Abstract
This paper summarizes a range of experimental data central for developing a science-based approach for hazard identification of monomeric and polymeric aliphatic 1,6-hexamethylene diisocyanate (HDI). The dose–response curve of HDI-induced pulmonary responses in naïve or dermally sensitized rats after one or several inhalation priming exposures was examined in the Brown Norway (BN) rat asthma model. Emphasis was directed to demonstrate the need and the difficulty in selecting an appropriate pulmonary dose when much of the inhaled chemically reactive vapor may concentration dependently be retained in the upper airways of obligate nose-breathing rats. The course taken acknowledges the experimental challenges in identifying an elicitation threshold for HDI-monomer near or above the saturated vapor concentration or in the presence of a HDI-polymer aerosol. The inhalation threshold dose on elicitation was determined based on a fixed concentration (C) × variable exposure duration (t) protocol for improving inhalation dosimetry of the lower airways. Neutrophilic granulocytes (PMN) in bronchoalveolar lavage (BAL) fluid in equally inhalation primed naïve and dermally sensitized rats were used to define the inhalation elicitation threshold C × t. Sensitized rats elaborated markedly increased PMN challenged sensitized rats relative to equally challenged naïve rats at 5625 mg HDI/m3 × min (75 mg/m3 for 75 min). PMN were essentially indistinguishable at 900 mg HDI/m3 × min. By applying adjustment factors accounting for both inter-species differences in inhalation dosimetry and intra-species susceptibility, the workplace human-equivalent threshold C × t was estimated to be in the range of the current ACGIH TLV® of HDI. Thus, this rat “asthma” model was suitable to demonstrate elicitation thresholds for HDI-vapor after one or several inhalation priming exposures and seems to be suitable to derive occupational exposure values (OELs) for diisocyanates in general.
Introduction
Monomeric HDI is a volatile aliphatic diisocyanate which is almost exclusively used in its homo-polymeric forms in the manufacture of glues, paints, and surface coatings. Hence, potential workplace exposure is predominated to polyisocyanate aerosol. In the past, these products contained a small amount of residual HDI monomer, usually in the range of 0.3%, with a tendency to increase with extended storage (Myer et al., Citation1993; Pauluhn, Citation1994; Pauluhn et al., Citation2002). Along with technical progress, this residual content was further reduced. There are numerous occupational studies involving spray painters that analyze and assess the role of the monomer (vapor), the polyisocyanate (aerosol), and the vapor partitioned to the aerosol for acquiring contact dermatitis and/or asthma following occupational contact with HDI polyisocyanates (Aalto-Korte et al., Citation2011; Alexandersson et al., Citation1987; Baur, Citation2007; Baur et al., Citation1994; Bello et al., Citation2007; Cassidy et al., Citation2010; Cockcroft & Mink, Citation1979; Janko et al., Citation1992; Liu et al., 2000; Pisaniello & Muriale, Citation1989; Pronk et al., Citation2006a,Citationb, Citation2007; Redlich, Citation2010; Redlich et al., Citation2002; Thomasen et al., Citation2011; Tornling et al., Citation1990; Vandenplas, Citation2011; Vandenplas et al., 1993a–c). Despite the vast amount of studies on HDI-based polyisocyanates, the association between asthma-related health effects to the role of the monomer, polyisocyanate, and the most critical exposure intensity/route remains unclear (Bello et al., Citation2004; Maître et al., Citation1996; Myer et al., Citation1993; Pauluhn & Mohr, Citation2005; Rando & Poovey, Citation1999; Pronk et al., Citation2007). In experimental laboratory models, aerosols are targeted to be highly respirable. Typically, any larger aerosol fraction is eliminated from a polydisperse aerosol prior to entering inhalation exposure systems. In minimizing the large-size fraction of aerosol, an unaccounted enrichment of volatile HDI in exposure atmospheres is a common accompaniment in such studies. The presence of HDI-vapor or partitioning to the liquid aerosol phase has major impact on the site of HDI-retention and respiratory tract dosimetry. Hence, the local dosimetry of inhaled HDI in two differing physical forms may potentially render the unequivocal distinction of responses attributable to irritant and allergic airway inflammation dosimetrically difficult.
Due to the increasing health concerns associated with occupational asthma and the impending directives on the regulation of respiratory sensitizers and allergens, an experimental approach is urgently needed to identify these compounds not only as asthmagen but also to derive safe workplace limits. The challenges that chemical respiratory allergy pose for toxicologists are substantial. No validated methods are available yet for hazard identification and characterization, and this is due in large part to the fact that there remains considerable uncertainty and debate about the mechanisms through which sensitization and hypersensitivity of the respiratory tract is acquired and aggravated (Boverhof et al., Citation2008; Kimber & Dearman, Citation2005; Kimber et al., Citation2014; Pauluhn & Mohr, Citation2005). Despite this uncertainty, there is a need to establish models and dose descriptors that integrate the thresholds of respiratory tract irritation and elicitation response. Any holistic threshold of sensitization appears to be more complex to define due to exposure route-specific differences in immune response. As the elicitation-related dose descriptor seems to be less dependent on uncharacterized sensitization encounters, preference is given to this endpoint for deriving an Occupational Exposure Level (OEL) or DNEL (for definitions see ECHA, Citation2012).
The protocols applied to date for the hazard identification of respiratory sensitizers employ modeling systems that evaluate preferentially the acute etiopathology rather than the chronic allergic airway inflammation typical of asthma. Chronic allergic inflammation of the airways is a common accompaniment and may underlie airway hyperresponsiveness observed following non-specific or specific inhalation elicitation challenges (Pauluhn & Mohr, Citation2005). Especially in surrogate animal models of asthma, pathophysiological reactions typifying asthma focus on increased inflammatory cells (neutrophils) in bronchoalveolar lavage fluid (BAL). This endpoint was shown to interrelate with asthma-like pathophysiological responses delayed in onset in both humans and rats (Lemière et al., Citation2002; Pauluhn, Citation2008; Vandenplas et al., Citation1993a–c). The “gold standard” for any objective assessment of occupational asthma in an individual worker is the specific inhalation elicitation bronchoprovocation test (Malo et al., Citation1991; Raulf-Heimsoth et al., Citation2013). Attempts were made to adopt this principle in the bioassay devised.
Surprisingly, the bioassays that were used most frequently have not appropriately taken into account that allergic asthma is a chronic disease with acute manifestations. This etiopathology was modeled in the Brown Norway (BN) rat using cutaneous sensitization followed by recurrent inhalation priming to induce and amplify the allergic characteristics of airway inflammation. Emphasis was directed on the analysis of the concentration × time (C × t)–response relationship on elicitation-based endpoints by employing a highly rationalized dose–escalation-like protocol which was structured somewhat similar to clinical bronchoprovocation tests (Raulf-Heimsoth et al., Citation2013). This concept assumes that sensitization via the skin can serve as a predisposing factor for subsequent inhalation encounters. Published evidence demonstrates that systemic sensitization can more readily be achieved through skin exposure than via the respiratory tract because the former is recognized to be inherently predestined for immunological response (Pauluhn, Citation2005, Citation2014; Pauluhn & Poole, Citation2011; Pauluhn et al., Citation2005).
The aim of this study was to extend published evidence from past respiratory sensitization inhalation studies with the HDI-polyisocyanate on guinea pigs (Pauluhn et al., Citation2002) and to better understand the interrelationship between pulmonary irritation and sensitization/allergy using a more chronic respiratory sensitization/priming BN rat protocol. This animal model was shown to be suitable to demonstrate elicitation thresholds for aromatic diisocyanates (MDI-aerosol, TDI-vapor; Pauluhn, Citation2005, Citation2014; Pauluhn & Poole, Citation2011; Pauluhn et al., Citation2005). This study complements previously published evidence using HDI-vapor, an aliphatic, highly reactive diisocyanate preferentially captured in the more proximal airways of obligate nasal breathing rodents. Alike previous studies, also this study utilized a heuristic approach for quantifying the elicitation-based Point of Departure (POD) on pre-disposed, “asthmatic” rats, rather than paying any particular attention to the involved mechanisms.
