Abstract
Context: US EPA proposed a Reference Concentration for Libby amphibole asbestos based on the premise that pleural plaques are adverse and cause lung function deficits.
Objective: We conducted a systematic review to evaluate whether there is an association between pleural plaques and lung function and ascertain whether results were dependent on the method used to identify plaques.
Methods: Using the PubMed database, we identified studies that evaluated pleural plaques and lung function. We assessed each study for quality, then integrated evidence and assessed associations based on the Bradford Hill guidelines. We also compared the results of HRCT studies to those of X-ray studies.
Results: We identified 16 HRCT and 36 X-ray studies. We rated six HRCT and 16 X-ray studies as higher quality based on a risk-of-bias analysis. Half of the higher quality studies reported small but statistically significant mean lung function decrements associated with plaques. None of the differences were clinically significant. Many studies had limitations, such as inappropriate controls and/or insufficient adjustment for confounders. There was little consistency in the direction of effect for the most commonly reported measurements. X-ray results were more variable than HRCT results. Pleural plaques were not associated with changes in lung function over time in longitudinal studies.
Conclusion: The weight of evidence indicates that pleural plaques do not impact lung function. Observed associations are most likely due to unidentified abnormalities or other factors.
Introduction
Exposure to asbestos can cause lung inflammation and fibrotic conditions, such as asbestosis and diffuse pleural thickening (DPT). These conditions are associated with lung function decrements, which are typically restrictive but may be obstructive or both (Antonescu-Turcu & Schapira, Citation2010; Currie et al., Citation2009; Craighead, Citation2008; Miles et al., Citation2008; Weill, Citation2008). Asbestos exposure is also associated with pleural plaques, which may be present on the lateral chest wall or on the pleural surface of the diaphragm (ATS, Citation2004; Craighead, Citation2008; Weill, Citation2008). Pleural plaques (sometimes called localized pleural thickening or circumscribed pleural thickening) are comprised of collagen fibers in an open basket-weave pattern and covered by a layer of mesothelial cells, and they may or may not be calcified (ATS, Citation2004). They correlate with time from first asbestos exposure and are typically seen 20 years after first exposure (Weill, Citation2008). Pleural plaques are distinct from DPT, which consists of extensive fibrosis of the visceral pleura, often presenting as fibrous strands that extend into the lung parenchyma (Miles et al., Citation2008). Whether pleural plaques cause deficits in lung function or are simply markers of asbestos exposure is controversial. While the American Thoracic Society (ATS, Citation2004) and the British Thoracic Society (BTS, Citation2011) consider pleural plaques markers of asbestos exposure, a few epidemiology studies have reported that they are associated with reduced lung function (Bourbeau et al., Citation1990; Larson et al., Citation2012; Miller et al., Citation2013).
In 2011, the United States Environmental Protection Agency (US EPA, Citation2011) released a draft Toxicological Review of Libby Amphibole Asbestos that included a Reference Concentration (RfC) calculation. An RfC is the concentration of a substance for which continuous inhalation exposure over a lifetime is likely to be without an appreciable risk of deleterious effects. US EPA based its proposed RfC on pleural plaques. As the presence of pleural plaques is considered by many to be a biomarker of exposure, rather than an established adverse health effect per se, US EPA's decision to base the RfC on pleural plaques is controversial.
Historically, pleural plaques were identified by X-ray radiography (ILO, Citation2000). Some limitations of this method include the inability to detect some plaques and early lung parenchymal fibrosis and pleural thickening, as well as the misdiagnosis of extrapleural fat pads as pleural plaques (ATS, Citation2004). High resolution computed tomography (HRCT), a more sensitive tool for identification of pleural plaques and other lung abnormalities, has come into use in the last 25 years or so as a state-of-the-art method for lung imaging (ATS, Citation2004). It has been suggested that studies using X-ray radiography to identify pleural plaques lack sensitivity because lung function decrements observed in these studies may actually be attributable to the presence of other pleural or parenchymal fibrosis that was undetected by the radiographs (Schwartz et al., Citation1990a; Weill, Citation2008). Studies using HRCT to identify pleural plaques are less likely to suffer from this potential limitation and may be more reliable for the purpose of determining whether pleural plaques affect lung function.Footnote1
We identified and critically reviewed studies that used either HRCT or X-ray radiography to identify people with and without pleural plaques to determine whether the weight of evidence indicates pleural plaques are associated with lung function decrements and hence biomarkers of effect or are more likely markers of asbestos exposure. We also compared the findings of HRCT studies with those of X-ray studies to ascertain whether the results were dependent on the method used to identify pleural plaques.
Methods
Literature search strategy and study selection
We searched the PubMed database for studies published through May 2014 that evaluated pleural plaques and lung function using several search terms: (lung function tests OR pulmonary function tests OR spirometry OR fev OR fev1 OR fvc OR residual volume OR rv OR total lung capacity OR tlc OR erv OR expiratory reserve volume OR DLCO OR gas diffusion) AND (pleural plaques OR pleural thickening OR asbestos). In addition, we checked references in review articles of pleural plaques and asbestos effects to identify any studies that may not have been identified by our literature search.
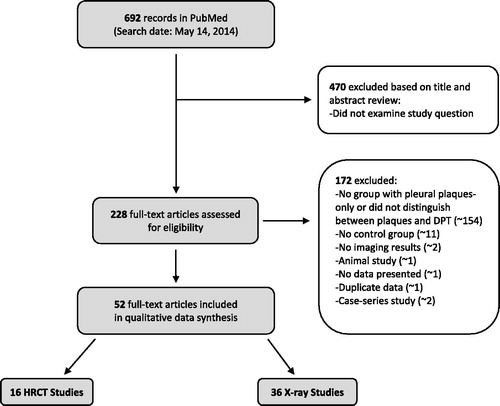
We included peer-reviewed observational studies of adult humans, with pleural plaques as the potential risk factor of interest and lung function tests as the outcomes of interest. We did not consider studies that met any of our exclusion criteria: animal or in vitro studies; observational studies conducted in children; studies that did not have either subjects with pleural plaques alone (i.e. with no other abnormalities) or a regression analysis for pleural plaques adjusting for other abnormalities; studies that did not report lung function test or regression results for individuals with pleural plaques alone; studies that investigated pleural plaques resulting from a disease, a medication or a medical intervention; or review articles. Two investigators independently reviewed each study for inclusion, first by reviewing study titles and abstracts, and then the full text. When there was a disagreement, a third investigator was consulted to resolve the discrepancy. details the literature search and study selection process.
Data extraction
One investigator extracted data from each study using a standardized data extraction form, and a second investigator independently reviewed each entry. When there was a disagreement regarding a particular entry, a third investigator was consulted to resolve the disagreement. The data we extracted included study design (i.e. cross-sectional or longitudinal), study size (including the number of subjects with no lung abnormalities and pleural plaques alone) and location, population from which subjects were drawn, participation rate, number of image readers, number of measures of lung function, standards for conducting lung function tests, number of times lung function tests were conducted, results of lung function tests, whether there was an association of lung function with extent of plaques and whether analyses were adjusted for exposure, smoking or body mass index (BMI).
Assessment of study quality and risk of bias
Two investigators independently evaluated study quality and risk of bias for each study. When there was a disagreement, a third investigator was consulted to resolve the disagreement. The characteristics we evaluated included study design, study size, participation rate, whether or not the referents were exposed to asbestos, whether the study included an exposure assessment, imaging method, number of image readers, standards used for lung function testing, number of lung function trials, number of lung function parameters tested, statistical methods and whether the analyses were adjusted for confounders (BMI, smoking and asbestos exposure).
