesalazine or E. coli Nissle 1917 on microsatellite instability in ulcerative colitis. Aliment Pharmacol Ther 2009;30:634–42.
- Kruis W, Fric P, Pokrotnieks J, Lukas M, Fixa B, Kascak M, . Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004;53:1617–23.
- Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res 1983;113:173–215.
- Speit G, Hartmann A. The comet assay: a sensitive genotoxicity test for the detection of DNA damage. Methods Mol Biol 2005;291:85–95.
- Sonnenborn U, Janosch D, Dubbert S, Stroff T, Schulze L, Müller H. Antimutagenic activity of the probiotic E. coli strain Nissle 1917. J Crohn's Colitis 2009;3:136.
- Schultz M, Watzl S, Ölschläger T, Rath HC, Göttl C, Lehn N, . Green fluorescent protein for detection of the probiotic microorganism Escherichia coli strain Nissle 1917 (EcN) in vivo. J Microbiol Methods 2005;61:389–98.
- Westendorf AM, Gunzer F, Deppenmeier S, Tapadar D, Hunger JK, Schmidt MA, . Intestinal immunity of Escherichia coli Nissle 1917: a safe carrier for therapeutic molecules. FEMS Immunol Med Microbiol 2005;43:373–84.
- Nagy G, Dobrindt U, Grozdanov L, Hacker J, Emödy L. Transcriptional regulation through RfaH contributes to intestinal colonization by Escherichia coli. FEMS Microbiol Lett 2005;244:173–80.
- Monteiro C, Saxena I, Wang X, Kader A, Bokranz W, Simm R, . Characterization of cellulose production in Escherichia coli Nissle 1917 and its biological consequences. Environ Microbiol 2009;11:1105–16.
- Brombacher E, Dorel C, Zehnder AJB, Landini P. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 2003;149:2847–57.
- Kuhnert P, Hacker J, Mühldorfer I, Burnens AP, Nicolet J, Frey J. Detection system for Escherichia coli-specific virulence genes: absence of virulence determinants in B and C strains. Appl Environ Microbiol 1997;63:703–9.
- Ott M, Hoschützky H, Jann K, Van Die I, Hacker J. Gene clusters for S fimbrial adhesin (sfa) and F1C fimbriae (foc) of Escherichia coli: comparative aspects of structure and function. J Bacteriol 1988;170:3983–90.
- Zingler G, Ott M, Blum G, Falkenhagen U, Naumann G, Sokolowska-Köhler W, . Clonal analysis of Escherichia coli serotype O6 strains from urinary tract infections. Microb Pathog 1992;12:299–310.
- Zingler G, Blum G, Falkenhagen U, Orskov I, Orskov F, Hacker J, . Clonal differentiation of uropathogenic Escherichia coli isolates of serotype O6:K5 by fimbrial antigen typing and DNA long-range mapping techniques. Med Microbiol Immunol 1993;182:13–24.
- Valdebenito M, Crumbliss AL, Winkelmann G, Hantke K. Environmental factors influence the production of enterobactin, salmochelin, aerobactin, and yersiniabactin in Escherichia coli strain Nissle 1917. Int J Med Microbiol 2006;296:513–20.
- Malchow H, Sonnenborn U, Greinwald R, Körner A. Colonization of adults by an apathogenic E. coli strain administered after gut decontamination. Gastroenterology 1995; 108:A-869.
- Jöres-Nguyen-Xuan TH, Boehm SK, Joeres L, Schulze J, Kruis W. Survival of the probiotic Escherichia coli Nissle 1917 (EcN) in the gastrointestinal tract given in combination with oral mesalamine to healthy volunteers. Inflamm Bowel Dis 2009 July 27 [Epub ahead of print].
- Prilassnig M, Wenisch C, Daxboeck F, Feierl G. Are probiotics detectable in human feces after oral uptake by healthy volunteers? Wien Klin Wochenschr 2007;119:1–7.
- Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol Rev 2006;30:487–513.
- Besselink MGH, van Santvoort HC, Buskens E, Boer-meester MA, van Goor H, Timmerman HM, . Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008;371:651–9.
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr 2006;83:1256–64.
- Ezendam J, Van Loveren H. Probiotics: immunomodulation and evaluation of safety and efficacy. Nutr Rev 2006;64:1–14.