Methods
Test material and chemicals
1,6-Hexamethylene diisocyanate (HDI, CAS-no.: 822-06-0, trade name DESMODUR® H) used in this study was from Bayer MaterialScience AG, Leverkusen, Germany). The purity was 99.9% with an analytically confirmed content of free isocyanate (NCO) of 50%. During handling and storage, the headspace of HDI containers was purged with dry nitrogen to remove air and humidity to prevent its decomposition. All calibrations used a mass-based metric (mg/m3). Volumetric concentrations utilized the following conversion: 1 mg/m3 ∼0.14 ppmV (parts per million volume). In pre-studies, the polyisocyanate DESMODUR® N 3300, which is a HDI-homopolymer of the isocyanurate type (0.08% HDI-monomer), was used.
Animals, diet, and housing conditions
Male Brown Norway (BN) rats of the strain BN/Crl BR were from Charles River, Sulzfeld, Germany. Wistar rats, strain Hsd Cpb:WU (SPF), were used in the ancillary pre-studies for sensory irritation measurements (Breeder Harlan-Nederland, AD Horst, The Netherlands). Animals were maintained in polycarbonate cages with one rat per cage, which contained bedding material (low-dust wood shavings), and were provided with a standard fixed-formula diet (ssniff® R/M-H pellets maintenance diet for rats and mice; ssniff Spezialdiäten GmbH, http://www.ssniff.de) and municipality tap water in drinking bottles. Both feed and water were given ad libitum except during inhalation exposures. At the commencement of study, BN-rats were approximately 3 months old with an average body weight of 240 g. Animals were quarantined for at least 5 d prior to being placed on study. The health status of each animal was repeatedly assessed during this period (and thereafter) without evidence of any signs of infection or health impairment. Rats were subjected to specific screens for pathogens by the breeder who was bound by contract to follow the animal hygiene guidelines detailed by FELASA (Mähler et al., Citation2014). Each batch of rats was certified according to the information made openly available by the breeder (http://www.criver.com/products-services/basic-research/health-reports/europe-asia/germany-by-strain). Animal rooms were maintained at approximately 22 °C with a relative humidity at 40–60% and a 12-h light cycle beginning at 0:600 h.
The principal study described in this paper was in methodological accordance with contemporary, internationally harmonized testing standards/guidelines (OECD, Citation2009) and Good Laboratory Practice standards (OECD, Citation1998). The experiments were performed in an animal care-approved laboratory in accordance with the German Animal Welfare Act and European Council Directive 2010/63/EU as of 22 September 2010.
Pre-studies for selecting C × t regimens of inhalation challenge exposures
Several ancillary pre-studies in naïve Wistar rats preceded this sensitization study with the objective to better understand, characterize, and quantify the etiopathologies attributable to reflexively induced upper airway sensory irritation by HDI-vapor and HDI-polyisocyanate aerosol-induced alveolar irritation. The mixture of both was also examined to evaluate whether HDI vapor (preferentially retained in the upper airways) is shuttled into lower airways by the liquid polyisocyanate aerosol. Previous inhalation studies with the HDI-polyisocyanate aerosol in rats demonstrated that the acute irritant-alveolitis-based (probed by bronchoalveolar lavage) and the histology-based no-observed-adverse effect level (NOAELs) after subchronic 90-d inhalation exposure were similar (Pauluhn, Citation2002; Pauluhn & Mohr, Citation2001). In order to enhance the penetration of HDI-vapor into the lower airways, the inhalation priming protocol utilized aerosolized HDI at concentrations in the range and above vapor saturation and/or mixtures of HDI-monomer and HDI-polyisocyanate aerosol. The pre-tests executed for this study were designed to assess both the upper airway sensory irritation potency of HDI-monomer as a vapor or a mixture of the vapor and aerosol in the absence and presence of a HDI-based polyisocyanate-aerosol. Wistar rats were used in previous studies with diisocyanates that focused on respiratory tract irritation (Pauluhn, Citation2002; Pauluhn & Mohr, Citation2001). With regard to irritation-related changes in pulmonary function, appreciable differences between Wistar and BN rats were not identified (Pauluhn, Citation2004).
Due to the high retention (scrubbing) of HDI-vapor within the upper airways of rats, the concentration of HDI-monomer used for inhalation priming and challenge exposures has to be selected judiciously otherwise insufficient amounts of HDI may gain access to the anatomical target of diisocyanate asthma. Concentrations of HDI-monomer bracketed a range from HDI-vapor (4–27 mg/m3; exposure duration 0.5 h) to HDI-vapor/aerosol (112–190 mg/m3). While upper respiratory tract sensory irritation was quantified by lung function measurements, lower respiratory tract irritation was probed by bronchoalveolar lavage 1-h, 1 and 3 d post-exposure (exposure duration 0.5 h to either 112 or 190 mg HDI-vapor-aerosol/m3).
The shuttle-effect into the lower respiratory tract of HDI-monomer partitioned to polyisocyanate aerosol was examined as a vapor–aerosol mixture at 100 and 200 mg/m3 polyisocyanate–aerosol in the presence/absence of 8 mg/m3 HDI-vapor (exposure duration 0.5 h). These data demonstrated that minute amounts of HDI-vapor partitioned to the aerosol increased the alveolar irritation potency of the aerosol. However, this alternative approach was abandoned due to contemporaneous depression in ventilation and associated difficulties to interrelate “inhaled dose” and “effect”. Consequently, under such testing conditions, acute pulmonary irritation could not be differentiated from allergic outcomes.
Experimental protocol and justification of provocation dose
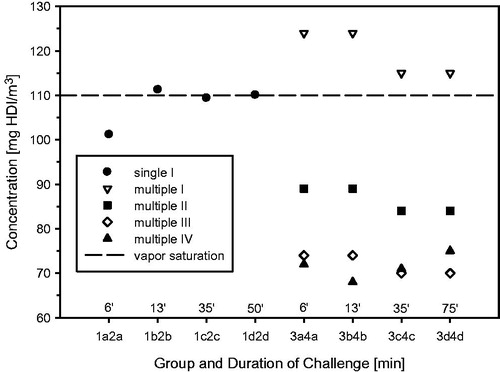
The protocols applied focused on the analysis of the concentration × time (C × t)–response relationship on elicitation-based endpoints by employing a highly rationalized dose-escalation-like protocol. For the HDI-vapor, a Cconst × tvar regimen was selected with a “C” high enough to overcome the scrubbing capacity of the nasal passages of the obligate nasal breathing rats (Schroeter et al., Citation2013; Shiotsuka et al., Citation2006, Citation2010). Ancillary pre-studies served the purpose of identifying that “C” of HDI-vapor gains access to the lower airways at stable breathing conditions. Throughout all priming inhalation exposures, the exposure duration was 30 min whereas for the terminal dose-escalation challenge ramped exposure durations from tvar = 6 to 75 min were used.
The principle sequence of steps used in this study is conceptualized in . Step I was structured based on the ancillary pre-test studies bearing in mind two major objectives: (i) maintenance of dosimetric stability (attainment of maximum depression in ventilation) and (ii) sufficient dosing intensity to the lower airways in the absence of marked alveolitis. The concentrations used for the ramped challenge-exposures I– IV are shown in . Protocol steps I and II were designed to investigate the impact of recurrent inhalation priming exposures at minimally alveolar irritant conditions. The terminal escalation challenge in the absence (step I) and presence (step II) of prior lung-priming inhalation exposures served the purpose to identify induction of tolerance and/or any aggravating responses at high exposure intensities. The lower elicitation dose was targeted to be the elicitation threshold (no-observed-adverse-effect level, NOAEL). The duration of challenge followed the rationale of procedures commonly applied in human-specific bronchoprovocation tests to reveal diisocyanate-induced occupational asthma (Raulf-Heimsoth et al., Citation2013).
Figure 1. Protocols used to test for respiratory sensitization/allergy in a topical-induction and repeated inhalation priming/elicitation Brown Norway rat model. Two weeks after the last sensitization encounter, the rats were subjected to a dose–escalation type (Cconst × tvar) of bronchoprovocation challenge at 110 mg HDI/m3 for either 6, 13, 35, or 50 min duration (step I). At step II, similar sensitized rats were subjected to three successive inhalation priming/challenge exposures at about 120–87–72 mg/m3 × 30 min () followed by bronchoprovocation challenge at about 72 mg/m3 for either 6, 13, 35, or 75 min duration. The time periods between priming inhalation challenges was selected that irritant inflammation regresses from one interval to the next whereas the allergic-type inflammation progresses. The asthma phenotype was probed by lung function measurements overnight post-challenge and PMN in bronchoalveolar lavage fluid (BAL) 1 d after the respective escalation inhalation challenge. Nitric oxide in exhaled air was analyzed before and after lung function measurements.