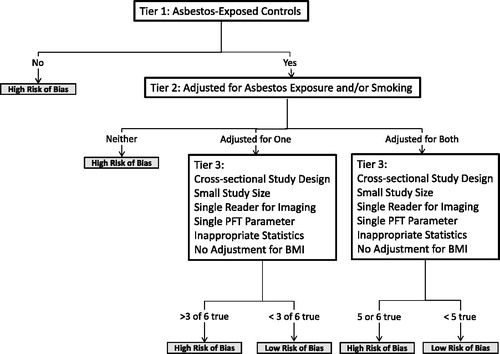
We conducted risk of bias analyses separately for HRCT and X-ray studies based on study quality characteristics that may have impacted the validity of the study findings (). We first considered whether control subjects were exposed to asbestos (Tier 1). Because non-exposed subjects do not appropriately represent the source population subjects with pleural plaques are selected from, studies that have non-exposed controls have a high risk of bias (likely away from the null) and are of lower quality. We next evaluated whether studies (with asbestos-exposed controls) adjusted for asbestos exposure and/or smoking (Tier 2). If a study did not adjust for either of these factors, we considered it to have a high risk of bias and to be of lower quality. If a study adjusted for one or both of these factors, we assessed six factors that can potentially bias results: cross-sectional study design (versus longitudinal study), small study size (below the median of 120 subjects), use of a single person reading images (versus two or three), examination of a single pulmonary function test (PFT) parameter, no reported statistical methods and/or no adjustment for BMI (Tier 3). Cross-sectional studies have higher risk of bias than longitudinal studies as the former does not take into account within-person variation in measured lung function parameters. Smaller studies are less precise and more prone to chance findings than larger studies. A single reader of all imaging results may introduce systematic error in identification of pleural plaques, thus increasing the risk of bias. A single type of spirometry measure [e.g. forced vital capacity (FVC) or forced expiratory volume in one second (FEV1)] does not give a complete picture of pulmonary function and may lead to disease misclassification. Because obese people have lower lung function (Salome et al., Citation2010), a study has a higher risk of bias (and is of lower quality) if there is no adjustment for BMI. Overall, each of these factors is likely to have a smaller impact on a study's risk of bias than having non-exposed controls or no adjustment for asbestos exposure and smoking. Thus, we concluded that a study with asbestos-exposed controls and adjustment for both asbestos exposure and smoking had a high risk of bias, and was of lower quality if it met five or more Tier 3 criteria; on the other hand, if it met only one or none of these criteria, it had a low risk of bias and was considered of higher quality. We also concluded that studies with asbestos-exposed controls that adjusted for asbestos exposure or smoking (but not both) had high risk of bias (and were of lower quality) if they satisfied three or more Tier 3 criteria, and a low risk of bias and higher quality otherwise.
Evidence integration
HRCT and X-ray studies were first analyzed separately. We focused on spirometry, gas diffusion, and lung volume findings. Because FVC (or VC [vital capacity]), FEV1, the FEV1/FVC (or FEV1/VC) ratio and total lung capacity (TLC) are the basic parameters needed to identify a functional deficit (i.e. restriction or obstruction; Pellegrino et al., Citation2005), we primarily focused on these, along with diffusing capacity of carbon monoxide (DLCO), for our analysis. We looked at each endpoint separately and in combination to determine whether there was an indication of restriction, obstruction, mixed effect or gas diffusion effect in each study. We focused on differences between individuals with pleural plaques and those with no lung abnormalities.
We considered both the statistical and clinical significance of lung function results. According to ATS (Citation1986), clinical respiratory function impairment is defined as a reduction in the capacity to exercise or work. Clinical significance is usually evaluated on an individual level by a clinician; thus, the general guidelines for what constitutes clinical significance cannot be directly applied to a population. The “gold standard” approach to define individual clinical significance is based on the lower limit of normal (LLN), i.e. normal spirometric values falling between the population mean and ±2.5 standard deviations. Some clinicians and researches use an arbitrary cutoff point (e.g. <80% of predicted) to define functional deficits; however, the LLN is considered more accurate because what defines “normal” varies within populations based on the age, ethnicity and sex distributions. In studies that reported the mean FVC, FEV1 and other spirometric values in each population, it is impossible to know how much decline or improvement any one member of the population experienced.
When evaluating results across studies, we placed more weight on higher quality studies and, consistent with the recommendations of the National Research Council (NRC, Citation2014), focused on strength of association, consistency, directness (the extent to which the study directly addresses the study question), precision, magnitude of effect, possible confounding, population or study consistency, plausibility and coherence. We then compared the integrated results of X-ray and HRCT studies to determine whether the two different diagnostic methods for pleural plaques provided differing results.
To evaluate consistency of results across studies, we tabulated the effects in the higher quality studies, and whether patterns of effects were evident across studies, regardless of magnitude or statistical significance. If no pattern was observed, then a causal association is unlikely. If a pattern was observed, it could mean either a causal association or a consistent bias or confounding factor across studies. We also looked at magnitude of effect, to determine whether any observed changes in lung function were large enough to likely signify a clinically relevant effect.
We also tabulated the longitudinal studies in a separate table to evaluate whether there was any effect of pleural plaques on lung function over time.
Results
Literature search and study selection
We identified 695 studies from the initial literature search (). Based on a review of titles and abstracts, we narrowed the list down to 228 studies for full text review. Fifty-two studies met our inclusion criteria and were included in the final analysis. and describe the characteristics of included studies, and and present the results of each study.
Table 1. HRCT study characteristics.
Table 2. X-ray study characteristics.
Table 3. HCRT study results for pleural plaques and lung function.
Table 4. X-ray study results for pleural plaques and lung function.
Study quality and risk of bias
We evaluated study quality separately for the 16 HRCT and 36 X-ray studies we identified. Many of the studies were limited by characteristics such as small study size, inappropriate referents, incomplete reporting of methods and lack of adjustments for confounding factors. Based on our criteria, we identified six and 16 higher quality HRCT and X-ray studies, respectively (). Most of the analyses discussed below and in and are focused on these 22 studies.
Table 5. Risk of bias.
HRCT studies
Sixteen studies used HRCT to identify pleural plaques. Two were longitudinal, with mean follow-up times of 3.7 years (Rui et al., Citation2004) and 5 years (Damian et al., Citation2007), respectively; the rest were cross-sectional. Study sizes ranged from 31 to 2743 participants. In most studies, the participation rate of eligible subjects was not reported. Participation rates ranged from 42% to 95% in three studies that either reported the information or gave enough data for the rate to be calculated. Two studies assessed the effect of pleural plaques in regression analyses only (Copley et al., Citation2001; Valkila et al., Citation1995) and evaluated pleural plaques as an independent variable for all participants regardless of the presence or absence of other lung abnormalities. In the other 14 studies, lung function measurements in a group of pleural-plaques-only participants were compared with those of a reference population. Because all participants with pleural plaques were exposed to asbestos, the most appropriate reference populations are asbestos-exposed participants without lung abnormalities. Thirteen of the studies included asbestos-exposed reference populations, and one included only unexposed controls (Sandrini et al., Citation2006).
In eight of the HRCT studies, two or more readers interpreted the results of the HRCT scans. A single reader was employed in four of the studies, and the number of readers was not reported in the remaining four. In six studies, investigators assessed the effects of increasing number, thickness or area of plaques on lung function. Fourteen of the 16 studies reported some measure of asbestos exposure, most often duration of employment. Thirteen of the studies reported the standard protocols by which the lung function measurements were taken, most often ATS or European Respiratory Society standards. All except one of the studies included testing of multiple lung function parameters (Sette et al., Citation2004 reported only gas exchange).
Body mass index, smoking status and asbestos exposure can all impact lung function. Only two of the studies (Clin et al., Citation2011; Rui et al., Citation2004) adjusted for BMI in their statistical evaluations. Adjustment for smoking was more common (10 studies), while five (Clark et al., Citation2014; Clin et al., Citation2011; Rui et al., Citation2004; Spyratos et al., Citation2012; Van Cleemput et al., Citation2001) included adjustments for asbestos exposure ().
X-ray studies
We identified 36 studies that used X-ray to identify pleural plaques. Four were longitudinal, with mean follow-up times of 2 (Schwartz et al., Citation1994), 4 (Ohlson et al., Citation1985), 7 (Ostiguy et al., Citation1995) and 10 years (Glencross et al., Citation1997). The remaining 32 were cross-sectional. Study sizes ranged from 23 to 6476 participants. In 20 studies, participation rate was not reported. In the 16 studies that either reported it or gave enough data for the rate to be calculated, the participation rate ranged from 3.4% to 97%. Four studies assessed pleural plaques by regression analysis only (Broderick et al., Citation1992; Kennedy et al., Citation1991; Larson et al., Citation2012; Schwartz et al., Citation1994), evaluating pleural plaques as an independent variable for all participants regardless of the presence or absence of other lung abnormalities. In the other 32 studies, lung function measurements in a group of pleural-plaques-only participants were compared with those of a reference population. Thirty-one of the studies included asbestos-exposed reference populations, and five did not.
In 22 of the X-ray studies, at least two readers interpreted the results of the X-ray films. In the other 14 studies, either some or all of the films were read by one reader, or the number of readers was not reported. In seven studies, investigators assessed the effects of increasing number, thickness or area of plaques on lung function. Thirty-one of the studies included some measure of exposure, usually duration. Standard protocols for measurements of lung function (most often ATS standards) were reported in 19 studies. Four studies reported measurements for only one lung function parameter (FVC); all other studies included multiple parameters.
Six studies included adjustments for BMI in their statistical analyses, most (29 of 36) adjusted for smoking, and 13 adjusted for exposure to asbestos.