- Mattia A, Merker R. Regulation of probiotic substances as ingredients in foods: premarket approval or “generally recognized as safe” notification. Clin Infect Dis 2008;46(Suppl 2): 115–8.
- Snydman DR. The safety of probiotics. Clin Infect Dis 2008;46(Suppl 2):104–11.
- Vankerckhoven V, Huys G, Vancanneyt M, Vael C, Klare I, Romond M-B, . Biosafety assessment of probiotics used for human consumption: recommendations from the EUPROSAFE project. Trends Food Sci Tech 2008;19:102–14.
- Wassenaar TM, Klein G. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J Food Protect 2008;71:1734–41.
- Blum G, Falbo V, Caprioli A, Hacker J. Gene clusters encoding the cytotoxic necrotizing factor type 1, Prsfimbriae and alpha-hemolysin form the pathogenicity island II of the uropathogenic Escherichia coli strain J96. FEMS Microbiol Lett 1995;126:189–95.
- Evans DJJr, Evans DG. Classification of pathogenic Escherichia coli according to serotype and the production of virulence factors, with special reference to colonization-factor antigens. Rev Infect Dis 1983;5(Suppl 4):S692–701.
- Levine MM. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis 1987;155:377–89.
- Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev 1998;11:142–201.
- Orskov I, Orskov F. Escherichia coli in extra-intestinal infections. J Hyg 1985;95:551–75.
- Karch H, Meyer T. Evaluation of oligonucleotide probes for identification of Shiga-like-toxin-producing Escherichia coli. J Clin Microbiol 1989;27:1180–6.
- Mandel L, Travnicek J. The minipig as a model in gnotobiology. Die Nahrung 1987;31:613–17.
- Sereny B. Experimental keratoconjunctivitis Shigellosa. Acta Microbiol Hung 1957;4:367–76.
- Beutin L, Gleier K, Kontny I, Echeverria P, Scheutz F. Origin and characteristics of enteroinvasive strains of Escherichia coli (EIEC) isolated in Germany. Epidemiol Infect 1997;118:199–205.
- Small PLC, Isberg RR, Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yers-inia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within Hep-2-cells. Infect Immun 1987;55:1674–9.
- Tröger H, Richter J, Beutin L, Epple HJ, Fromm M, Zeitz M, . Wege der bakteriellen Translokation in Kolon-Epithelzellen unter dem Einfluss pro-inflammatorischer Zytokine. Z Gastroenterol 2003;41:771.
- Reeves P. Role of O-antigen variation in the immune response. Trends Microbiol 1995;3:381–6.
- Cassels FJ, Wolf MK. Colonization factors of diarrheagenic E. coli and their intestinal receptors. J Ind Microbiol 1995;15:214–26.
- Zingler G, Schmidt G, Orskov I, Orskov F, Falkenhagen U, Naumann G. K-antigen identification, hemolysin production, and hemagglutination types of Escherichia coli O6 strains isolated from patients with urinary tract infections. Zbl Bakt 1990;274:372–81.
- Gaastra W, Svennerholm AM. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol 1996;4:444–52.
- Klemm P. Fimbrial adhesins of Escherichia coli. Rev Infect Dis 1985;7:321–40.
- Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev 1991;4:80–128.
- Marre R, Hacker J, Henkel W, Goebel W. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect Immun 1986;54:761–7.
- NCCLS(National Committee for Clinical Laboratory Standards). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th edn. Approved standard M7–A4. 1997.
- Frick JS, Zahir N, Kirschning C, Bohn E, Autenrieth IB. Commensal bacteria differentially modulate cytokine expression in dendritic cells. Int J Med Microbiol 2005;295:131.
- Bleich A, Sundberg JP, Smoczek A, Von Wasielewski R, De Buhr MF, Janus LM, . Sensitivity to Escherichia coli Nissle 1917 in mice is dependent on environment and genetic background. Int J Exp Pathol 2008;89:45–54.
- Burmeister M, Ulanovsky L. 1992. Pulsed-field gel electrophoresis; protocols, methods, and theories. Totowa, NJ: Humana Press.
- Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res 1991;19:6823–31.