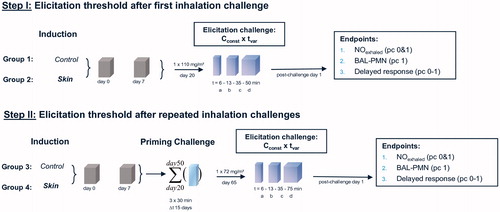
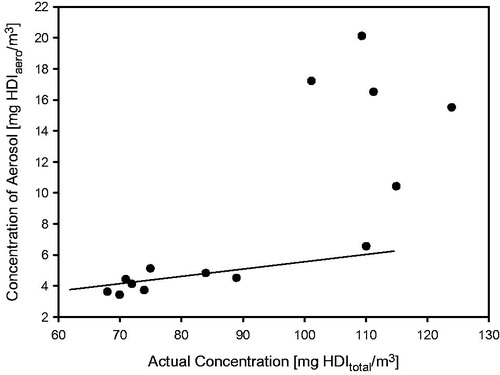
Figure 2. Analytically determined concentrations of HDI in naïve control (groups 1 and 3) and sensitized rats (groups 2 and 4) at the durations of bronchoprovocation of “a–d” ().
When constructing the BN-rat challenge protocol (step II), 75 mg HDI/m3 × 30 min translates to 2250 (mg/m3 × min) × 1/100 (default rat to human conversion) = 23 (mg/m3 × min) human-equivalent dose. The workday and ppmV-adjusted equivalent were estimated to be 23 (mg/m3 × min) × 1/7 (conversion from mg to ppmV) × 1/480 min−1 = 0.007 ppmV. The derivation of the rat to human conversion factor assumed that bronchial airways of oronasally breathing humans are subjected to a 3-times higher dose as compared with nasally breathing rats (Schroeter et al., Citation2013). Likewise, due to the reflexively-induced depression of ventilation occurring in rats, the inhaled dose of rats exposed to HDI-vapor at concentrations penetrating the lower respiratory tract is likely to be about one-third of normally breathing rats. These two factors make rats dosimetrically less sensitive than humans. By using an additional intra-species susceptibility factor of 10 as default, at the outset of study the “healthy” and not “predisposed” rat to human adjustment factor was estimated to be 3 × 3 × 10 = 100. Consequently, the lung-priming dose of 0.007 ppmV used in this study was assumed to be within the upper range of the adjusted human-equivalent challenge–dose response curve. Of note is that this animal model uses “predisposed” asthmatic rats which may justify a lower than 10-fold intra-species susceptibility factor. This aspect will be addressed in detail in the discussion.
Experimental procedures
Sensitization and challenge
BN-rats were exposed in directed-flow nose-only restraining tubes (for details, see Pauluhn & Thiel, Citation2007). Animals were sensitized topically (contralateral flanks) on days 0 and 7 to HDI dissolved in the vehicle acetone:olive oil (4:1 mixture, 100 μl vehicle/administration; 2% w/v). This induction regimen was similar to that used for TDI (Pauluhn, Citation2014). Based on the potency comparison of HDI and TDI in mice after topical administration, HDI was found to be markedly more potent than TDI (Thorne et al., Citation1987). Animals were exposed “open epicutaneously” for 24 h. Naive control and sensitized rats were simultaneously primed/challenged by inhalation (for details, see and ). Each sub-group per stepped challenge consisted of eight male rats. When executing step II (), the first inhalation priming encounter was to the vapor saturation concentration of HDI (approximately 120 mg/m3, see and ). At this concentration, a precipitous increase of pulmonary irritation occurred. Accordingly, inhalation exposures were continued at lower concentrations in the range of 72–87 mg/m3. Ramped inhalation challenges, including terminal examinations, were executed on target days 20 (step I) and 65 (step II). In the step II study, animals were re-challenged on days 20, 35, and 50 prior to the dose–response analysis on day 65 (grace period ± 2 d). The time spacing between each priming exposure was long enough to minimize acute irritation-related carry-over effects. Nitric oxide in exhaled air (eNO) was determined shortly after each terminal provocation challenge and 20 h later. This endpoint provided supportive evidence in the BN-rat TDI-study (Pauluhn, Citation2014) and was judged to indicate increased airway inflammation in atopic, non-smokers exposed to higher levels of isocyanates (Jonaid et al., Citation2014). eNO has also been shown to be a sensitive, non-invasive biomarker of airway inflammation of rats (Liu et al., Citation2013) and is generally considered as a diagnostic biomarker in occupational asthma (Ewald-Kleimeier et al., Citation2013).
Generation and characterization of exposure atmospheres
HDI-vapor concentrations were generated using the method described for TDI-vapor (Pauluhn, Citation2014). HDI-vapor/aerosol mixtures utilized an atomizing/baffle (pre-separator) system (Casella, Buffalo, NY) (water-jacketed spray nozzle Series 970/form-S 3; modified Schlick, http://www.duesen-schlick.de/index.php?cat=66&cl2=66&prod_cat=1&prod=1; conditioned compressed air (600 kPa) at 15 L/min (ambient pressure). HDI was metered into the nozzle system using a digitally controlled pump (Harvard PHD 2000 infusion pump, Beckman Coulter Incorporated, Brea, CA). The water-jacketed nozzle was maintained at 10 °C using a thermostat (JULABO UC, Julabo, Seelbach, Germany) to prevent evaporation within the nozzle and pulsed aerosolization. Based on the applied generation conditions, a major fraction of the aerosol was shown to spontaneously evaporate below the vapor saturation concentration (). Atmospheres of the polyisocyanate (isocyanurate) aerosol for inhalation exposures were generated under similar conditions as described above for the HDI-aerosol. In order to decrease the viscosity of the polyisocyanate, the nozzle was maintained at 40 °C. This aerosol was then allowed to re-equilibrate with the separately generated HDI-vapor atmosphere in a tube-like mixing chamber (length: 110 cm, inner diameters outer/inner tubes: 9.7/6.8 cm, inner tube distance to bottom: 5 cm). This arrangement allowed a controlled re-equilibration of the liquid aerosol with vapor of HDI-monomer over a total travel distance approximately 210 cm. The time available for re-equilibration was in total ≈0.5 min.
Polyisocyanate–aerosol concentrations were determined by gravimetric analysis (filter: Glass–Fibre–Filter, Sartorius, Göttingen, Germany). HDI-vapor/aerosol in exposure atmospheres were determined by adsorption collection tubes filled with glass powder (coated with N-4-nitrobenzyl-N-n-propylamine) and elution with acidified acetonitrile. The resultant urea derivative was quantified by high-performance liquid chromatography (HPLC; with DAD-Detection wave length: 276 nm). Standard solutions of the urea derivative of HDI were used for calibration. The recovery of HDI–vapor was 95% (a coefficient of variation was 1.2–6.7). The polyisocyanate–aerosol particle-size distribution was analyzed using a Berner-type, low-pressure critical orifice cascade impactor (Hauke, Gmunden, Austria). HDI-aerosol was quantified by the TSI-Laser Velocimetty (TSI-APS 3321, including diluter TSI Model 3302; TSI Inc., St. Paul, MN; Remiarz et al., Citation1983). While the analytical procedure used integrates both phases of HDI, the necessary dilution required for the analysis of HDI-aerosol is biased to underestimate the concentration of HDI-aerosol. The mass median aerodynamic diameter (MMAD) and the geometric standard deviation (GSD) were calculated as described previously (Pauluhn & Thiel, Citation2007).
General observations
Body weights of animals were recorded once per week. The appearance and behavior of all animals were examined systematically in an ordinal (categorical) manner.
