Abstract
Blockade of the complement cascade at the C5a/C5a receptor (C5aR)-axis is believed to be an attractive treatment avenue in rheumatoid arthritis (RA). However, the effects of such interventions during the early phases of arthritis remain to be clarified. In this study we use the murine delayed-type hypersensitivity arthritis (DTHA) model to study the very early effects of a blocking, non-depleting anti-C5aR mAb on joint inflammation with treatment synchronised with disease onset, an approach not previously described. The DTHA model is a single-paw inflammatory arthritis model characterised by synchronised and rapid disease onset driven by T-cells, immune complexes and neutrophils. We show that a reduction in paw swelling, bone erosion, cartilage destruction, synovitis and new bone formation is apparent as little as 60 h after administration of a single dose of a blocking, non-depleting anti-mouse C5aR mAb. Importantly, infiltration of neutrophils into the joint and synovium is also reduced following a single dose, demonstrating that C5aR signalling during the early stage of arthritis regulates neutrophil infiltration and activation. Furthermore, the number of T-cells in circulation and in the draining popliteal lymph node is also reduced following a single dose of anti-C5aR, suggesting that modulation of the C5a/C5aR axis results in effects on the T cell compartment in inflammatory arthritis. In summary, these data demonstrate that blockade of C5aR leads to rapid and significant effects on arthritic disease development in a DTHA model strengthening the rationale of C5aR-blockade as a treatment strategy for RA, especially during the early stages of arthritis flare.
Introduction
The complement system is part of the innate humoral immune system, which reacts immediately to defend against pathogens. However, complement is also implicated in various auto-inflammatory diseases, including rheumatoid arthritis (RA), where it contributes to disease pathology [Citation1]. Immune complexes in the articular space of RA patients activate the complement system which results in the production of complement components of which many are highly active inflammatory proteins [Citation2]. The complement anaphylatoxin C5a, which is produced upon complement activation when C5 convertase cleaves C5 into C5a and C5b, is thought to be the main complement component responsible for tissue damage in RA [Citation3]. Levels of C5a protein have been found to be increased in the synovial fluid of RA patients [Citation4], and expression levels of C5a receptor (C5aR) on macrophages and fibroblasts in the synovium of RA patients have shown to correlate with the number of swollen joints [Citation5]. C5aR is expressed on many cell types, including granulocytes, monocytes, macrophages, dendritic cells, mast cells, osteoblasts and osteoclasts [Citation6–8]. Expression on T cells, and epithelial end endothelial cells [Citation9,Citation10], has also been reported. Dependent on the cell type, ligation of C5a to C5aR leads to endothelial adhesion, transendothelial migration and chemotaxis of cells [Citation6,Citation11,Citation12] and results in cell activation, evident by oxidative burst, granule release and release of inflammatory mediators such as TNFα, IL-6, IL-1β, vasoactive amines, matrix metalloproteinases [Citation11,Citation13,Citation14] and decreased apoptosis of neutrophils [Citation15]. C5a can also induce osteoclast differentiation from peripheral blood mononuclear cells (PBMCs) [Citation8]. All these events are thought to contribute to the tissue inflammation and destruction seen in RA. Data from animal models also implicate the C5a-C5aR axis in arthritis pathology. A vaccine that induces generation of anti-C5a antibodies has been shown to be effective in murine collagen-induced arthritis (CIA) in mice [Citation16], and oral treatment with a small molecule C5a antagonist reduced joint damage in the rat antigen-induced arthritis model [Citation17]. In several mouse models of arthritis C5aR-deficient mice show reduced disease development [Citation18–21]. In human C5aR knock-in mice, a mouse anti-human C5aR monoclonal antibody effectively ameliorated neutrophil-dependent K/B × N serum transfer-induced arthritis [Citation22], and it has been shown that anti-mouse C5aR mAb lowers disease activity when administered in the early stages of murine CIA [Citation23]. C5aR-blockade could potentially prevent or abrogate an arthritis flare in patients, and therefore the purpose of the present study was to study the early effects of a blocking, non-cell-depleting anti-C5aR mAb. For this purpose we chose the highly synchronised delayed-type hypersensitivity arthritis (DTHA) model, previously described by us [Citation24]. It is a single-paw arthritis model in C57BL/6 mice, induced by modifying a classical methylated bovine serum albumin (mBSA)-induced DTH foot pad response by i.v. administration of an 8–10-fold lower dose of the anti-type II collagen antibodies (anti-CII) than is required to induce collagen antibody-induced arthritis (CAIA) in the C57BL/6 strain. The mice display systemic manifestations and severe paw inflammation and bone erosion, but only in the antigen-challenged paw. The dose of anti-CII given to induce DTHA does not have any arthritogenic effect without combining it with the DTH foot pad response [Citation24]. The model is ideally suited for testing compounds thought to have an effect in the earliest stages of an arthritis flare development, as treatment onset can be synchronised precisely with disease onset, which is defined exactly by the experimenter as the time of foot pad challenge with mBSA. In addition, disease incidence is 100% and the variation is low. In contrast, treatment in the CIA model is often initiated at first sign of disease, a time when early disease processes have been ongoing for a while before the appearance of joint swelling. Additionally, disease incidence in the CIA model is less than 100% and the variation is relatively large.
In contrast to CAIA and the K/BxN models, disease development in DTHA is dependent on CD4+ T cells, and thus adds the dimension of the adaptive immune system while still being significantly immune-complex and neutrophil-driven [Citation24]. Depletion of CD4+ T cells prior to immunisation in DTHA completely prevents disease development [Citation24] and depletion post induction of arthritis reduces disease severity significantly (our unpublished data). However, depletion of CD4+ T cells after initiation of CIA does not ameliorate disease [Citation25], indicating that T cells are important for CIA primarily in the immunisation phase after which the B cells drive disease progression through the production of anti-type II collagen antibodies. Previously, we have shown that etanercept (a TNFα-inhibitor) could ameliorate disease in DTHA both when administered at arthritis induction and post arthritis induction [Citation24], suggesting predictive validity of the model for efficacy of new anti-arthritic biologics. Not all preclinical data generated in the CIA model has been directly transferrable to RA, including data on anti-IL-1, which showed good efficacy in CIA, but limited therapeutic potential in human RA [Citation26]. So, although this anti-C5aR mAb has previously been tested in the CIA model [Citation23], we also wished to test its efficacy in the DTHA model, as we believe that efficacy data with this particular anti-C5aR mAb from different arthritis models increase the probability of translatable preclinical data.