Evidence integration
shows lung function measurements reported in the HRCT studies, and lists those reported in the X-ray studies. Spirometry measurements (FVC or VC, FEV1 and FEV1/FVC or FEV1/VC) can indicate restriction or obstruction, while TLC, residual volume (RV) and DLCO measurements can help confirm a diagnosis and determine the severity of a functional deficit (Pellegrino et al., Citation2005). Evaluating whether there is a decline in pulmonary function involves comparing the mean test results in a population of interest to the average values for asymptomatic, non-smoking individuals of a comparable age, height, race/ethnicity and sex. Typically, characterizing a functional deficit (i.e. restriction or obstruction) requires an evaluation of FEV1/FVC first, followed by FVC and/or TLC to confirm whether lung function is abnormal in an individual or the result of normal variation or possible test error (Pellegrino et al., Citation2005). Measures of lung function such as maximal expiratory flow (MEF), peak expiratory flow (PEF) and maximal voluntary ventilation (MVV) can help diagnose specific disorders when other measurements give abnormal results, but these are not generally used for initial determinations of function (Pellegrino et al., Citation2005).
HRCT studies
Spirometry
All but one of the HRCT studies reported measurements for some or all of the following spirometric parameters: FVC or VC; FEV1; FEV1/FVC or FEV1/VC (). Most studies (11/15) did not report any statistically significant differences in any of these parameters between populations with and without pleural plaques. In addition, the magnitude and direction of effect varied among all the studies. Of the six higher quality studies, three reported statistically significant but very small (3–7%) mean decrements and three did not (). In the three studies that did not, there was no consistent pattern in direction of effect for FVC (or VC) or for FEV1, although the ratio of these two measurements was consistently lower.
Table 6. Differences in spirometry, gas diffusion, and lung volume in participants with pleural plauqes compared to controls in higher quality HRCT and X-ray studiesa.
Lung volumes
Of eight HRCT studies that reported TLC measurements, three reported statistically significant differences between populations with and without pleural plaques. Three of the eight did not report values, only significance levels. Each of the other five showed a slight decrement in TLC for the pleural plaques group. All of these were among the higher quality studies. Of four studies that measured RV, two did not report values, one reported a slight increase, and one reported a slight decrease. The latter two were in the higher quality study category. None of the differences were statistically significant.
DLCO
Nine HRCT studies reported DLCO. Of the five reported values, three showed small increases and two showed small decreases in populations with pleural plaques compared to referents. One of the increases was statistically significant. Among the higher quality studies, one reported an increase and two reported decreases in DLCO in pleural plaques groups.
Other
Among various other types of lung function measurements reported in the HRCT studies (e.g. MEF, FEF [forced expiratory flow]), there were no consistent findings, and no statistically significant results were reported.
X-ray studies
Spirometry
Thirty-three of 36 X-ray studies reported measurements for some or all of the following spirometric parameters: FVC or VC; FEV1; FEV1/FVC or FEV1/VC (). Just over half (17/33) of these reported a statistically significant decrement in one or more of these measures in populations with pleural plaques. Although most of the differences were small, overall, they tended to be larger than the differences reported in the HRCT studies. Among the 14 higher quality studies, seven reported statistically significant differences in one or more of these parameters. The general pattern across all higher quality studies was a decrease for each of these parameters.
Lung volumes
Seven studies measured TLC. Three of these reported small, statistically significant decreases. The other four studies did not report statistically significant differences. Among the higher quality studies, one reported a significant decrease and two reported slight decreases that were not significant. Of five studies that measured RV, only one reported a statistically significant difference (decrease) in the pleural plaque group compared to the referents. One reported a slight non-significant decrease, two a slight increase, and one did not report the direction of effect. Among the higher quality studies, neither of two RV measurements was significantly different from controls, with one small increase and one small decrease.
DLCO
Eleven studies measured DLCO (also called carbon monoxide transfer factor, or TLCO). Four reported significant decreases in the pleural plaques groups. The seven remaining non-significant results included two decreases and five increases in DLCO. Among the higher quality studies, there was a more consistent pattern. Five studies reported small decreases; two were statistically significant. One non-significant increase was also reported.
Obstruction/restriction
Five X-ray studies evaluated whether pleural plaques were associated with restrictive or obstructive effects, either by calculating odds ratios or the percentage of participants who fit the criteria for these conditions. In each of these studies, restriction and obstruction were defined by measurements of FVC, FEV1, FEV and/or FEV1/FVC. Of four higher quality studies, three reported a significant increase in restriction among populations with pleural plaques and one reported an increase in mixed restriction/obstruction (Dujic et al., Citation1993; Garcia-Closas & Christiani, Citation1995; Larson et al., Citation2012; Oliver et al., Citation1988). One lower quality study did not report any associations (Kennedy et al., Citation1991).
Other
Several other parameters were measured in one or more studies but not reported frequently enough to display patterns of significance, direction of effect or magnitude of effect ().
Longitudinal studies
Longitudinal studies are the optimal way to determine whether pleural plaques cause a greater loss of lung function over time than would be anticipated from aging alone. Thus, if plaques are associated with lung function decrements, we would expect to see a greater loss of lung function over time in individuals with plaques compared to those without plaques. We identified six longitudinal studies of pleural plaques and lung function. Two of these were HRCT studies (Damian et al., Citation2007; Rui et al., Citation2004) and four were X-ray studies (Glencross et al., Citation1997; Ohlson et al., Citation1985; Ostiguy et al., Citation1995; Schwartz et al., Citation1994). All of these studies were higher quality, and follow-up times ranged from 2 to 10 years. None of these six studies showed a significantly greater reduction in lung function values over time in participants with pleural plaques compared to participants with normal lung scans (). Although not significant, the direction of effect was toward slightly lower lung function in the pleural plaques groups over time compared to the referents in five of these studies. The exception, Ohlson et al. (Citation1985), showed slightly less lung function loss in the pleural plaques group compared to the referents.
Table 7. Differences in lung function in participants with pleural plaques compared to those with no abnormalities in higher quality longitudinal HRCT and X-ray studies.
Extent of pleural plaques and lung function
If pleural plaques can impact lung function, there should be a clear relationship between extent of plaques (in terms of number or size) and lung function. This association was assessed in six HRCT studies. Five of these (Clark et al., Citation2014; Copley et al., Citation2001; Oldenburg et al., Citation2001; Sette et al., Citation2004; Van Cleemput et al., Citation2001) reported no significant effect of the extent of plaques, measured in number or size, on lung function. One study (Clin et al., Citation2011) reported statistically significant associations between the sum of circumferences of plaques, but not thickness, and FVC and TLC. Directions of effect were mixed, but the values for FVC and FEV1 were slightly lower with increasing extent of pleural plaques (about 4% difference between greatest- and smallest-extent groups) in the two higher quality HRCT studies with reported values (Clark et al., Citation2014; Clin et al., Citation2011).
Three X-ray studies showed no effect of extent of pleural plaques on lung function (Hillerdal et al., Citation1990; Ostiguy et al., Citation1995; Zavalic & Bogadi-Sare, Citation1993), while three reported associations. Fridriksson et al. (Citation1981) observed an association with the extent of plaques and TLC, RV, airways resistance (Raw), volumetric airways conductance (Gaw/V), maximal elastic recoil pressure [Pst(max)] and the ratio of static lung compliance (Cst) to TLC. Oliver et al. (Citation1988) reported that FVC and the occurrence of pulmonary restriction were associated with quantitative pleural score. Larson et al. (Citation2012) found an association between the presence of pulmonary restriction and degree of extent and width of plaques.
Direction and magnitude of effect
shows the direction and magnitude of effects associated with pleural plaques in the higher quality HRCT and X-ray studies. Some studies reported the difference between the group with pleural plaques compared with the controls, whereas others only reported the predicted value for each group; for the latter, we calculated the difference in values. In addition, we calculated the confidence interval using the sample mean, standard deviation and sample size provided in the study.
Among the six higher quality HRCT studies, there were no consistent patterns for effects on FVC (or VC) or FEV1. The differences in mean FEV1 between pleural plaques groups and normal groups ranged from −7% to +3.2% predicted; for FVC (or VC), the differences ranged from −6% to +3% predicted. For FEV1/FVC (or FEV1/VC), there is a pattern of very slight decreases in the pleural plaques groups, with a range of differences in mean predicted values from −3 to 0. depicts the differences in FVC, FEV1 and FEV1/FVC, respectively, in the five higher quality HRCT studies that reported spirometric values in groups (Spyratos et al., 2014, the sixth higher quality HRCT study, reported only the differences between groups, but not predicted values for each group). Three of these studies (Clin et al., 2001; Damian et al., Citation2007; Rui et al., Citation2004) reported statistically significant differences for both FVC and FEV1, but the differences are small and not clinically significant. For TLC, there is also a pattern of decrease in the pleural plaques groups, with values ranging from −6.1% to −0.1% predicted. These values may not be comparable across studies, however, because the methods used to measure lung volumes were not consistent across studies. There are no consistent patterns among the few studies that reported values for RV and DLCO.