Pulmonary function tests and nitric oxide in exhaled air
These methods were described in detail in context with previous studies. In brief, delayed-onset respiratory response were recorded for approximately 20 h in barometric whole-body plethysmographs on four naïve and four sensitized but equally challenged rats from pre-selected subgroups at all priming challenges time points and at the terminal escalation challenge in all groups. Data collection commenced shortly after challenge by placing the animals into the pre-calibrated barometric whole-body plethysmographs (for further details see Pauluhn, Citation2008, Citation2014; Pauluhn & Poole, Citation2011). Nitric oxide (NO) in exhaled breath (eNO) was analyzed real-time using a chemi-luminescence analyzer (Sievers 280B NOA; Sievers Instrument, Inc., Denver, CO) (for details, see Liu et al., Citation2013).
Bronchoalveolar Lavage
One day after the escalation challenge all rats were examined by BAL. After exsanguination and prior to BAL fluid collection the weights of lungs and lung-associated lymph nodes (LALN) were determined. The applied methods and endpoints were similar to those used in previous studies (Pauluhn, Citation2008, Citation2014; Pauluhn & Poole, Citation2011).
Data analysis
Body weights, organ weights, and BAL data were analyzed using a one-way analysis of variance (ANOVA) followed by a Tukey–Kramer post hoc test. The time-related changes in respiratory minute volume were analyzed using the following sigmoidal non-linear regression function: y = y0 + a/(1+exp(−(x−x0)/b) where y is the respiratory minute volume relative to the pre-exposure period (=100%) and x is the time point of data collection during the 15-min pre-exposure period followed by the 30-min HDI exposure period. Non-linear regression parameters were estimated using SigmaPlot 12.5 software (Systat Software Inc., Point Richmond, CA). For all tests, the criterion for statistical significance was set at p < 0.05.
Results
Ancillary pre-studies and justification of protocol design
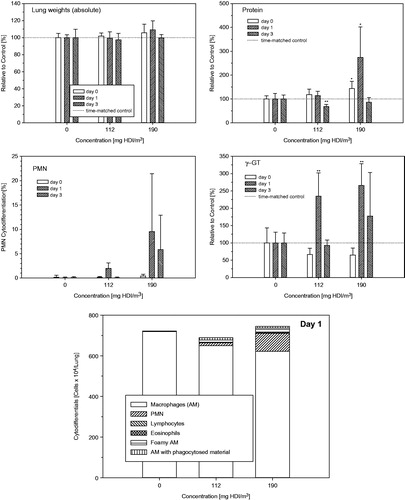
Naïve Wistar rats that were nose-only exposed to HDI vapor/aerosol for 30 min displayed an instant concentration-dependent decrease of the respiratory minute volume as shown in . A maximum depression in ventilation occurred at exposure concentrations exceeding 70 mg/m3. Decreased tidal volumes were observed at exposures to the vapor phase with reversal towards increased tidal volumes at concentrations () high enough to stabilize the aerosol phase (). This is taken as evidence that the HDI-aerosol gained access to the lower airways when present in concentrations in the range or above the vapor saturation concentration. This interpretation was empirically verified in Wistar rats exposed at 112 and 190 mg HDI-vapor/aerosol/m3 × 30 min (). Transient, minimally increased extravasated protein and PMN were observed at 112 mg/m3 on postexposure day 1 with reversibility on postexposure day 3. Definite pulmonary irritation was evidenced at 190 mg/m3; however, without gaining statistically increased lung weights or increased cell counts (). Increased γ-glutamyl transpeptidase (γ-GT) is taken as evidence of lower airway exposure and injury (Pauluhn, Citation2000).
Figure 4. Ancillary pre-study showing the time and concentration dependence of respiratory changes in naïve Wistar rats (5–6 animals/concentration) that were simultaneously exposed for 15-min to air, 30-min to either HDI-vapor (up to 27 mg/m3) or aerosol (at and above 109 mg/m3) followed by a 30-min recovery period. Changes in tidal volumes, minute volumes (MV), and the concentration dependence of ventilation (MV) are given in the top, middle, and lower panels, respectively. Measurements were made in head-out volume–displacement plethysmographs attached to a directed-flow nose-only inhalation chamber (Pauluhn & Thiel, Citation2007). The solid lines were derived using a sigmoid model fitted to measurements from the pre-exposure and exposure periods to estimate the degree of respiratory depression. All data were normalized to the pre-exposure period (=100%). The concentration dependence of the maximum stable depression of the respiratory minute volume is depicted in the lower panel. Depressed tidal volumes increased at concentrations with stable aerosol ().
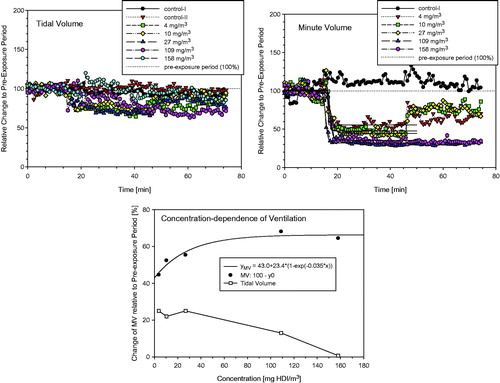
Figure 5. Concentration- and time-related changes of endpoints in bronchoalveolar lavage fluid (BAL) indicative of acute alveolar irritation 1-h, 1 and 3 d postexposure of Wistar rats exposed to HDI-vapor/aerosol at 112 and 190 mg HDI/m3 for 30 min. Absolute numbers of cytodifferentiated cells from BAL were from postexposure day 1. Analyses from day 0 and 3 revealed less pronounced changes. Data were presented as means ± SD (six rats/group). Asterisks denote significant difference to the equally exposed air time-matched control group (*p < 0.05, **p < 0.01).
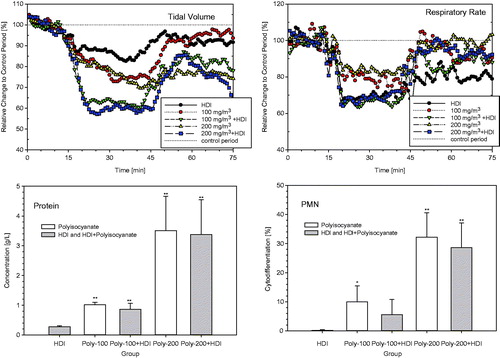
Additional groups of rats were exposed for 30-min to the mixture of HDI-vapor (8 mg/m3) and polyisocyanate-aerosol (100 and 200 mg/m3, MMAD/GSD 1.8 µm/1.7) with actually determined total mass concentrations of the polyisocyanate in the range of 100–108 and 191–222 mg/m3, respectively. Lung function measurements provided evidence of marked differences in aerosol-induced changes in respiratory patterns in the presence of HDI-vapor, especially for tidal volumes (, upper panel). Bronchoalveolar lavage (1 d postexposure) did not reveal appreciable differences (, lower panel) of the neat aerosol or that containing HDI which suggest that, in principle, HDI-vapor can be shuttled into the lower airways by the polyisocyanate aerosol; however, due to the HDI-driven changes in ventilation “inhaled dose”, adjustments become difficult. Based on the findings from these ancillary pre-studies, the conclusion was drawn that the objective of study can only be achieved when using challenge concentration of at least 70–80 mg HDI/m3 (a maximum depression in HDI vapor-induced ventilation). A precipitous increase in alveolar irritation occurred in the range of 120 mg HDI/m3 (), that is, a concentration above the vapor saturation of HDI-monomer favoring aerosol formation.
Figure 6. Inhalation exposure of Brown Norway rats to 100 or 200 mg/m3 polyisocyanate aerosol for 30-min with and without supplementation of 8 mg HDI/m3. Respiratory function measurements were made concomitant with exposure (15-min exposure to air for the collection of base-line data, 30 min exposure to the diisocyanate followed by air exposure for 30 min). Rats were subjected to bronchoalveolar lavage fluid (BAL) on postexposure day 1. Data were presented as means ± SD (six rats/group). Asterisks denote significant difference to the equally exposed air time-matched control group (*p < 0.05, **p < 0.01).