In the present study we found that twice weekly treatment with anti-C5aR mAb initiated at arthritis induction reduced paw swelling, bone erosion, cartilage destruction, synovitis, new bone formation and extra-articular inflammation. Our data also demonstrate that these effects were apparent already 60 h after a single dose of anti-C5aR given at arthritis induction. This shows that blocking C5aR is efficacious in the earliest stages of arthritis flare development before the appearance of joint swelling, which to our knowledge has not been demonstrated previously in a mouse model. In previous studies in the CIA model, dosing of the antibody either took place prior to or after the appearance of joint inflammation (REF). Due to the variable onset of disease in the CIA model, treatment at the exact onset of inflammation is not possible. Our data show reduced infiltration of neutrophils into the joint space and synovium after a single dose of anti-C5aR, demonstrating that one of the very early effects of blocking C5aR is reduced neutrophil chemotaxis into the inflamed joints. Levels of several neutrophil-associated proinflammatory mediators in the affected paw were also decreased indicating decrease in neutrophil activation. In addition, numbers of T cells in circulation and the draining popliteal lymph node were reduced following a single dose of anti-C5aR, which has not been shown previously in a mouse model of arthritis, and indicates interplay between the complement system and the T-cell compartment in arthritis. In summary, these data demonstrate that blockade of C5aR-signalling in DTHA leads to rapid-onset effects reducing leukocyte activation and joint infiltration of neutrophils, bone erosion, synovitis and cartilage destruction. Thus, these results strengthen the potential of C5aR-blockade as a treatment strategy for RA and demonstrate that C5aR signalling plays a role in the earliest stages of arthritis flare.
Materials and methods
Mice
Female C57BL/6J mice (Taconic, Denmark) of 8–10 weeks of age were used. Animals were housed in a facility with a 12 h light/dark cycle and with free access to water and standard rodent chow (Altromin®). All animal experiments were conducted according to Danish legislation and have been approved by the Danish Animal Inspectorate and the Novo Nordisk ethical review board.
Induction and assessment of DTH-arthritis
Mice were anaesthetised by isoflurane/O2/N2O and immunised intradermally (i.d.) with methylated bovine serum albumin (mBSA) (Sigma, St. Louis, MO) emulsified in complete Freund’s adjuvant (CFA) (Difco, Detroit, MI) at the base of the tail. Four days later they received 1000 µg (approx. 50 mg/kg) anti-mouse type II collagen antibody (anti-CII) cocktail (Chondrex, Redmond, WA) containing the clones A2-10 (IgG2a), F10-21 (IgG2a), D8-6 (IgG2a), D1-2G (IgG2b), and D2-112 (IgG2b) intravenously (i.v.) in 200 µl phosphate-buffered saline (PBS). Isotype control cocktail was mixed using mIgG2a and mIgG2b (BioXcell, West Lebanon, NH) in the same ratio as the anti-CII cocktail. Seven days after immunisation the mice were challenged with 200 µg mBSA subcutaneously in 20 μl PBS in the right foot pad. The left foot pad was given 20 μl PBS only and served as control. Baseline paw and ankle measurements were made on the right paw on day 0 prior to mBSA challenge. Paw and ankle swelling was measured using a dial thickness gauge (Mitutoyo, Japan), and was calculated as right paw or ankle thickness minus baseline measurement.
Test compounds and dosing of mice
Mice received either two weekly doses intraperitoneally (i.p.) over two weeks or one single dose (i.p.) of the following compounds: mouse anti-C5aR IgG2a.1, 0.5 mg/mouse corresponding to 25 mg/kg, or anti-TNP mouse IgG2a.1, 0.5 mg/mouse corresponding to 25 mg/kg, endotoxin levels <0.1 EU/mg. Mice were sacrificed after two weeks of treatment or 60 h (2.5 days) after a single dose.
Design of mIgG2a.1 antibody
The variable region for the heavy-chain (HC) and light-chain (LC) was derived from the rat anti-mouse anti-C5aR mAb 20/70, a kind gift from Prof. Dr. Jörg Zwirner [Citation27]. The constant region of LC is mouse kappa and the constant region of HC is mouse IgG2a designed with six mutations in the FC region, resulting in mIgG2a.1. The engineered mutations L234A, L235E, G237A (ADCC inactivation), D327Q, A330S and P331S (CDC inactivation) were made to reduce ADCC or CDC effector mechanisms. Lack of binding to mouse Fc-gamma receptors I–IV was determined by surface plasmon resonance analysis. The resulting anti-C5aR mIgG2a.1 mAb was non-depleting (Supplemental Figure S1). The anti-trinitrophenol (TNP) control mIgG2a.1 contained the same six mutations as the anti-C5aR antibody.
Histopathology
Paws were processed and stained with hematoxylin and eosin (H&E), Safranin O and for tartrate resistant acid phosphatase (TRAP) as previously described [Citation24]. TRAP stains osteoclasts red, and Safranin O stains cartilage red. The intensity of red in the Safranin O stain is inversely proportional to the degree of proteoglycan depletion from cartilage. Pathological changes in the paws were assessed on HE, TRAP and Safranin O stained sections. The extra-articular infiltration of inflammatory cells (assessed on a scale of 0–3) and arthritic changes were assessed separately. Arthritic changes were assessed on metatarsal and tarsal joints, respectively, where synovitis, cartilage destruction and bone erosion were scored separately on a 0–3 scale. For each of the three parameters of arthritic changes, an average between the two joint areas was calculated. In addition, new bone formation and extra-articular infiltration overall in the paw was scored on a 0-3 scale. The histology sum score was calculated by adding the five scores (extra-articular infiltration, synovitis, cartilage destruction, bone erosion and bone formation), whereas the extra-articular infiltration score is left out in the arthritis score. The person who performed the evaluation was blinded to the experimental setup.
Immunohistochemistry and digitalised image analysis
For immunohistochemical (IHC) detection of macrophages and neutrophils in the paws, paraffin sections were pre-treated with 10 µg/ml proteinase K for 10 min at 37 °C, and incubated overnight at 4 °C with primary antibodies. Macrophages were detected with 4 µg/ml rat anti-mouse F4/80 (IgG2b, Abcam, Cambridge, UK) and neutrophils with 2 µg/ml anti-mouse anti-Ly6B.2 (IgG2b, Ab Serotec, Nordic Biosite, Copenhagen, Denmark). Rat IgG2b and IgG2a isotype controls were applied on adjacent sections at the same concentrations. The primary antibodies were detected with rabbit–anti-rat antibodies (Dako, Glostrup, Denmark) followed by incubation with HRP labeled EnVision + System (Dako, Glostrup, Denmark). To visualise the target expression, sections were incubated with DAB (3-3′-diamino-benzidine-tetrahydrochloride) (Sigma-Aldrich) for 5 min and counterstained with hematoxylin. The sections were all digitally scanned and studied by using a NanoZoomer Digital Pathology Virtual Slide Viewer (Hamamatsu Photonic, Shizuoka, Japan). Automated digital image analyses of the infiltrating macrophages (F4/80) and neutrophils (Ly6B.2) in the paws were performed using the Visiopharm Integrator System (VIS, version 4.2.2.0, Visiopharm, Hoersholm, Denmark). On individual digital images of the arthritic paw, a region-of-interest (ROI) was automatically defined of the entire paw, and the bone marrow was outlined manually. Next, an analysis was run inside the ROI to detect the brown DAB staining of the specific IHC immunostaining, followed by a calculation step. The results are given as tissue area stained with F4/80 and Ly6B.2 of the entire paw area (%). Semi-quantitative visual scoring of neutrophil and macrophage influx into the synovium and joint space was performed on the Ly6B.2 and F4/80 stained sections, respectively, on a scale of 0-3, indicating the degree of neutrophil or macrophage influx into the synovium and joint spaces. The tarsal and metatarsal joints were scored as two separate areas, and the final score calculated as the average between the two. The evaluation of infiltration was performed by two individual people who were blinded to the experimental setup. Only the synovium tissue and joint space area was included in the semi-quantitative analysis, in contrast to the digitalised image analysis which analysed the entire paw section, leaving out the contribution from the bone marrow.