- Hardy KG. 1987. Plasmids – a practical approach. Washington, DC: IRL Press.
- Oswald S. Einführung von Virulenz-assoziierten Antigenen in den Escherichia coli-Carrier-Stamm DSM 6601. Dissertation, Bayr.Julius-Maximilians-Universität Würzburg, 1997.
- Datz M, Janetzki-Mittmann C, Franke S, Gunzer F, Schmidt H, Karch H. Analysis of the enterohemorrhagic Escherichia coli O157 DNA region containing lambdoid phage gene P and Shiga-like toxin structural genes. Appl Environ Micro-biol 1996;62:791–7.
- Magistrelli C, Colombo E, Tognoni A, Grandi G. Transfection of E. coli with lambda DNA by electroporation. Micro-biologica 1992;15:397–8.
- Smith HW, Green P, Parsell Z. Vero cell toxins in Escherichia coli and related bacteria: transfer by phage and conjugation and toxic action in laboratory animals, chickens, and pigs. J Gen Microbiol 1983;129:3121–37.
- Krammer HJ, Schlieger F, Harder H, Franke A, Singer MV. Probiotika in der Therapie des Reizdarmsyndroms. Z Gastroenterol 2005;43:467–71.
- Kruis W, Chrubasik S, Stange C, Schulze J. Therapeutische Wirksamkeit des probiotischen E. coli-Stammes Nissle 1917 bei Reizdarm-Subgruppen. Z Gastroenterol 2004;42:785.
- Plaßmann D, Schulte-Witte H. Therapie des Reizdarmsyndroms mit Escherichia coli Stamm Nissle 1917 (EcN). Eine retrospektive Untersuchung. Med Klin 2007;102:888–92.
- Tromm A, Niewerth U, Khoury M, Baestlein E, Wilhelms G, Schulze J, . The probiotic E. coli strain Nissle 1917 for the treatment of collagenous colitis: first results of an open-label trial. Z Gastroenterol 2004;42:365–9.
- Goerg KJ, Schlörer E. Probiotische Therapie einer pseudomembranösen Kolitis. Dtsch Med Wochenschr 1998;123:1274–8.
- Goerg KJ, Wybierala M, Rauen-Vossloh J, Hader C. A new approach in pseudomembranous colitis: probiotic Escherichia coli Nissle 1917 after intestinal lavage. Eur J Gastroenterol Hepatol 2008;20:155–6.
- Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Treatment of mild to moderate acute attacks of distal ulcerative colitis with rectally-administered E. coli Nissle 1917: dose-dependent efficacy. Gastroenterology 2006;130(4 Suppl 2):A-119.
- Malchow H. Crohn's disease and Escherichia coli. A new approach in therapy to maintain remission of colonic Crohn's disease? J Clin Gastroenterol 1997;25:653–8.
- Fric P, Zavoral M. The effect of non-pathogenic Escherichia coli in symptomatic uncomplicated diverticular disease of the colon. Eur J Gastroenterol Hepatol 2003;15:313–15.
- Kuzela L, Kascak M, Vavrecka A. Induction and maintenance of remission with nonpathogenic Escherichia coli in patients with pouchitis. Am J Gastroenterol 2001;96:3218–19.
- Henker J, Schuster F, Nissler K. Successful treatment of gut-caused halitosis with a suspension of living non-pathogenic Escherichia coli bacteria – a case report. Eur J Pediatr 2001;160:592–4.
- Lata J, Novotny I, Pribramska V, Jurankova J, Fric P, Kroupa R, . The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol 2007;19:1111–3.
- Wurzel RM. Prophylaxe der polymorphen Lichtdermatose. Aktuel Dermatol 1999;25:329–33.
- Hibi T, Inoue N, Ogata H, Naganuma M. Introduction and overview: recent advances in the immunotherapy of inflammatory bowel disease. J Gastroenterol 2003;38(Suppl 15): 36–42.
- Loftus EVJr. Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–17.
- Mathew CG, Lewis CM. Genetics of inflammatory bowel disease: progress and prospects. Hum Mol Genet 2004;13:R161–8.
- Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmunity Reviews 2004;3:394–400.
- Mahida YR, Rolfe VE. Host–bacterial interactions in inflammatory bowel disease. Clin Sci 2004;107:331–41.