Bronchoprovocation escalation challenge
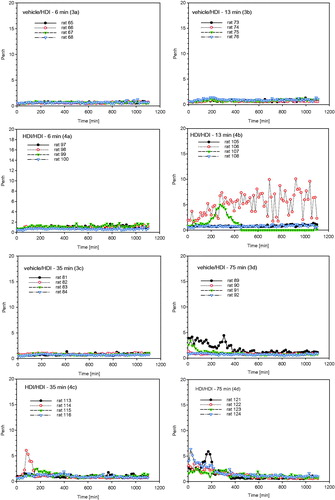
Groups of rats dermally induced with 2%-HDI were subjected to a terminal dose–escalation challenge of about 70–120 mg HDI/m3 without (, step I) and with lung-priming challenges (days 20, 35, and 50) (, step II) as conceptualized in and . At the first escalation challenge using a concentration of 110 mg/m3 for 6, 13, 35, and 50 min, irritation-related changes in respiration occurred at challenge durations of 35 and 50 min in both the control and sensitized groups over a time-period of about 10 h (). The escalation challenge executed after three prior priming inhalation encounters at a concentration at 75 mg/m3 for 6, 13, 35, and 75 min showed inconclusive responses at a challenge duration of 35 min () with minimal irritation-related changes in breathing patterns at 75 min for about 6 h post-challenge (). Collectively, lung function measurements did not reveal typical delayed-onset responses. Irritant inhalation doses produced changes in respiration in both naïve and sensitized rats () at concentrations close to the vapor saturation concentration of HDI. One rat of group 4b (step II, 13-min challenge duration) succumbed 7 h post-challenge with unclear etiopathology. Gross necropsy showed a red-discolorated liver and ascites without any macroscopic changes of the respiratory tract. These findings appear to be supportive of an incidental causality rather than any allergic etiopathology.
Figure 7. Measurement of Penh (enhanced pause) in whole body barometric plethysmographs after escalation challenge I – step I (see ) in naïve but challenged (vehicle/HDI) and skin-sensitized and challenged (HDI/HDI) Brown Norway rats. Data represent records from individual rats (four rats/subgroup) after challenge over a duration of approximately 20 h (Penh-AUC20 h). Note: Due to the limited number of plethysmographs available, only four out of eight rats/subgroup were examined to allow the simultaneous measurement of four naïve and four sensitized rats.
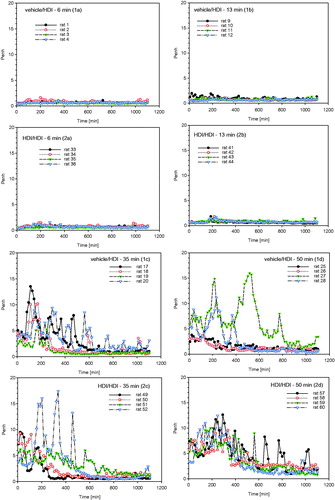
Figure 8. Measurement of Penh (enhanced pause) in whole body barometric plethysmographs after escalation challenge IV – step II (see ) in naïve but challenged (vehicle/HDI) and skin-sensitized and challenged (HDI/HDI) Brown Norway rats. Data represent records from individual rats (four rats/subgroup, for more details, see legend of ) after challenge over a duration of approximately 20 h (Penh-AUC20 h).
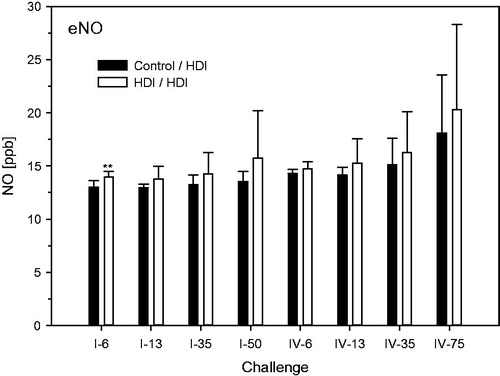
Protein in BAL was elevated at the highest escalation challenge dose in the absence of consistent differences between naïve and sensitized rats (). BAL–PMN were dose dependently increased especially when using the repeated inhalation priming protocol ( and ). Total cell counts were decreased at challenge-concentrations close to the vapor saturation of HDI (step I, 50- and 75-min; ). Eosinophilic granulocytes were observed both in naïve and in sensitized rats especially when using challenge protocol I (). With the exception of a small trend at higher challenge doses, nitric oxide in exhaled breath did not reveal significant differences between sensitized and non-sensitized rats (). Significantly increased lung or LALN weights were not observed in any group (data not shown). These findings support that PMN in BAL appears to be the endpoint of choice for dose–response analyses.
Figure 9. Protein and PMN in bronchoalveolar lavage at escalation challenges I (step I) and IV (step II) (for details, see ). The inhalation Cconst used at steps I and II were 110 and 70 mg/m3, respectively, at somewhat similar tvar, as shown in . The higher responsiveness observed at step I is attributed to the higher Cconst close to the vapor saturation concentration of HDI. Data were presented as means ± SD (eight animals/subgroup). Significant differences to the similarly challenged naïve control groups did not occur (p > 0.05).
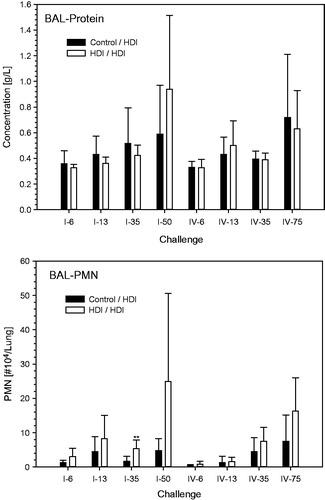
Figure 10. Absolute cytodifferentiation of cells in bronchoalveolar lavage at escalation challenges I (step I) and IV (step II) (for details, see ).
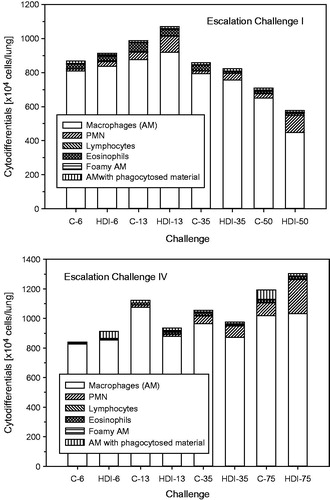
Figure 11. Nitric oxide (NO) in exhaled breath at escalation challenges I (step I) and IV (step II) (for details, see ). Data were presented as means ± SD (eight animals/subgroup). Asterisks denote a difference of p < 0.01.
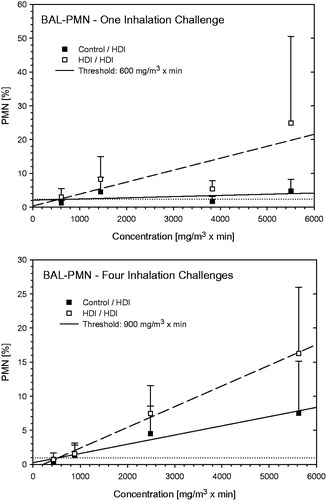
Rats from the naive control not receiving repeated priming inhalation exposures did not elaborate any escalation dose-dependent increase in BAL-PMN whereas those repeatedly primed showed increased susceptibility (). Despite this circumstance, sensitized rats without (step I)/with (step II) inhalation priming exposures elaborated comparable levels of BAL-PMN. Differences in PMN between equally challenged controls and sensitized groups were not observed at the 6- and 13-min challenges whereas distinct differences occurred at longer escalation challenge durations and three preceding priming-challenges. Based on the analyses given in , 900 mg HDI/m3 × min is considered to be the NOAEL for elicitation. Notably, sensitivity to inhalation challenge is generally assumed to increase with repeated exposure to the sensitizing agent. The POD of step I was slightly lower than that from step II. This apparent difference is conceived to be caused by the more irritant and variable challenge concentrations used at step I compared with step II. Accounting for these experimental factors, the outcome from either step is believed to be equivalent in this experimental model. The less irritant challenge dose used in step II is considered to deliver the most robust NOAEL.
Figure 12. Dependence of PMN in bronchoalveolar lavage on the escalation C × t-dose of HDI at escalation challenges I (step I) and IV (step II) (for details, see ). Data were presented as means ± SD (eight animals/subgroup). Equally challenged naïve and HDI-sensitized Brown Norway rats were considered indistinguishable at 600 and 900 mg HDI/m3 × min in rats without and with additional inhalation priming exposures.