Enzyme-linked immunosorbent assays (ELISAs)
Levels of serum amyloid P component (SAP) were measured in serum from mice with DTH-arthritis using sandwich ELISA kits (Genway, San Diego, CA) according to the manufacturer’s instructions. Levels of MPO in whole-paw homogenates were measured using sandwich ELISA kits (Hycult Biotech, Uden, The Netherlands) according to the manufacturer’s instructions. Paw homogenates for analysis of MPO were prepared in a custom-made buffer containing was a solution of 200 mM NaCl, 5 mM EDTA, 10 mM Tris, 10% glycerin, 1 mM PMSF, 1 μg/ml leupeptin and 28 μg/ml aprotinin with a pH value of 7.4 (Ampliqon, Skovlunde, Denmark). Levels of C5a in whole-paw homogenate supernatants were analysed by sandwich ELISA using the following reagents (all from BD Biosciences, Franklin Lakes, NJ): Capture rat anti-mouse C5a mAb, standard recombinant mouse C5a, detection biotin-conjugated rat anti-mouse C5a mAb, avidin-horseradish peroxidase (HRP) and reagent set B. The serine protease inhibitor FUT-175 (Calbiochem, Billerica, MA) was added to the homogenising buffer in order to preserve C5a protein. Levels of receptor-activator of nuclear factor kappa B (RANKL) were measured in serum from mice with DTH-arthritis using sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Multiplex analysis of inflammatory markers in paw homogenate
Paw homogenate supernatants from arthritic hind paws were prepared as previously described [Citation24]. The supernatants were analysed undiluted for levels of inflammatory markers using bead-based Luminex® xMAP® technology with Milliplex kits from Millipore (Billerica, MA) according to the manufacturer’s instructions. For statistical analysis, any values below the detection limit were set to the detection limit for the analyte in question and any values above detection limit was set to the upper detection limit for the analyte in question. In the case of vascular cell adhesion molecule 1 (VCAM-1), basic fibroblast growth factor (bFGF) and lymphotactin the analysis was performed using a 58-biomarker multi-analyte profile (RodentMAP®, Myriad RBM, Austin, USA).
Flow cytometry
Single-cell suspensions of whole-paws and lymph nodes were prepared as previously described [Citation24]. All samples were subjected to Fc-blocking prior to antibody staining using anti-CD16/32 (BD Bioscience). Dead cells in paw and lymph node preparations were excluded using Fixable Near IR Vital dye (Invitrogen, Carlsbad, CA). When analysing blood samples, red blood cells were lysed with FACS lysing solution (BD Bioscience) after antibody staining. The following antibody-fluorochrome conjugations were used for flow cytometry: Anti-CD88-PE, clone 20/70 (Biolegend, San Diego, CA), anti-CD45-PerCP, clone 30-F11 (BD Biosciences), anti-CD4-Qdot605, clone RM4-5 (Invitrogen, Carlsbad, CA), anti-TCRβ-Qdot 655, clone H57-597 (Invitrogen, Carlsbad, CA), anti-CD45-efluor450, clone 30-F11 (eBioscience, San Diego, CA), anti-TCRβ-PE-Cy7, clone H57-597 (Biolegend, San Diego, CA), anti-CD19-PerCP-Cy5.5, clone 1D3 (BD Biosciences), anti-Ly6G-PE, clone 1A8 (BD Biosciences), anti-CD11b-AF700, clone M1/70 (eBioscience, San Diego, CA), anti-CD88-FITC, clone 20/70 (Cedarlane, Ontario, Canada). For determination of blood cell counts, TruCount beads (BD Biosciences) were added to the samples prior to acquisition and the cell counts determined from the ratio of collected beads to total beads.
Statistics
Statistical analyses were conducted using GraphPad Prism software version 5.01 (La Jolla, CA). Non-parametric data or non-normal parametric data were analysed using the Mann–Whitney U-test, and parametric data were analysed using a two-sided unpaired Student’s t-test. For statistical analysis of the histology score data a two-sided unpaired Student’s t-test with Welch’s correction was used. Differences between groups were considered significant when p ≤ 0.05 and levels of significance were assigned as *: p ≤ 0.05, **: p ≤ 0.01 and ***: p ≤ 0.001.
Results
C5aR is expressed in inflamed paw tissue and on major leukocyte populations
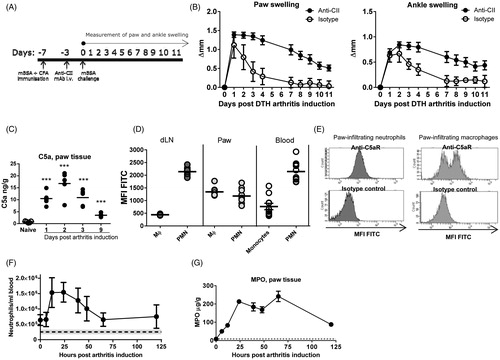
For induction of DTHA, mice were immunised with mBSA in CFA and 4 days later given an anti-CII cocktail i.v. Mice were challenged with mBSA in the right hind foot pad 7 days after immunisation (). The left hind foot pad received PBS and served as an intra-animal control. In this first part of experiment an isotype control group was included to illustrate the effect of the anti-CII cocktail on paw and ankle swelling in combination with the DTH response. The anti-CII cocktail does not induce any paw and ankle swelling without the mBSA challenge [Citation24]. To confirm the production of C5a in DTHA and the presence of C5aR on immune cell subsets, the expression levels in arthritic paws and major leukocyte subsets during disease were evaluated. Up-regulation of C5a was observed in the arthritic paw in the initial stages of the disease, by assaying whole-paw homogenates by ELISA (). Flow cytometry analysis showed C5aR+ neutrophils and macrophages in the affected paw. C5aR-expression on paw-infiltrating neutrophils was lower than the expression seen on neutrophils in the blood () and this down-regulation could be an effect of agonist-mediated receptor internalisation, which is known to occur [Citation28]. In the macrophage/monocyte compartment two distinct populations were seen; a C5aR+ and a C5aR−, while the neutrophil compartment consisted of one population with varying C5aR expression levels (). Marked neutrophilia in DTHA was observed, which peaked 12–24 h post arthritis induction, both in blood as measured by flow cytometry (), and in arthritic paws as measured indirectly as MPO in whole-paw homogenates (), where the peak occurred at 60 h post arthritis induction. Taken together, these data demonstrate that in the DTHA paw C5a levels are up-regulated, C5aR+ neutrophils and macrophages are present, and C5a ligation may have taken place with potential effects on activation and migratory activity in these cells.