- Betsi GI, Papadavid E, Falagas ME. Probiotics for the treatment or prevention of atopic dermatitis: a review of the evidence from randomized controlled trials. Am J Clin Dermatol 2008;9:93–103.
- Böhm SK, Kruis W. Probiotics. Do they help to control intestinal inflammation?Domschke WW, Kagnoff MF, Kucharzik TF, Mayer LF, Targan SR. Inflammatory bowel disease. Genetics, barrier function, immunologic mechanisms, and microbial pathways. Ann N Y Acad Sci 2006;1072:339–50.
- Duchmann R. The role of probiotics and antibiotics in regulating mucosal inflammation. Neurath MF, Blum-berg RS. Immune mechanisms in inflammatory bowel disease. Advances in Experimental Medicine and Biology, 579. Berlin: Springer Science. 2006.219–26.
- Ewaschuk JB, Dieleman LA. Probiotics and prebiotics in chronic inflammatory bowel diseases. World J Gastroenterol 2006;12:5941–50.
- Fedorak RN, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2008. J Clin Gastroenterol 2008;42(Suppl 2):S97–103.
- Floch MH. Probiotics, irritable bowel syndrome, and inflammatory bowel disease. Curr Treat Options Gastroenterol 2003;6:283–8.
- Floch MH, Montrose DC. Use of probiotics in humans: an analysis of the literature. Gastroenterol Clin North Am 2005;34:547–70.
- Gionchetti P, Rizzello F, Lammers KM, Morselli C, Sollazzi L, Davies S, . Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol 2006;12:3306–13.
- Jones JL, Foxx-Orenstein AE. Probiotics in inflammatory bowel disease. Pract Gastroenterol 2006;30:44–50.
- Kligler B, Hanaway P, Cohrssen A. Probiotics in children. Pediatr Clin North Am 2007;54:949–67.
- Kwon JH, Farrell RJ. Probiotics and inflammatory bowel disease. BioDrugs 2003;17:179–86.
- Marteau P, Seksik P, Jian R. Probiotics and intestinal health effects: a clinical perspective. Br J Nutr 2002;88(Suppl 1): S51–7.
- Marteau P, Seksik P, Jian R. Probiotics and health: new facts and ideas. Curr Opin Biotechnol 2002;13:486–9.
- Sartor RB. Therapeutic manipulation of the enteric micro-flora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology 2004;126:1620–33.
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94.
- Kruis W, Schütz E, Fric P, Fixa B, Judmaier G, Stolte M. Double-blind comparison of an oral Escherichia coli preparation and mesalazine in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther 1997;11:853–8.
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon ATR. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet 1999;354:635–9.
- Burke DA, Axon ATR. Adhesive Escherichia coli in inflammatory bowel disease and infective diarrhoea. BMJ 1988;297:102–4.
- Hoffmann JC, Zeitz M, Bischoff SC, Brambs HJ, Bruch HP, Buhr HJ, . Diagnostik und Therapie der Colitis Ulcerosa: Ergebnisse einer evidenzbasierten Konsensuskonferenz der Deutschen Gesellschaft für Verdauungs- und Stoffwechselerkrankungen zusammen mit dem Kompeten-znetz chronisch entzündliche Darmerkrankungen. Z Gastroenterol 2004;42:979–83.
- Stange EF, Riemann J, Von Herbay A, Lochs H, Fleig WE, Schölmerich J, . Diagnostik und Therapie der Colitis ulcerosa - Ergebnisse einer evidenzbasierten Konsensuskon-ferenz der Deutschen Gesellschaft für Verdauungs- und Stoffwechselkrankheiten. Leitlinien der DGVS. Z Gastro-enterol 2001;39:19–72.
- Travis SPL, Stange EF, Lémann M, Oresland T, Bemelman WA, Chowers Y. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohn's Colitis 2008;2:24–62.
- Vogelsang H, Tilg H, Petritsch W, Knoflach P. Colitis ulce-rosa: medikamentöse Rezidivprophylaxe. Österreichische Ärztezeitung 2003;1/2:40–1.
- Henker J, Müller S, Laass MW, Schreiner A, Schulze J. Probiotic Escherichia coli Nissle 1917 (EcN) for successful remission maintenance of ulcerative colitis in children and adolescents: an open-label pilot study. Z Gastroenterol 2008;46:874–5.