Discussion
The dose–response relationship of HDI-induced pulmonary airway inflammation following topical sensitization and repeated inhalation priming/elicitation exposures was examined in the Brown Norway rat asthma model. Previous studies with MDI-aerosol and TDI-vapor demonstrated that increased BAL-PMNs were most expedient to characterize inhalation dose–response relationships upon elicitation (Pauluhn, Citation2005, Citation2008, Citation2014; Pauluhn & Poole, Citation2011; Pauluhn et al., Citation2005). Comprehensive published evidence supports the supposition that neutrophilic granulocytes (probed by BAL) play a key role in diisocyanate-induced asthma both in animal models and in humans (De Vooght et al., Citation2011, Citation2013; Jatakanon et al., Citation1999; Lemière et al., Citation2002; Vanoirbeek et al., Citation2007). The often-reported differences of neutrophilic versus eosinophilic airway inflammation seem to be attributable to methodological factors. These include, but are not restricted to, the method of analysis per se (e.g. histopathology, BAL, induced sputum), severity of response and associated kinetics of cell influx into the fluids lining the airways as well as the time elapsed between the elicitation exposure and the analysis (Pauluhn, Citation2008, Citation2014; Pauluhn & Poole, Citation2011; Raulf-Heimsoth et al., Citation2013).
As already demonstrated in previous studies (Pauluhn, Citation2008), delayed-onset physiological responses typifying the allergic asthma response coincided with increased BAL-PMN. A complex sequence of biological events is expected to take place at both the induction and inhalation priming/aggravation exposures resulting eventually in disease patterns resembling that of diisocyanate occupational asthma. In the BN-rat model, the induction of these patterns required exposure at the predilection site of asthma (bronchial airways) in intensities above the irritant threshold dose. A separation of endpoints differentiating unequivocally the “irritant” from the “allergic” airway inflammation is experimentally complex, if possible at all. In this context, it should be recalled that lung macrophages are a type of antigen presenting cells (APC), including PMN, that infiltrate inflamed tissues and release prodigious quantities of reactive oxygen/nitrogen species (ROS/RNS) and inflammatory cytokines/chemokines. These orchestrate not only the inflammatory response, but also contribute to antioxidant depletion with further enhancement of inflammation and glutathione (GSH) depletion. Evidence suggests that depletion of GSH from APCs in vivo results in lowered Th1 and higher Th2 activity. Thus, macrophages with mostly oxidized GSH are effectively type 2 and could polarize Th2. Thus, it seems immune activity can have Th1 or Th2 character depending on the redox-status of the cell and the diverse biological activity they develop in response to pro-inflammatory mediators they encounter in their immediate microenvironment (Kidd, Citation2003; Laskin et al., Citation2010). In this context, it is important to recall that many reactive low molecular weight chemicals may undergo direct electrophilic reactions with GSH (Pauluhn, Citation2011), and hence are biased to polarize Th2 by an entirely different mechanism. Once initiated, APC and Th2 cells may undergo a self-reinforcing “autocrine” loop with amplification. Hence, despite the fact that the terminal phenotypic appearance speaks for an immunological sequence of events, it becomes increasingly demanding and complex to link “respiratory sensitization” unequivocally to any specific immunological so-called “master or fingerprint cytokines” or non-immunological process. Therefore, the course taken in this analysis focused on “integrating” diagnostic principles used for clinical bronchoprovocation tests rather than elaborating on additional endpoints characterizing any of the putatively involved allergy-related Adverse Outcome Pathways (AOPs) (Kimber et al., Citation2014; Villeneuve et al., Citation2014) which was beyond the focus of study.
The physicochemical properties of HDI-vapor favor its retention in the upper airways (Schroeter et al., Citation2013; Shiotsuka et al., Citation2006, Citation2010). Several equally spaced priming/aggravation inhalation exposures to mildly alveolar irritant concentrations of HDI were used at exposure durations long enough to deliver a sufficiently high inhaled dose (C × t) to the distal airways of the lung. The intensity of the emerging allergic response is conceived to depend on the C × t-related degree of local irritation/inflammation embedded in a complex network of nociceptive neurogenic and signal-transduction factors that will further modulate and perpetuate the release of pro-inflammatory neuropeptides, cytokines, and chemokines (Pauluhn & Mohr, Citation2005). This animal model was suitable to induce at least some features typical of asthma as well as that an elicitation threshold dose on BAL-PMN exists. While the resultant bronchial hypersensitivity from inhaled isocyanates is dependent on numerous factors, the interrelationship of the inhaled ramped C × t-provocation escalation dose-induced increased BAL-PMN was shown to be a unifying experimental variable in diisocyanate-aerosol/-vapor exposed rats (Pauluhn, Citation2008, Citation2014; Pauluhn & Poole, Citation2011) and humans (Lemière et al., Citation2002; Vandenplas et al., Citation1993a–c).
Comparative repeated 1-week inhalation exposure studies on naïve Wistar rats (exposure 6 h/d on 5 consecutive days) with TDI- and HDI-vapor demonstrated that at similar concentrations HDI-related irritant injury to the respiratory tract occurs preferentially within the nasal passages whereas TDI definitely gained access to the airways of the lung. Repeated respiratory function measurements of the type shown in yielded lower PODs (based on the benchmark concentration on the depression of ventilation) than nasal histopathology (unpublished data, Bayer AG). A mild time-related increase in susceptibility to irritation occurred from the first to last exposure day. This appears to be consistent with an irritation-related adjuvant effect, e.g. conveyed by transient receptor potential (TRP) channel-expressing cells of sensory nerves (Shiba et al., Citation2009). The sensory irritation-based average POD of HDI and TDI was ∼0.05 ppmV (PODTDI: 0.036 ppmV, PODHDI: 0.083 ppmV). This concentration was within that range eliciting marginal to marked eye irritation in humans (for details, see DFG, Citation2014; Pauluhn, Citation2014). Thus, with regard to “acute” sensory irritation, the derived irritation-related POD from rat repeated exposure inhalation studies was consistent with equivalent findings in humans. This study demonstrates that any “acute-on-chronic irritation” can lower the irritant threshold dose by non-allergic mechanisms once the inflammatory response has been initiated and amplified.
Extensive pre-studies were required for HDI to quantify the specific C × t relationship to sufficiently dose the typical target structures of asthma within the lung in the absence of any overriding acute irritation-related alveolitis. A precipitous increase in irritation potency of HDI-monomer occurred when attaining the vapor saturation concentration (). This appears to suggest that higher doses of HDI gained access to the distal airways when shifting the vapor-to-aerosol equilibrium towards the aerosol phase. However, under such experimental conditions, it becomes increasingly difficult to unequivocally differentiate irritant and asthma-like responses and data have to be judged cautiously (, upper panel). In contrast, at challenge concentrations below the vapor saturation concentration (, step II), a more pronounced dose-related increase in PMN occurred in sensitized rats (, lower panel) relative to similarly challenged control rats.
Repeated inhalation priming exposure in mice at 7.3 mg HDI/m3 for 180 or 360 min (1300–2600 mg/m3 × min on 3 d) were shown to increase the proliferation index of auricular lymph nodes more than ∼100-fold in the absence of any marked stimulation of lymph nodes draining the lung (Arts et al., Citation2008). This observation supports the conclusion of this study, namely that the anterior-to-posterior gradient of retention of HDI-vapor is highly concentration-dependent with difficulties to clearly differentiate the irritation-related “site-of-retention”-related response to irritant inflammation from any allergic etiopathology.
When using a HDI-homopolymer isocyanurate-type polyisocyanate as a carrier to shuttle HDI-vapor partitioned to the polyisocyanate aerosol into the lower airways, functional measurements provided evidence of increased pulmonary irritation at approximately 8 mg HDI/m3. However, the sensory irritation-related differences in ventilation and breathing patterns (see ) preclude any robust quantitative conclusion. Overall, the findings from the pre-studies as well as the single and repeated inhalation priming studies support the conclusion that the irritant and allergic etiopathology of HDI-vapor is difficult to disentangle experimentally in the presence of aerosol. Hence, in order to minimize the complexity of study, the focus was on HDI-vapor alone.