Figure 3 Spirometry results in high quality HRCT and X-ray studies. Percent predicted FVC and FEV1, and FVC/FEV1 ratio, among individuals with no lung lesions (light gray bars) or pleural plaques (dark gray bars). Error bars indicate standard deviations, except for Hilt et al. (Citation1987), where no standard deviations were reported. (A, B and C) show the results of higher quality HRCT studies and (D, E and F) show the results of the higher quality X-ray studies.
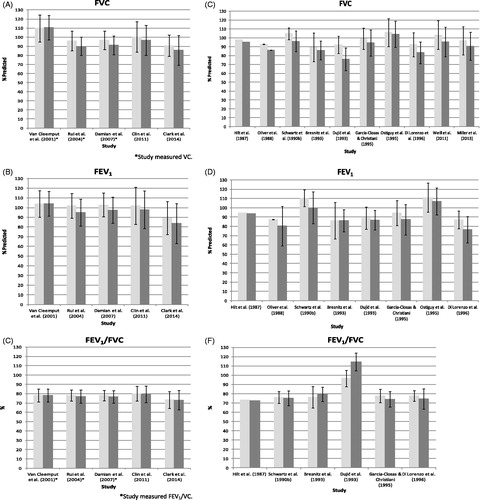
The patterns and magnitudes of effect in the 16 higher quality X-ray studies are somewhat different from those in the HRCT studies. With the exception of Ohlson et al. (Citation1985), the X-ray studies show patterns of decrease in the pleural plaques groups, with wider variations, for both the FVC (or VC) and FEV1 parameters. For FVC (or VC), the differences in mean percent predicted values compared to referents range from −16.4 to +13.3; for FEV1, they range from −10.4 to +11.5 (note that some of the reported values are regression values rather than percent predicted; these are not included in these ranges). Six studies included FEV1/FVC (or FEV1/VC) measurements with no consistency and a very wide range of differences; there are not enough reported RV values to determine any patterns. For DLCO, four of five studies reported decreases in the pleural plaques groups, with differences in mean values ranging from −7.6 to +0.2.
depicts the differences in FVC, FEV1, and FEV1/FVC, respectively, in the 10 higher quality X-ray studies that reported predicted values; other higher quality studies (Bourbeau et al., Citation1990; Glencross et al., Citation1997; Larson et al., Citation2012; Ohlson et al., Citation1985; Schwartz et al., Citation1994) were not included because the predicted values were not reported. Comparing to illustrates the higher variability in results across the X-ray studies versus HRCT studies.
Overall, there are clear differences between the HRCT and X-ray study results. The magnitude of differences in the HRCT studies has a much smaller range than in the X-ray studies, indicating that X-ray studies are less reliable for estimating the effects of pleural plaques.
HRCT results showed fewer consistent patterns regarding associations between pleural plaques and lung function, with inconsistent associations for FVC (or VC) and FEV1. Although the findings were more consistent for FEV1/FVC (or FEV1/VC) among studies, the changes were primarily non-significant decreases in the ratio; if pleural plaques were causing a reduction in volume, as in restrictive changes, one would expect the ratio to increase. Thus, the biological significance of the small reduction in the ratio should be questioned as the changes go in the direction opposite to that expected. The more clinically important findings are the observation that the changes in both parameters of lung volume, FVC and FEV1, are small and inconsistent.
Interpretation of lung function measurements
As the X-ray study results are less reliable, this section focuses on the higher quality HRCT studies. While the PFT results for these studies were mixed, some patterns were evident. For example, although there were both increases and decreases in mean FVC (or VC) and FEV1 values in the pleural plaques groups, the ratios of the two measurements showed a pattern of decrease. When FVC (or VC) and FEV1 are both increased, a decrease in the ratio between them is uninterpretable. In the four studies in which all the parameters were decreased, the mean changes were very small (≤1.5%) and generally either not statistically significant or not considered clinically significant by the study authors. The pattern of reduction in TLC for this group of studies indicated a trend toward restriction but, again, the results were too small to be clinically significant and more likely indicative of normal variation or a consistent bias among studies. It is also important to note that in each of the three studies that reported statistically significant decreases in TLC, the asbestos exposures for the groups with pleural plaques were substantially higher than for the referent groups. While two of these studies (Clin et al., Citation2011; Rui et al., Citation2004) adjusted for asbestos exposure, the substantial exposure differences indicate the decreases may have been at least in part due to residual confounding. There were not enough RV and DLCO results in this group of studies to allow for interpretation of effects, but the reported differences were also very small.
Longitudinal studies showed that lung function in individuals with pleural plaques did not decrease over time at a greater rate than lung function in those without plaques. This indicates that plaques have no effect on lung function independent of aging or other lung conditions. Similarly, there were no consistent findings regarding the effect of extent of plaques. The lack of a clear relationship between the extent of pleural plaques and lung function indicates that the very small associations observed in some of the studies may not be indicative of causation.
Most studies did not report FVC, FEV1 and other spirometric values at the individual level. Because an evaluation of these factors together is necessary to determine whether a particular individual has abnormal lung function, it is not possible to know how many, if any, individuals actually had abnormal lung function. A mean deficit seen in a group may be not be clinically relevant for some individuals, but it could correspond to a considerable increase in the proportion of the population that falls into the category of abnormal (i.e. below the LLN). It could also mean there is a reduction in the proportion of individuals on the higher end of the pulmonary function spectrum, but all individuals could still have normal lung function. However, without looking at the data on an individual level, one cannot know whether and, if so, how many people in the group (e.g. the group of people with pleural plaques) actually fall below the LLN. As stated in the previous section, in general, the studies evaluated showed mean declines of around 3–5% in the pleural plaque groups. Abnormal lung function is generally defined as below 80% of predicted values, depending on the age and ethnic makeup of the population, so it is unlikely that a 3–5% population change would shift any individuals within the population below 80% of predicted.
One way to remove some of the uncertainty surrounding the question of whether there is a true, clinically significant functional decline in a group is through categorical analysis of restrictive and obstructive effects. Several of the available studies (Dujic et al., Citation1993; Garcia-Closas & Christiani, Citation1995; Kennedy et al., Citation1991; Oliver et al., Citation1988) categorized each study participant as having either obstructive, restrictive or mixed effects based on individual lung function test results. The authors then compared the prevalence of such conditions between groups (e.g. between those with pleural plaques and those with no lung abnormalities). Kennedy et al. (Citation1991) and Garcia-Closas & Christiani (Citation1995) found no significant increase in restrictive lung function in pleural-plaque-only groups compared to normal exposed controls. Larson et al. (Citation2012) reported that the prevalence of restriction was higher in the group with pleural plaques compared to the normal group. These investigators used X-ray to identify pleural plaques, however, so undetected DPT and/or other fibrosis may have been responsible for the observed decrease in lung function. Alternatively, the differences between the groups might be accounted for by residual confounding. For example, in the Larson et al. (Citation2012) study, results were adjusted for exposure group (occupational, household contact or resident) and number of non-occupational exposure pathways but not duration of or cumulative exposure. Moreover, few studies adjusted for BMI, and differences in mean BMIs between the groups with pleural plaques and those without could account for small differences in lung function.
Discussion
We identified 16 studies that used HRCT to identify lung lesions (six were of higher quality). These studies showed no or very little change in lung function among people with pleural plaques versus those with no pulmonary abnormalities and, relative to controls, lung function in those with pleural plaques was generally well within the normal range. These studies also showed a smaller range of effects than the 36 X-ray studies (15 higher qualities) we identified, indicating that X-ray studies were less accurate. Longitudinal studies (using both HRCT and X-ray) indicated that pleural plaques do not cause longitudinal changes in lung function. Overall, the weight of evidence indicates that pleural plaques do not impact lung function.
Some researchers suggest that studies using X-ray radiography to identify pleural plaques are not reliable because lung function decrements observed with pleural plaques in these studies may be attributable to the presence of parenchymal fibrosis that was undetected in radiographs (Schwartz et al., Citation1990b; Weill, Citation2008). Indeed, ATS (Citation2004) stated:
Although pleural plaques have long been considered inconsequential markers of asbestos exposure, studies of large cohorts have shown a significant reduction in pulmonary function attributable to the plaques, averaging about 5% of FVC, even when interstitial fibrosis (asbestos) is absent radiographically… This has not been a consistent finding and longitudinal studies have not shown a more rapid decrement in pulmonary function in subjects with pleural plaques. Decrements, when they occur, are probably related to early subclinical fibrosis.
