Abstract
Apoptotic cells are recognized and cleared by the innate immune system. This process severely influences the outcome of several important functions of the latter. Apoptotic cells serve as sources for autoantigens employed by immature dendritic cells to maintain tolerance, excellent to prevent autoimmune diseases disastrous when this causes tumor tolerance. Apoptotic cells orchestrate healing and repopulation of the tissues, in which they died, again advantageous during wound healing and in the resolution phase of infections but a disaster after tumor reducing therapies. Many highly pathogenic microorganisms and viruses mimic changes of apoptotic cells like exposure of phosphatidyl serine and abuse their immune silencing signals to escape immune detection and eradication. If autologous chromatin is not properly cleared it is misinterpreted by the immune system as virus and causes the secretion by phagocytes of type-1 interferons. The continuous presence of these cytokines challenges tolerance and leads to SLE-like autoimmunity.
If one considers that about 50 × 109 cells die every day in the human body and that these cells possess around 300 mg of DNA that need to be disposed every single day by the phagocytic system, preferably without causing inflammation or triggering immune responses. If the clearance of dying cells and cell remnants fails, more than 300 mg of nucleic acids (DNA and RNA) tightly packed with proteins may turn into a dangerous endogenous cocktail. This is 7 and 10 orders of magnitude more than the antigenic load during an infection with hundred thousand bacteria or one million Herpes viruses, respectively. The latter conditions necessarily provoke protective immune responses that include inflammation and antigen presentation. Important signals that alert the innate immune sentinels are nucleic acids of the pathogens. To avoid continuous false alarms, the disposal of the endogenous chromatin requires mechanisms that mitigate these sentinels.
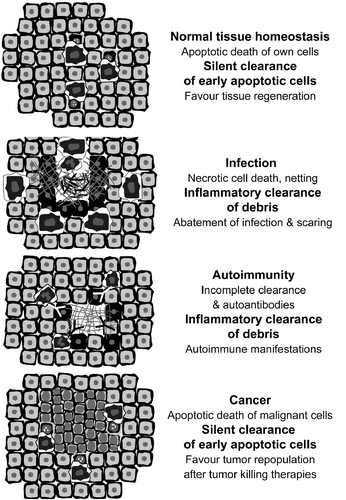
In normal conditions, apoptosis can be considered the starting event of the controlled elimination of superfluous cells. The macromolecules are pre-digested and the nuclear constituents are sequestered from the extracellular milieu. The completion of phagocytosis in early stages of apoptosis ensures the clearance of this material before it evolves to late stages of apoptosis or secondary necrosis. Accumulation of post-mortem cell remnants due to an inefficient clearance precipitates the initiation of autoimmunity in humans Citation[1–3].
There is an additional way of dying where chromatin plays further important roles post-mortem. This is the formation of neutrophil extracellular traps (NET). During bacterial infections or gout, chromatin is actively expelled from altruistic kamikaze-like neutrophils to avoid dissemination of pathogens and/or the inflammatory focus Citation[4,5]. The consequence is control of infection, confinement of inflammation and initiation of the scarring process. In the clearance deficiency scenario, this physiologic event may turn into a catastrophic incident that precipitates inflammation and autoimmune manifestations .
Figure 2. Apoptotic cells help to orchestrate the innate immune response, tissue healing and the maintenance of immunological tolerance.
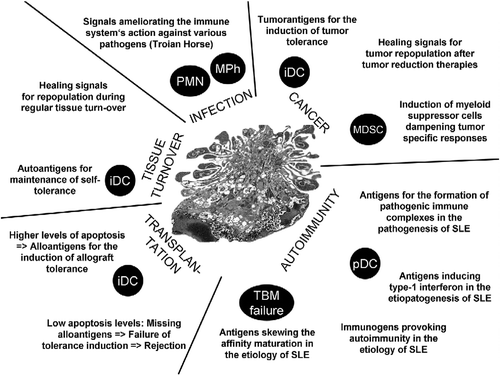
In patients with SLE (often deficient in the clearance of early apoptotic cells) extracellular chromatin is recognised by autoantibodies Citation[6] and forms nucleic acid containing immune complexes that turn out to be pro-inflammatory when taken up by the innate immune sentinels Citation[7,8]. Compared to the anti dsDNA autoantibodies and the nuclear remnants in separate, these immune complexes induce much more inflamemation and can, therefore, be considered binary pro-inflammatory pyrogens Citation[9,10].
The clearance of dying and dead own cells is a highly conserved process and a crucial homeostatic mechanism for multicellular organisms that still have many aspects to be elucidated. The molecular details of both cell death and phagocytosis have recently earned special attention of researchers in this field. The understanding of the immunomodulatory language of apoptotic cells results essential for the development of new classes of therapeutic and disease-modifying agents. The manipulation of this fundamental concept opens new avenues for research in the field of cancer, since malignant cells are often not recognized as “dangerous” and are even actively tolerated.
The induction of tumor cell death is a central goal of chemo- and radiotherapy. Recent evidence suggest that the induction of apoptosis by radiotherapy stimulates rapid tumor cell repopulation of surviving cells—a process crucially dependent on caspase 3 activity and partially mediated by PGE2 Citation[11]. Rendering malignant apoptotic cells immunogenic, e.g., by interfering with the clearance process is a future milestone of cancer research. The plethora of signals expressed or secreted by apoptotic cells and their influence on innate immune cells shall be carefully studied.
Declaration of interest : The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Herrmann M. Constant dripping wears away the stone. Autoimmunity. 2009; 42:249.
- Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, . Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002; 46:191–201.
- Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998; 41:1241–1250.
- Schorn C, Frey B, Lauber K, Janko C, Strysio M, Keppeler H, . Sodium overload and water influx activate the NALP3 inflammasome. J Biol Chem. 2011; 286:35–41.
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, . Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007; 176:231–241.
- Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, . Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010; 107:9813–9818.
- Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004; 50:1861–1872.
- Chaurio RA, Janko C, Munoz LE, Frey B, Herrmann M, Gaipl US. Phospholipids: key players in apoptosis and immune regulation. Molecules. 2009; 14:4892–4914.
- Munoz LE, Janko C, Grossmayer GE, Frey B, Voll RE, Kern P, . Remnants of secondarily necrotic cells fuel inflammation in systemic lupus erythematosus. Arthritis Rheum. 2009; 60:1733–1742.
- Munoz LE, Janko C, Chaurio RA, Schett G, Gaipl US, Herrmann M. IgG opsonized nuclear remnants from dead cells cause systemic inflammation in SLE. Autoimmunity. 2010; 43:232–235.
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, . Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011; 17:860–866.