Previous studies with TDI-vapor using the same protocol yielded an elicitation POD of 1000 mg TDI/m3 × min (143 ppmV × min), which is essentially similar to the POD of 900 mg HDI/m3 × min (129 ppmV × min) of this study (). Based on an average POD of 135 ppmV × min from both diisocyanates, the human workplace equivalent concentration (HEC) can be estimated as already rationalized in the method section: HEC = 135 ppmV × 1/(3 × 3)inhalation dosimetry × 1/480 min−1 = 0.03 ppmV (8-h working day time weighted average, TWA). In the absence of any adjustment for inter-species differences in healthy workers, this average estimate is six-fold higher than the current MAK-TWA of HDI (DFG, Citation2014) and TLV-TWA (2011) of HDI and TDI (0.005 ppmV) which aim at preventing diisocyanate asthma to occur in healthy subjects. A concentration of 0.01 ppmV TDI for 1–2 h (the following calculations used the average of 90 min) led in one of the 15 asthmatic persons to an asthma attack, while in healthy persons, even 0.02 ppmV did not have effects on the airways (Baur et al., Citation1994). Hence, the POD in asthmatic subjects can be estimated to be 0.02 ppmV × 90/480 min−1 ≤ 0.004 ppmV × 8 h, which is essentially identical with the current workplace standard. Based on these empirical data on diisocyanate vapors in asthmatic subjects, an additional intra-species susceptibility factor of 5 seems to be apt to extrapolate from this bioassay to the respective human threshold-TWA (asthmatic subjects). For the healthy subpopulation of workers, this would translate to a MAK/TLV-TWA of ≈0.006 ppmV as a safe workplace exposure level.
When applying the same algorithm to polymeric MDI-aerosol which was examined using a Cvariable × tconst protocol under otherwise comparable conditions (Pauluhn, Citation2008; Pauluhn & Poole, Citation2011), the respective POD in the BN-rat was 90 mg MDI/m3 × min with a HEC = 90 mg MDI/m3 × min × 1/3inhalation dosimetry × 1/480 min = 0.063 mg/m3-TWA-8 h. The inhalation dosimetry of MDI-aerosol is dependent on the aerosol size, which may differ to some extent in animal and human bronchoprovocation studies. MDI-did not cause any appreciable upper respiratory tract irritant-related depression in ventilation. Accordingly, an arbitrary rat-to-human inhalation–dose adjustment factor of 3 (conversion from nasal to oronasally breathing) appears to be a reasonably conservative estimate. This calculated threshold of 0.063 mg/m3-TWA-8 h is similar to the current TLV-TWA of polymeric MDI. Asthmatic human subjects were challenged with MDI-aerosol without symptoms at 0.15 mg/m3 × 30 min = 4.5 mg MDI/m3 × min; adjusted to 1/480 min−1 = ∼0.01 mg/m3-8 h (Leroyer et al., Citation1998) which is about five-fold lower than the current TLV-TWA to protect healthy workers. Hence, the empirical data of this bioassay on this polymeric diisocyanate aerosol would support an additional intra-species susceptibility factor of ∼6 to extrapolate from the POD of this bioassay to the TWA-averaged elicitation threshold of human subjects inflicted with mild asthma.
In summary, this comparative analysis of the inhalation elicitation threshold of two volatile isocyanates (aliphatic and aromatic) and one non-volatile aromatic diisocyanate in the BN-rat bioassay shows the existence of a threshold dose for the elicitation response in skin-sensitized and inhalation-primed rats predisposed to asthma. This comparison demonstrates further that the protocol structure is inherently conservative. These outcomes support inhalation dosimetry/intra-species susceptibility factors of 15 [dosimetry 3, intra-species 5] and 50 [dosimetry 3 × 3, intra-species 5] for non-volatile (aerosol) and volatile diisocyanates, respectively. Overall, the outcome of this analysis is in logical agreement with current occupational exposure standards of volatile and non-volatile diisocyanates. Of note is that the content of HDI-monomer in polyisocyanates commercialized for spray painting decades ago was much higher than to date. It cannot be excluded, in the absence of extensive phase-specific analytical characterization of HDI, those findings from the past were confounded by high residual HDI-content. Overall, the protocol devised is believed to be suitable to define a NOAEL on the elicitation response and to derive a safe OEL to both prevent from respiratory tract irritation and asthma to occur.
Conclusions
Thresholds of elicitation in sensitized individuals are important for safe use of diisocyanates or any respiratory sensitizer. Ideally, one would like to prevent the initial sensitization step; however, this would require in depth knowledge of the most critical route and putative route-specific intensity of exposure. It appears that the experimentally derived threshold of the elicitation response (in predisposed asthmatic rats) may lead to regulated levels that would prevent respiratory tract irritation and ensuing immunological sequelae called “sensitization” occurring from inhalation exposures superimposed on subjects with prior high-level skin exposures. This bioassay was devised to mimic the paradigms used in diagnostic human inhalation challenge tests and to reduce the dosimetric uncertainties associated with quantitative risk assessment for the “irritant” and “asthma-inducing” threshold. Thus, data from this bioassay can readily be put into human context for the purpose to derive safe occupational exposure levels of diisocyanates.
Acknowledgements
The author thanks P. Eidmann, Doris Zischka-Kuhbier, and A. Thiel for technical assistance.
Declaration of interest
The author received support from the European Aliphatic Isocyanates Producers Association (ALIPA) for the preparation of this manuscript. Bayer MaterialScience is a producer of HDI. The manuscript reflects solely the opinion of the author and not of any other funding source. The author reports that he has no conflicts of interests.
References
- Aalto-Korte K, Suuronen K, Kuuliala O, et al. (2011). Occupational contact allergy to monomeric isocyanates. Contact Dermatitis 67:78–88
- Alexandersson R, Hedenstierna G, Plato N, Kolmodin-Hedman B. (1987). Exposure, lung function, and symptoms in car painters exposed to hexamethylene diisocyanate and biuret modified hexamethylene diisocyanate. Arch Environ Health 42:367–73
- Arts JH, de Jong WH, van Triel JJ, et al. (2008). The respiratory local lymph node assay as a tool to study respiratory sensitizers. Toxicol Sci 106:423–34. Toxicology 1997;117:229–34
- Baur X. (2007). Evidence for allergic reactions in isocyanate asthma. J Allergy Clin Immunol 119:757–8
- Baur X, Marek W, Ammon J, et al. (1994). Respiratory and other hazards of isocyanates. Int Arch Occup Environ Health 66:141–52
- Bello D, Herrick CA, Smith TJ, et al. (2007). Skin exposure to isocyanates: reasons for concern. Environ Health Perspect 115:328–35
- Bello D, Woskie SR, Streicher RP, et al. (2004). Polyisocyanates in occupational environments: a critical review of exposure limits and metrics. Am J Ind Med 46:480–91
- Boverhof DR, Billington R, Gollapudi BB, et al. (2008). Respiratory sensitization and allergy: current research approaches and needs. Toxicol Appl Pharmacol 226:1–13
- Cassidy LD, Molenaar DM, Hathaway JA, et al. (2010). Trends in pulmonary function and prevalence of asthma in hexamethylene diisocyanate workers during a 19-year period. J Occup Environ Med 52:988–94
- Cockcroft DW, Mink JT. (1979). Isocyanate-induced asthma in an automobile spray painter. CMA 121:602–4
- De Vooght V, Haenen S, Verbeken E, et al. (2011). Successful transfer of chemical-induced asthma by adoptive transfer of low amounts of lymphocytes in a mouse model. Toxicology 279:85–90
- De Vooght V, Smulders S, Haenen S, et al. (2013). Neutrophil and eosinophil granulocytes as key players in a mouse model of chemical-induced asthma. Toxicol Sci 131:406–18