HRCT may miss some early subclinical fibrosis, but not to the extent that X-ray does (McGavin & Sheers, Citation1984; Paris et al., 2008; Staples et al., Citation1989). In general, HRCT is considered more sensitive and specific for the detection of pleural plaques, DPT and asbestosis (Miles et al., Citation2008). The sensitivity of the HRCT method is particularly important because, while they can co-occur, pleural plaques, other types of pleural thickening and interstitial fibrosis each have different etiologies and functional consequences. Physiologically, pleural plaques are different from other forms of pleural thickening; they have a distinct shape, whereas other forms are less distinct in pattern and shape. In general, plaques are specific to asbestos exposure, whereas other forms of pleural thickening can be caused by a wide range of previous or underlying illness and injury (e.g. pneumonia and chest trauma). Other forms of pleural thickening are sometimes associated with a functional decline, but evidence does not indicate that pleural plaques can cause this. DPT and interstitial fibrosis are more extensive lesions that are located on the interior of the lung, in addition to other locations. These conditions are strongly associated with decreased lung function (Miles et al., Citation2008; Schwartz et al., Citation1993). The proposed modes of action (MoAs) for lung function decrements with these latter conditions are the narrowing of the airways from the lesions causing reduced air flow (i.e. obstruction) or, for DPT, the ability to cause adherence of the parietal and visceral pleura to each another, restricting how far the lung can expand. Considering that other asbestos-related conditions (besides pleural plaques) are more accurately identified by HRCT than X-ray, and HRCT studies as a group show a smaller range of lung function decrements than X-ray studies, it is evident that even higher quality X-ray studies may not be as reliable as higher quality HRCT studies for conducting research on pleural plaques (versus individual screening).
Individuals with decrements in only one lung function parameter may not fall below the lower limit of normal lung function, and studies that evaluated these parameters independently (e.g. by comparing mean FVC across groups and then mean FEV1 across groups) did not address whether any individual actually fell below the lower limit of normal lung function. A few studies categorized each participant as having obstructive, restrictive or mixed effects based on clinical definitions that consider several parameters [i.e. FVC (or VC), FEV1, the FEV1/FVC (or FEV1/VC) ratio and TLC] (Pellegrino et al., Citation2005). In general, the results were inconsistent, with half of the studies reporting null results and half reporting a significant difference in function effects. It should be noted, however, that all of the available categorical analysis studies were conducted using X-ray. In general, as discussed in the “Results” section, studies with categorical comparisons reduce some of the uncertainty surrounding whether pleural plaques can cause clinically relevant lung function deficits. In these types of studies, each person in the study is assessed individually for the clinical relevance of his or her lung function changes, and one can then make a comparison between the group with plaques and the group with no lung abnormalities to determine if there is a higher prevalence of individuals with clinically significant lung function in either group.
The reference value equations used to calculate the percent predicted values for each of the lung function measures varied across studies and may be responsible for some of the variability in results. Of the 16 HRCT studies, two did not report the reference equations they used, and, among the remaining 14, eight different equations were used. Three of the 36 X-ray studies did not report reference equations, and it appears that 18 different reference equations were used among the other studies. Some of the variation is due to changes in equipment, software and measurement techniques, and population characteristics over time (Kuster et al., Citation2008; Stanojevic et al., Citation2010). Even now, however, there are different reference equations available for different populations, including for specific countries such as China and Brazil, or specific groups such as children and the elderly (Garcia-Rio et al., Citation2004). As each of these reference populations and corresponding equations are different, the definition of what is “normal” in a healthy population may vary quite substantially (Stanojevic et al., Citation2010). The same absolute decline in lung function could correspond to a much lower percent predicted value in one study compared to another that used different reference equations. As stated by Stanojevic et al. (Citation2010), “The use of inappropriate reference equations and misinterpretation, even when potentially appropriate equations are used, can lead to serious errors in both over- and under-diagnosis”. Unfortunately, the implications surrounding the choice of reference equations has often been overlooked in the literature (Crapo, Citation2004).
Many study results were not adjusted for asbestos exposure, age, BMI or smoking. All of these factors can impact lung function. Asbestos exposure is of particular concern because in almost every study where separate exposures were reported for both groups, the pleural plaques group exposure was higher than the referent group exposure. In some cases, the differences were quite striking. For example, in the Clin et al. (Citation2011) study, mean exposure for the pleural plaques group was more than twice as high as for the control group. With respect to BMI, Lee et al. (2001) found that BMI > 30 kg/m2 was associated with a higher prevalence of DPT on X-ray when compared to people with no pleural abnormalities in a group of almost 700 former asbestos workers. This suggests that BMI may vary between groups with radiological abnormalities, even within a cohort that is fairly homogenous. Finally, the ratio of smokers to non-smokers was not measured in all studies; however, several studies show substantial differences in smoking habits between the pleural plaques groups and referents (Clark et al., Citation2014; Clin et al., Citation2011; Ohlson et al., Citation1984, Citation1985; Oliver et al., Citation1988; Weill et al., Citation2011). Even studies that adjusted for these factors may have been impacted by residual confounding.
Moreover, even if one were to assume that the mean lung function deficits reported in these studies were caused by pleural plaques and not underlying subclinical fibrosis, the deficits reported in the studies – whether statistically significant or not – were small enough that they would likely not result in clinically significant lung function changes for most participants. This is reflected in the conclusions of many of the studies. For example, Clin et al. (Citation2011), the largest HRCT study, stated that while there was a statistically significant relationship between isolated plaques and restrictive pattern, “[t]he observed decrease in FVC and TLC is unlikely to be of real clinical relevance for the majority of subjects in this series”.
In one of the two higher quality HRCT studies that adjusted for confounders, Spyratos et al. (Citation2012) found that, while FVC and FEV1 were lower in those with pleural plaques (n = 29) than those with no abnormalities (n = 37), when results were adjusted for age, smoking, and duration of asbestos exposure, FVC and FEV1 were non-statistically increased in individuals with pleural plaques. In the other, Rui et al. (Citation2004) found non-significant slight decreases in lung function over time in people with pleural plaques compared to people without. (Note that Clin et al. (Citation2011) did not report adjusted lung function estimates, only adjusted p values.) In addition, two HRCT studies were longitudinal (Damian et al., Citation2007; Rui et al., Citation2004). Neither of these studies showed a significantly greater reduction in lung function values over time in subjects with pleural plaques compared to subjects with normal lung scans. Four X-ray studies were also longitudinal (Glencross et al., Citation1997; Ohlson et al., Citation1985; Ostiguy et al., Citation1995; Schwartz et al., Citation1994), with follow-up times of 2–10 years. None of these studies reported significant reductions in lung function over time in people with pleural plaques compared to people without lung abnormalities. Collectively, these studies indicated that pleural plaques do not impact lung function.
We considered conducting a meta-analysis of these studies but concluded that it would be inappropriate for several reasons. First and foremost, these studies are highly heterogeneous, likely due to differences in the participants, past exposures, reference equations and the way that studies were conducted (most notably, using HRCT versus X-ray to identify pleural plaques and the varying quality of individual studies). While the use of random-effects models helps to consider the heterogeneity in the effect estimates, it does not eliminate the heterogeneity. Second, there is a substantial amount of reporting bias among these studies (US EPA, Citation2014; National Toxicology Program, Citation2013). Several studies did not report data necessary for calculating mean difference and standard error of lung function parameters. For example, Ohlson et al. (Citation1985) did not report the numbers of individuals with and without pleural plaques within each category of exposure, and three studies (Hilt et al., Citation1987; Miller et al., Citation1992; Ohlson et al., Citation1985) did not report standard deviations or standard errors for respiratory measures. In addition, four studies reported there were no significant differences between pleural plaque groups and referents, but they did not provide any values (Copley et al., Citation2001; Neri et al., Citation1996; Ohlson et al., Citation1984; Staples et al., Citation1989). Furthermore, it is possible that in some studies with negative results for pleural plaques, the focus was on other lung conditions and the results for pleural plaques were never reported. Third, because pleural plaques typically do not cause functional effects, there is potential for publication bias in the body of literature on pleural plaques and lung function. Research has shown that studies with negative results are less frequently published than studies with positive associations, and this can present a major obstacle in drawing valid conclusions from a systematic review or meta-analysis (National Toxicology Program, Citation2013; Shea et al., Citation2007). Fourth, potential confounding by past asbestos exposure, smoking and BMI were not accounted for in the majority of studies. Therefore, although some individual studies may have adjusted for confounders when comparing lung function between groups with and without pleural plaques, it would have been impossible for us to do so in a meta-analysis based on published study results. Finally, a meta-analysis could not capture all of the relevant evidence that bears on the question of whether pleural plaques cause lung function decrements (e.g. by evaluating several lung function parameters together for each study, or considering all relevant studies).