Figure 1. C5aR is a therapeutic target in DTHA. (A) DTHA was induced by modification of a classical mBSA-induced DTH response. Mice were immunised with mBSA in CFA on day -7, and the modification consisted of giving the mice a sub-arthritogenic dose of an anti-type II collagen mAb cocktail (anti-CII) or isotype control cocktail i.v. on day-3. Challenge with mBSA in the right hind foot pad was done on day 0. (B) Temporal course of the DTH response. The group given isotype control Ab cocktail developed only an acute DTH response. Paw and ankle swelling was calculated as the swelling on a given day, minus the swelling on day 0. Mean ± 95% CI shown, n = 24 (anti-CII group) and n = 6 (isotype control group). (C) C5a-production is upregulated in the arthritic paw in DTHA compared to naïve controls, measured by ELISA in whole-paw homogenate supernatants. Mean shown, n = 5. ***: p ≤ 0.001, one-way ANOVA. (D) Median fluorescence intensity (MFI) of FITC conjugated to C5aR measured by flow cytometry on neutrophils (PMN) and macrophages (Mϕ) in the lymph node draining the arthritic paw (dLN), in paw infiltrate and in blood, measured 72 h after arthritis induction. Neutrophils were gated as CD45+CD19−CD11b+Ly6G+, macrophages as CD45+CD19−CD11b+Ly6G−F4/80+ and monocytes in blood as CD45+CD19−CD11b+Ly6G−SSClowFSCint. Mean shown, n = 5–10. (E) Representative flow cytometry histograms showing the mean fluorescence intensity (MFI) of FITC conjugated to either anti-C5aR or isotype control antibody. (F) Blood neutrophil count in DTHA measured by flow cytometry. Dotted line represents blood neutrophil count in naïve mice and grey shading 95% CI (mean: 251,968 cells/ml, 95% CI: 177,173–326,763 cells/ml). Mean ± 95% CI shown, n = 10. (G) Myeloperoxidase measured in whole-paw homogenate supernatants by ELISA. Mean ± 95% CI shown, n = 10. Dotted line represents levels in naïve mice and grey shading 95% CI (mean: 9.077 µg/g, 95% CI: 7.440–10.715 µg/g, n = 10).
Treatment with anti-C5aR mAb ameliorates DTH arthritis
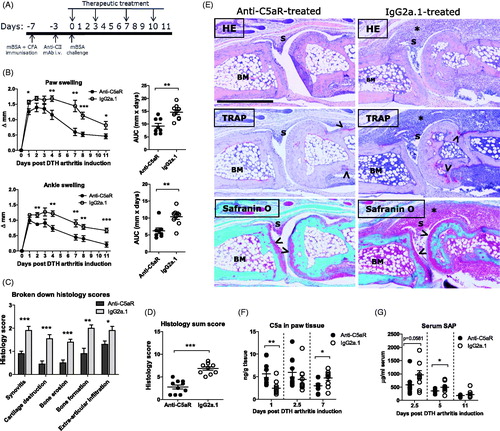
With confirmed activation of the target pathway, an efficacy study was performed. Mice with DTHA were dosed twice weekly with 500 µg anti-C5aR mAb or isotype-matched anti-TNP mAb (IgG2a.1) from the time of arthritis induction (). Treatment with anti-C5aR mAb significantly reduced both paw and ankle swelling in DTHA compared to the IgG2a.1 control group (). Histopathological evaluation at study end revealed a significant reduction in synovitis, cartilage destruction, bone erosion, new bone formation and extra-articular inflammation () and in the overall histopathology score in the anti-C5aR-treated mice (). C5a levels in paw tissue were increased in the C5aR-treated groups 24 h post arthritis induction, probably reflecting the blockade of C5aR, as this would inhibit the clearance of C5a by internalisation via the receptor [Citation28] (). On day 2.5 (60 h) post arthritis induction, levels of C5a were not significantly increased. This could reflect a lower production of C5a in the anti-C5aR group due to a generally lowered level of inflammation at this time (). Serum level of the murine acute-phase protein serum amyloid P component (SAP) was measured at 2.5 (60 h), 5 and 11 days post-arthritis induction to examine whether anti-C5aR-treatment had an effect on systemic inflammation. Anti-C5aR-treatment led to reduced SAP levels in serum on days 2.5 (60 h) and 5 post arthritis induction (). Taken together, these data demonstrate that treatment with anti-C5aR led to a significant reduction in disease activity in DTHA, both locally and systemically.
Figure 2. Treatment with anti-C5aR mAb ameliorates DTH arthritis. (A) Therapeutic treatment with 500 µg/mouse of anti-C5aR or isotype control Ab (IgG2a.1) was started at time of challenge and continued until day 11 post arthritis induction where the study was terminated. (B) Treatment with anti-C5aR ameliorated DTHA when begun at arthritis induction. Area under curve (AUC) values calculated from individual swelling curves over days 0–11. Mean ± SEM shown, n = 10. *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001, Student’s t-test. (C) Semi-quantitative histopathological scoring of arthritic and inflammatory parameters in the two treatment groups at study termination on a scale of 0–3 (see “Materials and methods” section for details), n = 10. *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001, Student’s t-test with Welch’s correction. (D) Sum of the individual scores in C). Maximum possible score is 15. n = 10. ***: p ≤ 0.001, Student’s t-test with Welch’s correction. (E) Images show the metatarsal joint from the paw of a mouse from the C5aR-treated group and a mouse from the IgG2a.1-treated control group with an arthritis sum score of 3.5 (mean: 2.8), and 8 (mean: 6.8), respectively, at study termination on day 11. Arrows point towards areas of cartilage destruction in the images of Safranin O stains and towards TRAP-positive osteoclasts in the images of TRAP stains. The stars indicate areas of extra-articular inflammation. BM: bone marrow; S: synovium. Magnification 10Χ, scale bar represents 600 µm. (F) Levels of C5a protein in whole-paw homogenate supernatants in the two treatment groups measured by ELISA. Mean ± SEM shown, n = 10. *: p ≤ 0.05; **: p ≤ 0.01, Student’s t-test. (G) Levels of the acute-phase protein serum amyloid P component (SAP) in serum from the two treatment groups by ELISA. Mean ± SEM shown, n = 10. *: p ≤ 0.05, Student’s t-test.