- Bruckschen E, Horosiewicz H. Chronische Obstipation, Vergleich von mikrobiologischer Therapie und Lactulose. Münch Med Wochenschr 1994;16:241–5.
- Möllenbrink M, Bruckschen E. Behandlung der chronischen Obstipation mit physiologischen Escherichia-coli-Bakterien. Med Klin 1994;89:587–93.
- Hernando-Harder AC, Von Bünau R, Nadarajah M, Singer MV, Harder H. Influence of E. coli Strain Nissle 1917 (EcN) on intestinal gas dynamics and abdominal sensation. Dig Dis Sci 2008;53:443–50.
- Henker J, Laass M, Blokhin BM, Bolbot YK, Maydannik VG, Elze M, . The probiotic Escherichia coli strain Nissle 1917 (EcN) stops acute diarrhoea in infants and toddlers. Eur J Pediatr 2007;166:311–8.
- Henker J, Laass MW, Blokhin BM, Maydannik VG, Bolbot YK, Elze M, . Probiotic Escherichia coli Nissle 1917 versus placebo for treating diarrhea of greater than 4 days duration in infants and toddlers. Pediatr Infect Dis J 2008;27:494–9.
- Fuller R. History and development of probiotics. 1992. Fuller R. Probiotics. London: Champman & Hall. 1–8.
- Bendali F, Sanaa M, Bichet H, Schelcher F. Risk factors associated with diarrhoea in newborn calves. Vet Res 1999;30:509–22.
- Schroeder B, Duncker S, Barth S, Bauerfeind R, Gruber AD, Deppenmeier S, . Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci 2006;51:724–31.
- Drisko JA, Giles CK, Bischoff BJ. Probiotics in health maintenance and disease prevention. Altern Med Rev 2003;8:143–55.
- Autieri SM, Lins JJ, Leatham MP, Laux DC, Conway T, Cohen PS. L-fucose stimulates utilization of D-ribose by Escherichia coli MG1655 (Delta)fucAO and E. coli Nissle 1917 (delta)fucAO mutants in the mouse intestine and in M9 minimal medium. Infect Immun 2007;75:5465–75.
- Brader P, Stritzker J, Riedl CC, Zanzonico P, Cai S, Burnazi EM, . Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clin Cancer Res 2008;14:2295–302.
- Coldewey SM, Hartmann M, Schmidt DS, Engelking U, Ukena S, Gunzer F. Impact of the rpoS genotype for acid resistance patterns of pathogenic and probiotic Escherichia coli. BMC Microbiol 2007;7:21.
- Hejnova J, Pages D, Rusniok C, Glaser P, Sebo P, Buchrieser C. Specific regions of genome plasticity and genetic diversity of the commensal Escherichia coli A0 34/86. Int J Med Microbiol 2006;296:541–6.
- Mundy R, Girard F, FitzGerald AJ, Frankel G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiol Lett 2006;265:126–32.
- Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol 2007;297:151–62.
- Duan F, March JC. Interrupting Vibrio cholerae infection of human epithelial cells with engineered commensal bacterial signaling. Biotechnol Bioeng 2008;101:128–34.
- Duan F, Curtis KL, March JC. Secretion of insulinotropic proteins by commensal bacteria: rewiring the gut to treat diabetes. Appl Environ Microbiol 2008;74:7437–8.
- Rao S, Hu S, McHugh L, Lueders K, Henry K, Zhao Q, . Toward a live microbial microbicide for HIV: commensal bacteria secreting an HIV fusion inhibitor peptide. Proc Natl Acad Sci U S A 2005;102:1993–8.
- Remer KA, Bartrow M, Roeger B, Moll H, Sonnenborn U, Oelschlaeger TA. Split immune response after oral vaccination of mice with recombinant Escherichia coli Nissle 1917 expressing fimbrial adhesin K88. Int J Med Microbiol 2009;299:467–78.
- Buddenborg C, Daudel D, Liebrecht S, Greune L, Humberg V, Schmidt MA. Development of a tripartite vector system for live oral immunization using a Gram-negative probiotic carrier. Int J Med Microbiol 2008;298:105–14.