Evaluation of evidence
US EPA calculated a draft RfC for Libby amphibole asbestos based on the premise that the presence of pleural plaques is an adverse health effect that causes lung function deficits. We conducted a weight-of-evidence evaluation regarding the effects of pleural plaques on lung function by applying the Bradford Hill guidelines for evaluating causation: strength of association, consistency, magnitude of effect, possible confounding, biological gradient, plausibility and coherence (Hill, Citation1965). We also considered study quality, the adversity of reported effects and alternate explanations of the evidence.
Strength of association/magnitude of effect
Reported findings of effects that are large and precise increase the confidence that an association is causal and not likely attributable to chance, bias, error or other factors. In the higher quality studies that reported statistically significant effects of pleural plaques and lung function, the sizes of the effects were generally quite small, especially in the more accurate HRCT studies (≤7%). Both the positive and negative effects were close to the null value in the vast majority of higher quality HRCT and X-ray studies. In many of the studies, including the higher quality studies, effects were in the opposite direction of adversity and often of similar magnitude as those findings that indicate adverse effects. As it is unlikely that pleural plaques are beneficial in some studies and harmful in others, this indicates that even the stronger, positive associations may not be indicative of causation. In addition, in six longitudinal studies (two higher quality HRCT and four higher quality X-ray), no associations were found between pleural plaques and any lung function parameter changes over time. Overall, because the vast majority of positive results for effects of pleural plaques are small in magnitude (and some are even in the opposite direction of adversity) and there is no evidence of longitudinal effects, the results are not particularly supportive of a causal relationship and indicate associations are likely due to a consistent bias among studies (e.g. confounding by early subclinical fibrosis).
Consistency and coherence
The strength of an inference of causality is greater when a consistent pattern of effects is observed across several independent studies. We assessed the consistency of results across studies for each of the most commonly reported endpoints. When results were discordant among studies, we considered the possible reasons for these discrepancies. Because an inference of causality for each lung function endpoint is stronger when other endpoints also support a causal interpretation of the association, we assessed the coherence of the results among the studies for all endpoints together.
Results were inconsistent for FVC or VC among the six higher quality HRCT studies. Three studies reported a statistically significant, but very small, decrease in the pleural plaques groups compared to the referents, one study reported a non-significant small decrease and two reported small increases. There was more consistency within the higher quality X-ray studies, with 11 of 12 reporting decreases in the pleural plaques groups (five of those were statistically significant), and one study reporting increases, but the X-ray results were not consistent with the HRCT results.
For FEV1, among the six higher quality HRCT studies, results were also inconsistent. As with the FVC measurements, three studies reported a statistically significant, but very small, decrease in the pleural plaques groups compared to the referents. One study reported a non-significant small decrease, and two studies reported small increases. Among the eight higher quality X-ray studies that measured FEV1, only two reported statistically significant decreases in the pleural plaques groups. Five others reported non-significant decreases ranging from 0.8% to 10%, and one study reported increases. Thus, the HRCT results showed inconsistent FEV1 effects, and again, the X-ray results were not consistent with the HRCT results.
Measurements of FEV1/FVC or FEV1/VC were more consistent across all studies. None of six higher quality HRCT studies or four higher quality X-ray studies reported significant decreases in the pleural plaques groups. All except one reported very small, non-significant decreases, and one higher quality X-ray study reported a large (18%), statistically significant increase.
An evaluation of the three different endpoints within each study, FEV1, FVC (or VC) and FEV1/FVC (or FEV1/VC), shows that, generally, the endpoints within a given study were coherent with each other. In most studies, particularly the HRCT studies, the measures of FVC or VC and FEV1 increased or decreased in the pleural plaques groups by the same approximate magnitudes, resulting in little or no effect in the FEV1/FVC or FEV1/VC ratios. This is consistent with normal lung function. If pleural plaques caused restrictive changes, the FEV1/FVC or FEV1/VC ratios would increase, which is the opposite of what we found.
The effects of pleural plaques on TLC were also consistent across studies, with all eight that reported measurements showing a very small decrease. Four of these were significant (three HRCT and one X-ray) and four were not (two HRCT and two X-ray). Few studies reported RV measurements and, among these, results were inconsistent. None of the RV results for pleural plaques were significantly different from controls, with two reporting increases (one HRCT and one X-ray) and two reporting decreases (one HRCT and one X-ray). DLCO measurements were also inconsistent across studies, with HRCT studies reporting no significant differences (one mean increase and two mean decreases); two of five X-ray studies reported significant decreases, two reported non-significant decreases and two reported increases.
Overall, the effects of pleural plaques on lung function were not coherent across studies, especially across the most reliable higher quality HRCT studies. Some of this may be due to the different reference equations used across studies, as described previously. Still, if pleural plaques caused clinically significant lung function deficits, coherent, substantial effects within each of the studies and across endpoints would be expected.
Confounding
As cofounders can be partially or fully responsible for observed associations between an exposure and health outcome, it is imperative that they are considered in epidemiology studies. The reference equations used to calculate predicted values of lung function incorporate the impact of age, race and sex; however, the characteristics of reference populations used in formulating these equations may not always be truly representative of those in the population of interest in each study. Further, the reference equations cannot account for differences in other characteristics between the population of interest and the reference population, such as level of asbestos exposure, BMI and smoking patterns, all of which are associated with reduced lung function. We considered studies that accounted for these confounders as higher quality than studies that did not.
Among epidemiology studies that accounted for confounders, we found that statistically significant effects were generally reduced or no longer statistically significant when confounding factors were controlled for. Furthermore, in almost all studies that reported different asbestos exposures for groups with pleural plaques compared to controls, higher asbestos exposure was reported for the groups with plaques, suggesting that residual confounding may have been present – even when results were adjusted for exposure. Moreover, as discussed previously, several studies reported substantial differences in smoking habits between the pleural plaques groups and referents (Clark et al., Citation2014; Clin et al., Citation2011; Ohlson et al., Citation1984, Citation1985; Oliver et al., Citation1988; Weill et al., Citation2011). Even those studies that adjusted for smoking often asked only whether participants were smokers but did not estimate smoking exposure using more precise measures, such as pack-years of smoking. Thus, it is possible that studies that adjusted for smoking may still have suffered from residual confounding due to differences in the intensity and duration of smoking between groups. Overall, our results indicate that residual confounding could be responsible for the small differences observed between the asbestos-exposed controls and participants with pleural plaques.
Biological gradient
If pleural plaques were causal for lung function deficits, we would expect to see a larger decrease in lung function with increasing size, area or number of plaques. Of two higher quality HRCT studies that measured this, one reported a significant effect of increasing extent of pleural plaques on FVC and TLC and one reported no effect. The results for X-ray studies were also mixed, with one higher quality study reporting no effect and three reporting significant effects. The mixed results in these studies do not support an association between increasing extent of pleural plaques and decreased lung function.
Biological plausibility
Although a known MoA is not necessary to conclude causation, there is no evidence to support a biologically plausible MoA for pleural plaques to impact lung function. While there are no experimental animal studies that evaluate whether pleural plaques could cause lung function decrements, the location and extent of pleural plaques (typically discrete areas on the outside of the pleura) indicate that they are not likely to have any impacts (BTS, Citation2011). As they are located on the exterior of the lung, if plaques could cause any effect at all, they would have to be restrictive, as restriction prevents the lungs from fully expanding and reduces one's ability to fully exhale. There is no consistent evidence from the epidemiology literature that supports restriction caused by pleural plaques.
Overall, our analysis was based on the methodology put forth by NRC (Citation2014) and considered the weight of evidence regarding the effects of pleural plaques on lung function. We applied the Bradford Hill guidelines for causation, considering study quality, the adversity of reported effects and alternate explanations of the evidence, and found that studies had a broad range of populations and study designs. In higher quality HRCT studies, some associations were relatively consistent but very small in magnitude, to the extent that they were not clinically relevant. For the most part, results for the different endpoints were coherent with each other. We conclude the effects are not biologically plausible owing to the physiology of pleural plaques, and these associations are most likely due to residual confounding from early subclinical fibrosis.