Single dose treatment with anti-C5aR results in reduced disease activity after 60 h
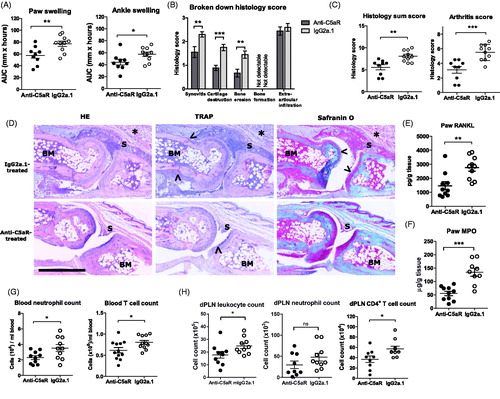
Having shown that treatment with anti-C5aR mAb could attenuate DTHA disease activity in a multiple dose study, we sought to investigate effects of the compound on the earliest stages of arthritis development, as this would allow us to more closely examine the early mechanistic effects of C5aR-blockade on cell activation and migration. A single dose of 500 µg anti-C5aR given at arthritis induction led to a reduced paw- and ankle swelling when measured over 60 h (). Histopathological evaluation revealed that synovitis, cartilage destruction and bone erosion were attenuated, but also that extra-articular infiltration of inflammatory cells was not affected (). The overall histopathology score was also reduced, as was the arthritis score, which is defined as the sum of all the individual evaluation parameters pertaining to an arthritic phenotype (). Levels of the osteoclast-activating receptor-activator of nuclear factor κB ligand (RANKL) were also significantly reduced in the paws of anti-C5aR-treated mice (), supporting the reduction in bone erosion observed. In addition, levels of MPO were also reduced in the paws of anti-C5aR-treated mice (), indicating either reduced neutrophil recruitment, activation or both. A reduction in circulating neutrophils and T cells was observed in the anti-C5aR-treated group () and total leukocyte count was reduced in the popliteal lymph node draining the arthritic paw (dPLN) in anti-C5aR-treated mice, as was CD4+ T cell count (). Furthermore, a tendency towards a reduction in neutrophil count in the dPLN was also observed (). Together, these data demonstrate that a single dose of anti-C5aR has the ability to significantly reduce early onset disease development and arthritic changes in DTHA by reducing numbers of CD4+ T cells in the draining lymph node, reducing circulating inflammatory cells and preventing arthritic changes in the affected paw.
Figure 3. Disease activity in DTHA is reduced 60 h following a single dose of anti-C5aR mAb. (A) Area under curve (AUC) of paw- and ankle swelling, measured every day until 60 h after arthritis induction. Mean ± SEM shown, n = 10. *: p ≤ 0.05; **: p ≤ 0.01, Student’s t-test. (B) Semi-quantitative histopathology scoring of arthritic and inflammatory parameters in the two treatment groups on a scale of 0–3 (see Material and methods for details). n = 10. *: p ≤ 0.05; **: p ≤ 0.01; ***: p ≤ 0.001, Student’s t-test with Welch’s correction. (C) Sum of the individual scores in B (arthritis sum score), and sum of the individual scores in B minus extra articular infiltration (arthritis score). Maximum possible score is 15 and 9, respectively. n = 10. *: p ≤ 0.05; **: p ≤ 0.01, Student’s t-test with Welch’s correction. (D) Images show the metatarsal joint from the paw of a mouse from the C5aR-treated group and a mouse from the IgG2a.1-treated control group with an arthritis sum score of 3 (mean: 5.6), and 6 (mean: 8.1), respectively, at study termination at 60 h post arthritis induction. Arrows point towards areas of cartilage destruction in the images of Safranin O stains and towards TRAP-positive osteoclasts in the images of TRAP stains. The stars indicate extra-articular inflammation. BM: bone marrow; S: synovium. Magnification 10×, scale bar represents 600 µm. (E) Levels of receptor-activator of nuclear factor κB ligand (RANKL) protein in whole-paw homogenate supernatants from the two treatment groups measured by ELISA. Mean ± SEM shown, n = 10. **: p ≤ 0.01, Student’s t-test. (F) Levels of myeloperoxidase (MPO) protein in whole-paw homogenate supernatants measured by ELISA. Mean ± SEM shown, n = 10, ***: p ≤ 0.001, Student’s t-test. (G) Neutrophil and T cell counts in blood of mice treated with anti-C5aR or IgG2a.1. Neutrophils were defined as CD45+CD11b+Ly6G+ and T cells as CD45+TCRβ+. Mean ± SEM shown, n = 10. *: p ≤ 0.05, Student’s t-test. (H) Total leukocyte, neutrophil and CD4+ T cell count in the popliteal lymph node draining the arthritic paw (dPLN) in mice treated with anti-C5aR or IgG2a.1. Neutrophils and T cells defined as above. Mean ± SEM shown, n = 10. *: p ≤ 0.05, Student’s t-test.
A single dose of anti-C5aR leads to reduced influx of neutrophils into the joint space
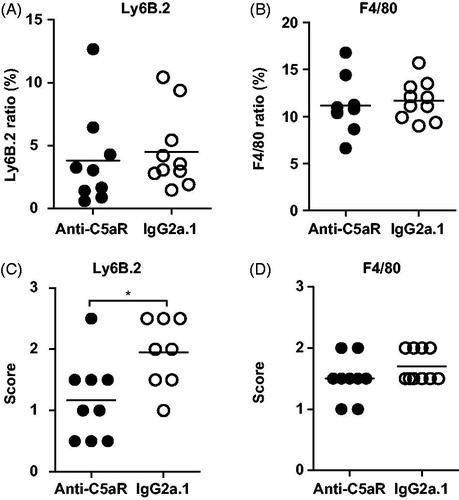
Having demonstrated that a single dose of anti-C5aR could reduce disease activity in the very early stages of DTHA, we now investigated the effect of a single dose more closely on infiltration of neutrophils and macrophages into the arthritic paw. Immunohistochemical stainings for macrophages and neutrophils on whole-paw tissue sections from both treatment groups were performed, and the numbers of these cells were quantified using Visiomorph digitalised image analysis. This method did not reveal a significant difference in total neutrophil (Ly6B.2-positive) or macrophage (F4/80-positive) infiltration into the arthritic paws (), despite there being an effect of a single dose of anti-C5aR on clinical disease parameters (). As automated digitalised image analyses are not suitable for quantifying neutrophil and macrophage infiltration specifically into the joint space and surrounding synovium due to varying nature of synovial tissue in arthritis, the Ly6B.2 and F4/80-stained sections were scored by eye in a semi-quantitative manner instead (see materials and methods for details of the scoring system). This analysis revealed a significant difference in neutrophil infiltration, with the anti-C5aR-treated animals displaying a lower degree of infiltration into the joint space and synovium compared to isotype control-treated mice (). No difference could be detected when the same scoring system was applied to F4/80-stained sections (). Together, these data indicate that anti-C5aR does not have an effect on neutrophil and macrophage recruitment to the total of the affected paw after a single dose and they support the finding that a single dose of anti-C5aR does not reduce total extra-articular inflammation (). On the other hand, a single dose of anti-C5aR reduced MPO protein levels in the arthritic paw, which is an indirect way of estimating neutrophil numbers in tissue, so a firm conclusion cannot be made. Importantly, the present data does indicate that anti-C5aR treatment has an effect on neutrophil spatial organisation locally in the inflamed synovium and in the joints.