Our critical review has several strengths. We followed NRC's (Citation2014) recommendations for systematic reviews, conducted a thorough literature search, included all relevant studies, conducted a formal study quality or risk-of-bias analysis (and placed more weight on higher quality studies), considered all relevant endpoints in studies evaluated and considered the impact of confounders. The studies we evaluated were quite broad and evaluated different populations from different countries that were exposed to different types of asbestos, including Libby amphibole asbestos. A major limitation of our study is that we could not overcome the limitations of individual studies described above. Of most concern is that studies did not provide information on parameters for each individual. In several studies, lung function measurements were not reported or were reported as regression coefficients, therefore lung function values could not be compared across all studies. For studies that reported only p values but not lung function measurements, we could not make any determinations about the direction of effect; analysis may have been biased in either direction as a result.
Conclusions
The epidemiology studies reviewed here can only evaluate statistical associations. They cannot indicate whether lung function reduction is caused by pleural plaques, only whether they are associated with pleural plaques. It is expected that lung function decrements could be associated with extensive pleural plaques as they are associated with asbestos exposure, which can cause pulmonary effects by other mechanisms. Studies that show lung function decrements indicate that they are very small and highly uncertain. Uncertainties due to study limitations may be greater than the reported small decrements in lung function (i.e. generally between ∼3 and 7%) that, notably, are well within the normal range of lung function for most people. Thus, it is more likely that reported decrements are attributable to other factors as opposed to pleural plaques. The weight of evidence indicates that pleural plaques do not cause lung function decrements and are not adverse.
US EPA is currently in the process of determining non-cancer health hazards from exposure to Libby amphibole asbestos. As discussed above, in its latest draft of the toxicological review of Libby amphibole asbestos, US EPA (Citation2011) proposed to base the RfC on pleural plaques. Pleural plaques are clinically relevant because they are biomarkers of exposure. The presence of pleural plaques indicates that an individual was likely exposed to asbestos and is therefore at risk for asbestos-related disease. Because pleural plaques do not cause lung function decrements themselves, the exposure level at which they occur should not be used to define the level of asbestos likely to cause adverse health effects. Pleural plaques should continue to be used in a clinical setting, but they should not be used in a regulatory setting to determine levels that will protect the general population, including sensitive individuals.
Acknowledgements
The authors wish to thank Joseph M. King and Sara Pacheco Shubin for technical assistance, and Bethany M. Allen, Shelby L. Condray, Jasmine Lai and Carla A. Walker for assistance with graphics and manuscript preparation.
Declaration of interest
The authors are employed by Gradient, a private environmental consulting firm, and the University of Rochester, School of Medicine, a private medical school. The work reported in this paper was conducted by the authors during the normal course of employment with financial support provided by W.R. Grace & Co. The authors have the sole responsibility for the writing, content, and conclusions in this article.
Notes
1Although HRCT scans are preferred for this purpose, X-rays may be more appropriate in the clinical setting. This is because HRCT scans are more costly and may identify benign nodules that then require repeated testing, causing unnecessary radiation exposure (Maxim et al., Citation2014).
References
- Antonescu-Turcu AL, Schapira RM. (2010). Parenchymal and airway diseases caused by asbestos. Curr Opin Pulm Med 16:155–61
- American Thoracic Society (ATS). (1986). Evaluation of impairment/disability secondary to respiratory disorders. Am Rev Respir Dis 133:1205–9
- American Thoracic Society (ATS). (2004). Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 170:691–715
- Bourbeau J, Ernst P, Chrome J, et al. (1990). The relationship between respiratory impairment and asbestos-related pleural abnormality in an active work force. Am Rev Respir Dis 142:837–42
- Bresnitz EA, Gilman MJ, Gracely EJ, et al. (1993). Asbestos-related radiographic abnormalities in elevator construction workers. Am Rev Respir Dis 147:1341–4
- Britton MG, Apps MC, Maxwell DL, et al. (1989). The value of ear lobe oximetry in the assessment of disability in asbestos-related disease. Respir Med 83:43–9
- Broderick A, Fuortes LJ, Merchant JA, et al. (1992). Pleural determinants of restrictive lung function and respiratory symptoms in an asbestos-exposed population. Chest 101:684–91
- British Thoracic Society (BTS). (2011). Pleural plaques: information for health care professionals. London: BTS; 11 p
- Clark KA, Flynn JJ III, Goodman JE, Zu K. (2014). Pleural plaques and their effect on lung function in Libby vermiculite miners. Chest 146:786–94
- Clin B, Paris C, Ameille J, et al. (2011). Do asbestos-related pleural plaques on HRCT scans cause restrictive impairment in the absence of pulmonary fibrosis? Thorax 66:985–91
- Copley SJ, Wells AU, Rubens MB, et al. (2001). Functional consequences of pleural disease evaluated with chest radiography and CT. Radiology 220:237–43
- Craighead JE. (2008). Benign pleural and parenchymal diseases associated with asbestos exposure. In: Craighead JE, Gibbs AR, eds. Asbestos and its diseases. Oxford, UK: Oxford University Press, 139–171
- Crapo RO. (2004). The role of reference values in interpreting lung function tests (Editorial). Eur Respir J 24:341–2
- Currie GP, Watt SJ, Maskell NA. (2009). An overview of how asbestos exposure affects the lung. Br Med J 339:506–10
- Damian A, Rui F, De Zotti R. (2007). Funzionalita respiratoria e fumo di sigaretta in lavoratori della navalmeccanica e del porto [Respiratory function and smoking habit among shipyard and dock workers]. G Ital Med Lav Ergon 29:828–30
- Di Lorenzo L, Mele M, Pegorari MM, et al. (1996). Lung cinescintigraphy in the dynamic assessment of ventilation and mucociliary clearance of asbestos cement workers. Occup Environ Med 53:628–35
- Dujic Z, Eterovic D, Tocilj J. (1993). Association between asbestos-related pleural plaques and resting hyperventilation. Scand J Work Environ Health 19:346–51
- Fridriksson HV, Hedenstrom H, Hillerdal G, Malmberg P. (1981). Increased lung stiffness of persons with pleural plaques. Eur J Respir Dis 62:412–24
- Garcia-Closas M, Christiani DC. (1995). Asbestos-related diseases in construction carpenters. Am J Ind Med 27:115–25
- Garcia-Rio F, Pino JM, Dorgham A, et al. (2004). Spirometric reference equations for European females and males aged 65-85 yrs. Eur Respir J 24:397–405
- Glencross PM, Weinberg JM, Ibrahim JG, Christiani DC. (1997). Loss of lung function among sheet metal workers: ten-year study. Am J Ind Med 32:460–6
- Hedenstierna G, Alexandersson R, Kolmodin-Hedman B, et al. (1981). Pleural plaques and lung function in construction workers exposed to asbestos. Eur J Respir Dis 62:111–22
- Hill AB. (1965). The environment and disease: association or causation? Proc R Soc Med 58:295–300
- Hillerdal G, Malmberg P, Hemmingsson A. (1990). Asbestos-related lesions of the pleura: parietal plaques compared to diffuse thickening studied with chest roentgenography, computed tomography, lung function, and gas exchange. Am J Ind Med 18:627–39
- Hilt B, Lien JT, Lund-Larsen PG. (1987). Lung function and respiratory symptoms in subjects with asbestos-related disorders: a cross-sectional study. Am J Ind Med 11:517–28
- Hjortsberg U, Orbaek P, Aborelius M Jr, et al. (1988a). Railroad workers with pleural plaques: I. Spirometric and nitrogen washout investigation on smoking and nonsmoking asbestos-exposed workers. Am J Ind Med 14:635–41
- Hjortsberg U, Orbaek P, Aborelius M Jr, et al. (1988b). Railroad workers with pleural plaques: II. Small airway dysfunction among asbestos-exposed workers. Am J Ind Med 14:643–7
- International Labour Organization (ILO). (2000). Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses. International Labour Office, Geneva, Occupational Safety and Health Series No. 22, 43 p
- Jarvholm B, Larsson S. (1988). Do pleural plaques produce symptoms? A brief report. J Occup Med 30:345–7