Figure 4. Anti-C5aR treatment reduces neutrophil infiltration into joints, but not total quantity of neutrophils and macrophages in paws. Digitalised image analysis (see “Materials and Methods” section for details) of (A) neutrophils (Ly6B.2) and (B) macrophages (F4/80) in DTHA paws at 60 h post arthritis induction after a single dose of anti-C5aR or isotype control antibody. The results are given as tissue area stained positive for F4/80 and Ly6B.2 of the entire paw area (%). Mean shown, n = 9–10. Semi-quantitative visual scoring (see Materials and Methods for details) of (C) neutrophil infiltration and (D) macrophage infiltration into the joint space and synovium of DTHA paws at 60 h post arthritis induction after a single dose of anti-C5aR or isotype control antibody. Evaluation was performed on Ly6B.2-stained (C) and F4/80 (D) sections. Range 0–3. Mean ± SEM shown, n = 9–10. *: p ≤ 0.05, Student’s t-test with Welch’s correction.
A single dose of anti-C5aR leads to changes in inflammatory biomarkers locally in the arthritic paw
To further investigate the pathways affected by blockade of C5aR, and to investigate whether anti-C5aR leads to a reduction in activation of inflammatory cells, arthritic paws were harvested and homogenised 60 h post arthritis induction and analysed for protein levels of a range of cytokines and chemokines. The inflammatory cytokine IL-6 was significantly decreased in the paws of anti-C5aR-treated mice and there was a tendency towards a reduction in IL-17, while no change in IL-1β or IL-10 was observed. Furthermore, the chemoattractants CXCL1/KC, CXCL2/MIP-2, CXCL5/LIX and lymphotactin were significantly reduced in the paws of anti-C5aR-treated animals, as was G-CSF. Finally, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and vascular cell VCAM-1 were also significantly reduced in the paws of anti-C5aR-treated mice (). These data demonstrate that a single dose of anti-C5aR could rapidly mediate changes in local inflammatory mediators, which are both markers of and important for the activation and infiltration of a variety inflammatory cell subsets, and for angiogenesis. Although a single dose of anti-C5aR does not reduce neutrophil infiltration into the affected paw at this time point, it could reduce the activation level of the infiltrating neutrophils.
Table 1. Reduction in protein biomarkers of inflammation in paw tissue following a single dose of anti-C5aR or IgG2a.1.
Discussion
The present study demonstrates the efficacy of a blocking, non-cell-depleting anti-mouse C5aR mAb in the early stage of arthritis development in the DTHA model, a single-paw arthritis model with synchronised onset, low variation and predictive validity for efficacy of anti-arthritic therapeutics [Citation24]. A multiple dose study showed reduction in paw swelling and histopathological evaluation revealed reduced synovitis, bone erosion, cartilage destruction, reduced new bone formation and extra-articular inflammation. Importantly, rapid onset effects of a single dose of anti-C5aR in DTHA were apparent as little as 60 h after a single dose administered at arthritis induction. Significant reduction in paw swelling, synovitis, bone erosion, cartilage destruction and recruitment of neutrophils to the joints and synovium was demonstrated. The reduction in paw swelling observed was similar to the reduction observed after treatment with etanercept or dexamethasone in a previous study in the DTHA model [Citation24]. RANKL levels locally in the affected paw were also reduced, indicating reduced osteoclast activation, which supports the reduction in osteoclast activity observed in the histopathological evaluation. Numbers of neutrophils in circulation and the draining popliteal lymph node were reduced following a single dose of anti-C5aR, as was MPO and G-CSF protein levels in the affected paw, indicating reduced neutrophil recruitment from the bone marrow. The reduction in paw swelling could also be a result of impaired activation of already infiltrating C5aR-positive cells, thus reducing overall tissue inflammation. The first wave of neutrophils to reach the paw after arthritis induction is most likely not fully dependent on the C5a-C5aR axis, as it is also not in the delayed-type hypersensitivity (DTH) and K/BxN models [Citation29–32]. Indeed, the initial extra-articular inflammation observed in DTHA is likely to be a result of the DTH response in isolation and unrelated to the anti-CII cocktail given, as a previous study has shown that paw swelling 24 h after mBSA challenge was comparable whether anti-CII had been given or not [Citation24]. Complement activation and C5a production in DTHA most likely takes place at the cartilage surfaces where the anti-CII administered during induction localises and forms immune complexes with cartilage CII. In the CAIA and CIA models anti-CII antibodies deposit on cartilage surfaces and activate complement [Citation19,Citation33], and in the K/BxN and K/BxN serum transfer models glucose-6-phosphate isomerase (GPI)-anti-GPI immune complexes deposit on the cartilage surfaces and activate complement [Citation34]. Cartilage surfaces are not very rich in cells and thus lack the cell membrane-bound C3 inactivators decay-acceleration factor (DAF) and membrane cofactor protein (MCP), so only soluble inhibitors and surface sialic acid residues are present to dampen the activation of complement pathways [Citation34]. In addition, the breakdown of cartilage that occurs during joint inflammation can unmask molecules that can further contribute to the activation of complement [Citation33]. Together these circumstances make the inflamed joint a location where the complement equilibrium is unsteady and excessive activation is favoured, which could be the reason for the pronounced effect of blocking C5aR signalling at this site. In line with this, the effect of anti-C5aR on neutrophil migration in the single-dose study was isolated to the cells infiltrating the joints and synovial tissue.
Levels of CXCL2/MIP-2 were lower in the paws of anti-C5aR-treated mice at 60 h post arthritis induction in the present study and this could indicate a lower level of activation of the infiltrating neutrophils, as CXCL2/MIP-2 is secreted by activated neutrophils themselves [Citation29] as well as by macrophages and mast cells [Citation35]. The chemoattractants CXCL1/KC, CXCL5/LIX and lymphotactin, which were also reduced in paws of anti-C5aR-treated mice 60 h after a single dose, have been demonstrated to be upregulated in both RA synovial tissue/fluid and other murine arthritis models [Citation36,Citation37]. In addition, it is suggested that lymphotactin can exert immunomodulatory effects through MMP release from synovial fibroblasts and cytokine release from macrophages in addition to its chemotactic properties [Citation37]. The present data indicate that C5aR signalling is important for the expression of leukocyte chemoattractants in the earliest stages of arthritis development and that a blockade of the receptor could potentially represent a way of lowering leukocyte migration and activation in the disease.
The observed reduction in G-CSF could be a contributing factor to the more rapid resolution of inflammation observed in DTHA after multiple doses of anti-C5aR, as fewer neutrophils are thus released into circulation while neutrophils present in the inflamed tissue undergo apoptosis. G-CSF is a major cytokine for proliferation and survival of neutrophils and exerts its effecs by down-regulating CXCL12 and CXCR4, which leads to reduced neutrophil retention in the bone marrow [Citation38]. C5a amplifies the production of G-CSF in vivo and in vitro, and G-CSF in turn up-regulates C5aR on neutrophils [Citation39]. G-CSF can be produced by leukocytes, endothelial cells and fibroblasts, and the reduction observed in the present study could be the direct result of blocking of C5aR on these cells.