- Jarvholm B, Sanden A. (1986). Pleural plaques and respiratory function. Am J Ind Med 10:419–26
- Kee ST, Gamsu G, Blanc P. (1996). Causes of pulmonary impairment in asbestos-exposed individuals with diffuse pleural thickening. Am J Respir Crit Care Med 154:789–93
- Kennedy SM, Vedal S, Muller N, et al. (1991). Lung function and chest radiograph abnormalities among construction insulators. Am J Ind Med 20:673–84
- Kilburn KH, Warshaw RH. (1990). Abnormal pulmonary function associated with diaphragmatic pleural plaques due to exposure to asbestos. Br J Ind Med 47:611–14
- Kouris SP, Parker DL, Bender AP, Williams AN. (1991). Effects of asbestos-related pleural disease on pulmonary function. Scand J Work Environ Health 17:179–83
- Kuster SP, Kuster D, Schindler C, et al. (2008). Reference equations for lung function screening of healthy never-smoking adults aged 18-80 years. Eur Respir J 31:860–8
- Larson TC, Lewin M, Gottschall EB, et al. (2012). Associations between radiographic findings and spirometry in a community exposed to Libby amphibole. Occup Environ Med 69:361–6
- Lee YC, Runnion CK, Pang SC, et al. (2001). Increased body mass index is related to apparent circumscribed pleural thickening on plain chest radiographs. Am J Ind Med 39:112--6
- Lumley KPS. (1977). Physiological changes in asbestos pleural disease. In: Walton WH, McGovern B, eds. Inhaled particles. IV. Proceedings of an International Symposium Organized by the British Occupational Hygiene Society, Edinburgh, 22–26 September 1975, 781–8
- Maxim LD, Niebo R, Utell MJ. (2014). Screening tests: a review with examples. Inhal Toxicol doi: 10.3109/08958378.2014.955932
- Mazziotti S, Gaeta M, Costa C, et al. (2004). Computed tomography features of liparitosis: a pneumoconiosis due to amorphous silica. Eur Respir J 23:208–13
- McGavin CR, Sheers G. (1984). Diffuse pleural thickening in asbestos workers: disability and lung function abnormalities. Thorax 39:604–7
- Miles SE, Sandrini A, Johnson AR, Yates DH. (2008). Clinical consequences of asbestos-related diffuse pleural thickening: a review. J Occup Med Toxicol 3:20
- Miller A, Lilis R, Godbold J, et al. (1992). Relationship of pulmonary function to radiographic interstitial fibrosis in 2,611 long-term asbestos insulators. An assessment of the International Labour Office profusion score. Am Rev Respir Dis 145:263–70
- Miller A, Warshaw R, Nezamis J. (2013). Diffusing capacity and forced vital capacity in 5,003 asbestos-exposed workers: relationships to interstitial fibrosis (ILO profusion score) and pleural thickening. Am J Ind Med 56:1383–93
- National Research Council (NRC). (2014). Review of EPA's integrated risk information system (IRIS) process. Washington, DC: National Academies Press, 204 p. Available at http://www.nap.edu/catalog.php?record_id=18764 [last accessed 17 Sept 2014]
- National Toxicology Program (NTP). (2013). Draft OHAT approach for systematic review and evidence integration for literature-based health assessments – February 2013. Office of Health Assessment and Translation (OHAT), February 26, 15 p
- Neri S, Boraschi P, Antonelli A, et al. (1996). Pulmonary function, smoking habits, and high resolution computed tomography (HRCT) early abnormalities of lung and pleural fibrosis in shipyard workers exposed to asbestos. Am J Ind Med 30:588–95
- Neuberger M, Ambrosch P. (1985). Lungenfunktionsuntersuchungen in einer population mit endemischen pleuraplaques und umweltbedingter asbestexposition [Pulmonary function tests in a population with endemic pleural plaques and environmental asbestos exposure]. Wien Klin Wochenschr 97:289–93
- Ohlson CG, Rydman T, Sundell L, et al. (1984). Decreased lung function in long-term asbestos cement workers: a cross-sectional study. Am J Ind Med 5:359–66
- Ohlson CG, Bodin L, Rydman T, Hogstedt C. (1985). Ventilatory decrements in former asbestos cement workers: a four year follow up. Br J Ind Med 42:612–16
- Oldenburg M, Degens P, Baur X. (2001). Asbest-bedingte lungenfunktionseinschrankungen mit und ohne pleuraplaques [Asbestos-induced lung function restrictions with and without pleural plaques]. Atemwegs Lungenkrankheiten 27:422–3
- Oliver LC, Eisen EA, Greene R, Sprince NL. (1988). Asbestos-related pleural plaques and lung function. Am J Ind Med 14:649–56
- Ostiguy G, Vaillancourt C, Begin R. (1995). Respiratory health of workers exposed to metal dusts and foundry fumes in a copper refinery. Occup Environ Med 52:204–10
- Paris C, Thierry S, Brochard P, et al. (2009). Pleural plaques and asbestosis: dose- and time-response relationships based on HRCT data. Eur Respir J 34:72–9
- Pellegrino R, Viegi G, Brusasco V, et al. (2005). Interpretative strategies for lung function tests. Eur Respir J 26:948–68
- Rosenstock L, Barnhart S, Heyer NJ, et al. (1988). The relation among pulmonary function, chest roentgenographic abnormalities, and smoking status in an asbestos-exposed cohort. Am Rev Respir Dis 138:272–7
- Rui F, De Zotti R, Negro C, Bovenzi M. (2004). A follow-up study of lung function among ex-asbestos workers with and without pleural plaques. Med Lav 95:171–9
- Salome CM, King GG, Berend N. (2010). Physiology of obesity and effects on lung function. J Appl Physiol 108:206–11
- Sandrini A, Johnson AR, Thomas PS, Yates DH. (2006). Fractional exhaled nitric oxide concentration is increased in asbestosis and pleural plaques. Respirology 11:325–9
- Schwartz DA, Fuortes LJ, Galvin JR, et al. (1990a). Asbestos-induced pleural fibrosis and impaired lung function. Am Rev Respir Dis 141:321–6
- Schwartz DA, Galvin JR, Dayton CS, et al. (1990b). Determinants of restrictive lung function in asbestos-induced pleural fibrosis. J Appl Physiol 68:1932–7
- Schwartz DA, Galvin JR, Yagla SJ, et al. (1993). Restrictive lung function and asbestos-induced pleural fibrosis. A quantitative approach. J Clin Invest 91:2685–92
- Schwartz DA, Davis CS, Merchant JA, et al. (1994). Longitudinal changes in lung function among asbestos-exposed workers. Am J Respir Crit Care Med 150:1243–9
- Sette A, Neder JA, Nery LE, et al. (2004). Thin-section CT abnormalities and pulmonary gas exchange impairment in workers exposed to asbestos. Radiology 232:66–74
- Shea BJ, Grimshaw JM, Wells GA, et al. (2007). Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 7:10
- Sider L, Holland EA, Davis TM, Cugell DW. (1987). Changes on radiographs of wives of workers exposed to asbestos. Radiation 164:723–6
- Singh B, Eastwood PR, Finucane KE, et al. (1999). Effect of asbestos-related pleural fibrosis on excursion of the lower chest wall and diaphragm. Am J Respir Crit. Care Med 160:1507–15
- Soulat JM, Lauque D, Esquirol Y, et al. (1999). High-resolution computed tomography abnormalities in ex-insulators annually exposed to asbestos dust. Am J Ind Med 36:593–601
- Spyratos D, Chloros D, Haidich B, et al. (2012). Chest imaging and lung function impairment after long-term occupational exposure to low concentrations of chrysotile. Arch Environ Occup Health 67:84–90
- Stanojevic S, Wade A, Stocks J. (2010). Reference values for lung function: past, present and future. Eur Respir J 36:12–19
- Staples CA, Gamsu G, Ray CS, Webb WR. (1989). High resolution computed tomography and lung function in asbestos-exposed workers with normal chest radiographs. Am Rev Respir Dis 139:1502–8
- US EPA. (2011). Toxicological review of Libby amphibole asbestos in support of summary information on the integrated risk information system (IRIS) (External Review Draft). EPA/635/R-11/002A, August, 467 p
- US EPA. (2014). Draft development materials for the integrated risk information system (IRIS) toxicological review of inorganic arsenic [CASRN 7440-38-2]. National Center for Environmental Assessment (NCEA), EPA/630/R-14/101, April, 731 p
- Valkila EH, Nieminen MM, Moilanen AK, et al. (1995). Asbestos-induced visceral pleural fibrosis reduces pulmonary compliance. Am J Ind Med 28:363–72
- Van Cleemput J, De Raeve H, Verschakelen JA, et al. (2001). Surface of localized pleural plaques quantitated by computed tomography scanning: no relation with cumulative asbestos exposure and no effect on lung function. Am J Respir Crit Care Med 163:705–10
- Weill D. (2008). Diagnostic features and clinical evaluation of the asbestos-associated diseases. In: Craighead JE, Gibbs AR, eds. Asbestos and its diseases. Oxford, UK: Oxford University Press, 253–68
- Weill D, Dhillon G, Freyder L, et al. (2011). Lung function, radiological changes and exposure: analysis of ATSDR data from Libby, MT, USA. Eur Respir J 38:376–83
- Zavalic M, Bogadi-Sare A. (1993). Lung functions and chest radiographs in shipyard workers exposed to asbestos. Arh Hig Rada Toksikol 44:1–8