IL-6 levels in paw tissue were also reduced after a single dose of anti-C5aR. Neutrophils [Citation40] and macrophages [Citation41] express IL-6 upon stimulation with C5a, and production of this cytokine was attenuated when C5a was blocked by antibodies or in C5aR−/− mice [Citation40]. The reduction in IL-6 in paw tissue observed in the present study might therefore reflect a direct effect of anti-C5aR on activation of neutrophils and macrophages in DTHA. IL-6 induces RANKL expression by synovial fibroblasts and activates osteoclast precursor cells and thus contributes to arthritis development [Citation42], and so the reduced IL-6 observed following anti-C5aR-treatment could contribute to the reduction in bone erosion observed. IL-17 is also a key mediator in inflammation-driven bone erosion and is upstream from RANKL in arthritis [Citation43]. The observed reduction in IL-17 following anti-C5aR treatment in the present study indicates that reduction of Th17 effector T-cell activity is a mechanism of C5aR-blockade in experimental arthritis. Vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) were also significantly reduced in paws of anti-C5aR-treated mice. Both are implicated in RA pathology through their angiogenic and proliferative effects in inflamed synovium [Citation44–47]. The infiltration and retention of inflammatory cells in RA synovium is facilitated by upregulation of VCAM-1 on endothelial cells and synovial fibroblasts in this tissue [Citation48]. VCAM-1 was reduced in DTHA paws following anti-C5aR treatment, and as C5aR is expressed on endothelial cells and synovial fibroblasts [Citation7] this reduction in VCAM-1 could represent a direct effect of anti-C5aR treatment on these cells. In support of this, it has been shown that C5aR−/− mice have lower expression of VCAM-1 mRNA than wild-type mice after induction of CAIA [Citation19].
Finally, we also observed an effect of C5aR-blockade on the T cell compartment, which to our knowledge has not previously been demonstrated in a murine arthritis model. Numbers of total T cells in circulation was reduced, as was the numbers of CD4+ T cells in the dPLN. Recent findings have implicated C5a in the regulation of T-cell immunity [Citation9,Citation10,Citation49], but from our data it was not further possible to determine whether the observed reductions in T cell subsets following C5aR-blockade were a primary or secondary effect.
In summary, we demonstrate that treatment with anti-C5aR mAb reduces disease activity in DTHA and prevents development of severe arthritis. Importantly, rapid onset effects of C5aR-blockade were apparent 60 h after a single dose. These included a decrease in inflammatory cytokines and chemoattractants locally in the affected paws, indicating that anti-C5aR-treatment could reduce inflammatory cell activation. Reduction in the neutrophil marker MPO indicates impaired neutrophil recruitment and activation. Immunohistochemistry showed a reduced influx of neutrophils specifically into the joint spaces and synovium, demonstrating that C5aR-signalling is important for neutrophil recruitment into the inflamed joints during the early stages of arthritis. Finally, an effect of anti-C5aR on the T-cell compartment was also shown. In conclusion, our study demonstrates that C5aR-blockade has early-onset effects on recruitment, infiltration and activation of inflammatory cells in experimental arthritis. Thus, data from this preclinical study strengthen the potential of C5aR-blockade as a treatment strategy for RA, and indicates that blocking C5aR could be an option for treating flaring of the disease, if treatment is initiated before full-blown joint inflammation is present and perhaps prevent severe arthritis from developing.
Supplementary material available online Supplementary Figure S1
supplemental figure 1
Download MS Power Point (220.6 KB)Acknowledgements
The authors sincerely thank Kirstine Smedenfors, Mie Berndorff, Julie Jensen, Malik Nygaard Nielsen, Jette Mandelbaum, Pia Rothe, and Birthe Jørgensen for excellent technical assistance, Josephine Hebsgaard for coordinating the histopathology processing and evaluation, and the staff of Laboratory Animal Science, Novo Nordisk A/S, Maaloev for taking care of the animals and assisting with blood sampling.
Declaration of interest
Anneline Nansen, Pernille Autzen Usher, Bodil-Cecilie Søndergaard, Birgitte Friedrichsen, Chih-Chuan Chang, Claus Haase and Lars Hornum are employees of Novo Nordisk A/S and minor shareholders in the company. The study was funded in full by Novo Nordisk A/S, except the salary of Sara Marie Atkinson, which was funded by the Novo Nordisk LIFE In Vivo Pharmacology Centre (LIFEPHARM) at the University of Copenhagen.
References
- Sturfelt, G., and L. Truedsson. 2012. Complement in the immunopathogenesis of rheumatic disease. Nat. Rev. Rheumatol. 8: 458–468
- Chen, M., M. R. Daha, and C. G. Kallenberg. 2010. The complement system in systemic autoimmune disease. J. Autoimmun. 34: J276–J286
- Okroj, M., D. Heinegård, R. Holmdahl, and A. M. Blom. 2007. Rheumatoid arthritis and the complement system. Ann. Med. 39: 517–530
- Jose, P. J., I. K. Moss, R. N. Maini, and T. J. Williams. 1990. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann. Rheum. Dis. 49: 747–752
- Yuan, G., J. Wei, J. Zhou, et al. 2003. Expression of C5aR (CD88) of synoviocytes isolated from patients with rheumatoid arthritis and osteoarthritis. Chin. Med. J. (Engl.) 116: 1408–1412
- Guo, R. F., and P. A. Ward. 2004. Role of C5a in inflammatory responses. Annu. Rev. Immunol. 23: 821–852
- Wetsel, R. A. 1995. Expression of the complement C5a anaphylatoxin receptor (C5aR) on non-myeloid cells. Immunol. Lett. 44: 183–187
- Ignatius, A., P. Schoengraf, L. Kreja, et al. 2011. Complement C3a and C5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with IL-1β. J. Cell. Biochem. 112: 2594–2605
- Strainic, M. G., J. Liu, D. Huang, et al. 2008. Locally produced complement fragments C5a and C3a provide both costimulatory and survival signals to naive CD4+ T cells. Immunity 28: 425–435
- Lalli, P. N., M. G. Strainic, M. Yang, et al. 2008. Locally produced C5a binds to T cell-expressed C5aR to enhance effector T-cell expansion by limiting antigen-induced apoptosis. Blood 112: 1759–1766
- DiScipio, R. G., I. U. Schraufstatter, L. Sikora, et al 2006. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int. Immunopharmacol. 6: 1109–1118
- Kolaczkowska, E., and P. Kubes. 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13: 159–175
- Zhou, W. 2012. The new face of anaphylatoxins in immune regulation. Immunobiology 217: 225–234
- Gonzalez, J. M., C. W. Franzke, F. Yang, et al. 2011. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am. J. Pathol. 179: 838–849
- Guo, R. F., L. Sun, H. Gao, et al. 2006. In vivo regulation of neutrophil apoptosis by C5a during sepsis. J. Leukoc. Biol. 80: 1575–1583
- Kessel, C., K. S. Nandakumar, F. B. Peters, et al. 2014. A single functional group substitution in c5a breaks B cell and T cell tolerance and protects against experimental arthritis. Arthritis Rheumatol. 66: 610–621
- Woodruff, T. M., A. J. Strachan, N. Dryburgh, et al. 2002. Antiarthritic activity of an orally active C5a receptor antagonist against antigen-induced monarticular arthritis in the rat. Arthritis Rheum. 46: 2476–2485
- Banda, N. K., S. Hyatt, A. H. Antonioli, et al. 2012. Role of C3a receptors, C5a receptors, and complement protein c6 deficiency in collagen antibody-induced arthritis in mice. J. Immunol. 188: 1469–1478
- Grant, E. P., D. Picarella, T. Burwell, et al. 2002. Essential role for the C5a receptor in regulating the effector phase of synovial infiltration and joint destruction in experimental arthritis. J. Exp. Med. 196: 1461–1471
- Hashimoto, M., K. Hirota, H. Yoshitomi, et al. 2010. Complement drives Th17 cell differentiation and triggers autoimmune arthritis. J. Exp. Med. 207: 1135–1143
- Sadik, C. D., N. D. Kim, Y. Iwakura, and A. D. Luster. 2012. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc. Natl. Acad. Sci. 109: E3177–E3185
- Lee, H., D. Zahra, A. Vogelzang, et al. 2006. Human C5aR knock-in mice facilitate the production and assessment of anti-inflammatory monoclonal antibodies. Nat. Biotech. 24: 1279–1284
- Andersson, C., C. S. Wenander, P. A. Usher, et al. 2014. Rapid-onset clinical and mechanistic effects of anti-C5aR treatment in the mouse collagen-induced arthritis model. Clin. Exp. Immunol. 177: 219–233
- Atkinson, S. M., P. A. Usher, P. H. Kvist, et al. 2012. Establishment and characterization of a sustained delayed-type hypersensitivity model with arthritic manifestations in C57BL/6J mice. Arthritis Res. Ther. 14: R134
- Goldschmidt, T. J., M. Andersson, V. Malmström, and R. Holmdahl. 1992. Activated type II collagen reactive T cells are not eliminated by in vivo anti-CD4 treatment: implications for therapeutic approaches on autoimmune arthritis. Immunobiology 184: 359–371
- Gabay, C., C. Lamacchia, and G. Palmer. 2010. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 6: 232–241
- Shushakova, N., J. Skokowa, J. Schulman, et al. 2002. C5a anaphylatoxin is a major regulator of activating versus inhibitory FcγRs in immune complex-induced lung disease. J. Clin. Invest. 110: 1823–1830
- Naik, N., E. Giannini, L. Brouchon, and F. Boulay. 1997. Internalization and recycling of the C5a anaphylatoxin receptor: evidence that the agonist-mediated internalization is modulated by phosphorylation of the C-terminal domain. J. Cell. Sci. 110: 2381–2390
- Chou, R. C., N. D. Kim, C. D. Sadik, et al. 2010. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33: 266–278
- Monach, P. A., P. A. Nigrovic, M. Chen, et al. 2010. Neutrophils in a mouse model of autoantibody-mediated arthritis: critical producers of Fc receptor gamma, the receptor for C5a, and lymphocyte function-associated antigen 1. Arthritis Rheum. 62: 753–764
- Nigrovic, P. A., B. A. Binstadt, P. A. Monach, et al. 2007. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc. Natl. Acad. Sci. USA 104: 2325–2330
- Biedermann, T., M. Kneilling, R. Mailhammer, et al. 2000. Mast cells control neutrophil recruitment during T cell-mediated delayed-type hypersensitivity reactions through tumor necrosis factor and macrophage inflammatory protein 2. J. Exp. Med. 192: 1441–1452
- Happonen, K. E., D. Heinegard, T. Saxne, and A. M. Blom. 2012. Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology 217: 1088–1096
- Matsumoto, I., M. Maccioni, D. M. Lee, et al. 2002. How antibodies to a ubiquitous cytoplasmic enzyme may provoke joint-specific autoimmune disease. Nat. Immunol. 3: 360–365
- De Filippo, K., A. Dudeck, M. Hasenberg, et al. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937
- Grespan, R., S. Y. Fukada, H. P. Lemos, et al. 2008. CXCR2-specific chemokines mediate leukotriene B4-dependent recruitment of neutrophils to inflamed joints in mice with antigen-induced arthritis. Arthritis Rheum. 58: 2030–2040
- Blaschke, S., P. Middel, B. G. Dorner, et al. 2003. Expression of activation-induced, T cell−derived, and chemokine-related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum. 48: 1858–1872
- von Vietinghoff S., and K. Ley. 2008. Homeostatic regulation of blood neutrophil counts. J. Immunol. 181: 5183–5188
- Bosmann, M., M. D. Haggadone, F. S. Zetoune, et al. 2013. The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur. J. Immunol. 43: 1907--1913
- Riedemann, N. C., R. Guo, T. J. Hollmann, et al. 2004. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 18: 370–372
- Scholz, W., M. R. McClurg, G. J. Cardenas, et al. 1990. C5a-mediated release of interleukin 6 by human monocytes. Clin. Immunol. Immunopathol. 57: 297–307
- Takayanagi, H. 2012. New developments in osteoimmunology. Nat. Rev. Rheumatol. 8: 684–689
- Chao, C. C., S. J. Chen, I. E. Adamopoulos, et al. 2010. Anti-IL-17A therapy protects against bone erosion in experimental models of rheumatoid arthritis. Autoimmunity 44: 243–252
- Yoo, S. A., S. K. Kwok, and W. U. Kim. 2008. Proinflammatory role of vascular endothelial growth factor in the pathogenesis of rheumatoid arthritis: prospects for therapeutic intervention. Mediators. Inflamm. 2008: 129873
- Marrelli, A., P. Cipriani, V. Liakouli, et al. 2011. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun. Rev. 10: 595–598
- Malemud, C. J. 2007. Growth hormone, VEGF and FGF: involvement in rheumatoid arthritis. Clin. Chim. Acta 375: 10–19
- Szekanecz, Z., T. Besenyei, G. Paragh, and A. E. Koch. 2009. Angiogenesis in rheumatoid arthritis. Autoimmunity 42: 563–573
- Ahmed, S., S. Riegsecker, M. Beamer, et al. 2013. Largazole, a class I histone deacetylase inhibitor, enhances TNFα-induced ICAM-1 and VCAM-1 expression in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharm. 270: 87–96
- Heeger, P. S., and C. Kemper. 2012. Novel roles of complement in T effector cell regulation. Immunobiology 217: 216–224