- DFG (Deutsche Forschungsgemeinschaft). (2014). MAK-und BAT-Werte-Liste 2014: Maximale Arbeitsplatzkonzentrationen und Biologische Arbeitsstofftoleranzwerte. MAK Values of Hexamethylene diisocyanate (HDI) and Diphenylmethane diisocyanate (MDI). Available from: http://onlinelibrary.wiley.com/doi/10.1002/9783527682010.ch2/pdf). [Last accessed: 23 Jun 2014]
- Directive 2010/63/EEC. (2010). Guideline of the council dated September 22, 2010 on the reconciliation of legal and administrative regulations of the member countries for the protection of animals used for studies and other scientific purposes. J Eur Commun, Legal Specifications L276, pp. 33–76
- ECHA. (2012). Guidance on information requirements and chemical safety assessment Chapter R.8: characterization of dose [concentration]–response for human health, version: 2.1. APPENDIX R. 8-11 Respiratory sensitization, p. 130. Available from: http://echa.europa.eu/documents/10162/13632/information_requirements_r8_en.pdf. [Last accessed: Nov 2012]
- Ewald-Kleimeier S, Lotz A, Merget R, et al. (2013). Exhaled nitric oxide in specific inhalation challenge. Adv Exp Med Biol 788:255–64
- Janko M, McCarthy K, Fajer M, van Raalte J. (1992). Occupational exposure to 1,6-hexamethylene diisocyanate-based polyisocyanates in the state of Oregon, 1980–1990. Am Ind Hyg Assoc 53:331–8
- Jatakanon A, Uasuf C, Maziak W, et al. (1999). Neutrophilic inflammation in severe persistent asthma. Am J Respir Crit Care Med 160:1532–9
- Jonaid BS, Pronk A, Doekes G, Heederik D. (2014). Exhaled nitric oxide in spray painters exposed to isocyanates: effect modification by atopy and smoking. Occup Environ Med 71:415–22
- Kidd P. (2003). Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev 8:223–46
- Kimber I, Dearman RJ. (2005). What makes a respiratory sensitizer? Curr Opin Allergy Clin Immunol 5:119–24
- Kimber I, Dearman RJ, Basketter DA, Boverhof DR. (2014). Chemical respiratory allergy: reverse engineering an adverse outcome pathway. Toxicology 318:32–9
- Laskin DL, Suni VR, Gardner CR, Laskin JD. (2010). Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 51:267–88
- Lemière C, Romeo P, Chaboillez S, et al. (2002). Airway inflammation and functional changes after exposure to different concentrations of isocyanates. J Allergy Clin Immunol 10:641–6
- Leroyer C, Perfetti L, Cartier A, Malo JL. (1998). Can reactive airways function syndrome (RADS) transform into occupational asthma due to “sensitization” to isocyanates? Thorax 53:152–3
- Liu FF, Li WL, Pauluhn J, et al. (2013). Rat models of acute lung injury: exhaled nitric oxide as a sensitive, noninvasive real-time biomarker of prognosis and efficacy of intervention. Toxicology 310C:104–14
- Maître A, Leplay A, Perdirix A, et al. (1996). Comparison between solid sampler and impinger for evaluation of occupational exposure to 1,6-hexamethylene diisocyanate (HDI) polyisocyanates during spray painting. Am Ind Hyg Assoc J 57:153–60
- Mähler M, Berard M, Feinstein R, et al. (2014). FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 48:178–92
- Malo J-L, Ghezzo H, L'archevêque J, et al. (1991). Is the clinical history a satisfactory means of diagnosing occupational asthma? Am Rev Respir Dis 143:528–32
- Myer HE, O'Block ST, Dharmarajan V. (1993). A survey of airborne HDI, HDI-based polyisocyanate and solvent concentrations in the manufacture and application of polyurethane coatings. Am Ind Hyg Assoc J 54:663–70
- OECD. (1998). Organisation for Economic Cooperation and Development (1998). OECD Principles on Good Laboratory Practice (as revised in 1997). ENV/MC/CHEM (98) 17, distributed 26 January 1998. Paris: Organisation for Economic Cooperation and Development
- OECD. (2009). Organization for Economic Cooperation and Development-Environment, Health and Safety Publications, Series on Testing and Assessment No. 39: Guidance Document for Acute Inhalation Toxicity Testing, [ENV/JM/MONO 28]. Available from: http://oberon.sourceoecd.org. [Last accessed: 21 Jul 2009]
- Pauluhn J. (1994). Assessment of chemicals for their potential to induce respiratory allergy in guinea pigs: a comparison of different routes of induction and confounding effects due to pulmonary hyperreactivity. Toxicology In Vitro 8:981–5
- Pauluhn J. (2000). Acute inhalation toxicity of polymeric diphenyl-methane-4,4'-diisocyanate (MDI) in rats: time course of changes in bronchoalveolar lavage. Arch Toxicol 74:257–69
- Pauluhn J. (2002). Short-term inhalation toxicity of polyisocyanate aerosols in rats: comparative assessment of irritant-threshold concentrations by bronchoalveolar lavage. Inhal Toxicol 14:287–301
- Pauluhn J. (2004). Comparative analysis of pulmonary irritation by measurements of Penh and protein in bronchoalveolar lavage fluid in Brown Norway rats and Wistar rats exposed to irritant aerosols. Inhal Toxicol 16:159–75
- Pauluhn J. (2005). Brown Norway rat asthma model of diphenylmethane-4,4′-diisocyanate. Inhal Toxicol 17:729–39
- Pauluhn J. (2008). Brown Norway rat asthma model of diphenylmethane-4,4′-diisocyanate (MDI): the elicitation dose–response relationship. Toxicol Sci 104:320–31
- Pauluhn J. (2011). Interrelating the acute and chronic mode of action of inhaled methylenediphenyl diisocyanate (MDI) assisted by computational toxicology. Regul Pharmacol Toxicol 61:351–64
- Pauluhn J. (2014). Development of a respiratory sensitization/elicitation protocol of toluene diisocyanate (TDI) in Brown Norway rats to derive an elicitation-based occupational exposure level. Toxicology 319:10–22
- Pauluhn J, Mohr U. (2001). Inhalation toxicity of 1,6-hexamethylene diisocyanatehomopolymers (HDI-IC and HDI-BT): results of subacute and subchronic repeated exposure inhalation exposure studies. Inhal Toxicol 13:513–32
- Pauluhn J, Mohr U. (2005). Review: experimental approaches to evaluate respiratory allergy in animal models. Exp Toxic Pathol 56:203–34
- Pauluhn J, Poole A. (2011). Brown Norway rat asthma model of diphenylmethane-4,4-diisocyanate (MDI): determination of the elicitation threshold concentration of after inhalation sensitization. Toxicology 281:15–24
- Pauluhn J, Thiel A. (2007). A simple approach to validation of directed-flow nose-only inhalation chambers. J Appl Toxicol 27:160–7
- Pauluhn J, Eidmann P, Mohr U. (2002). Respiratory hypersensitivity in guinea pigs sensitized to 1,6-hexamethylene diisocyanate (HDI): comparison of results obtained with the monomer and homopolymers of HDI. Toxicology 171:147–60
- Pauluhn J, Woolhiser MR, Bloemen L. (2005). Repeated inhalation challenge with diphenylmethane-4,4′-diisocyanate in brown Norway rats leads to a time-related increase of neutrophils in bronchoalveolar lavage after topical induction. Inhal Toxicol 17:67–78
- Pisaniello DL, Muriale L. (1989). The use of isocyanate paints in auto refinishing – a survey of isocyanate exposures and related work practices in South Australia. Am Occup Hyg 33:563–72
- Pronk A, Preller L, Raulf-Heimsoth M, et al. (2007). Respiratory symptoms, sensitization, and exposure response relationships in spray painters exposed to isocyanates. Am J Respir Crit Care Med 176:1090–7
- Pronk A, Tielemans E, Skarping G, et al. (2006a). Inhalation exposure to isocyanates of car body repair shop workers and industrial spray painters. Ann Occup Hyg 50:1–14
- Pronk A, Yu F, Vlaanderen J, et al. (2006b). Dermal, inhalation, and internal exposure to 1,6-HDI and its oligomers in car body repair shop workers and industrial spray painters. Occup Environ Med 63:624–31
- Rando RJ, Poovey HG. (1999). Development of a dichotomous vapor/aerosol sampler for HDI-derived total reactive isocyanate group. Am Ind Hyg Assoc J 60:737–46
- Raulf-Heimsoth M, Liebig R, Marczynski B, et al. (2013). Implementation of non-invasive methods in the diagnosis of diisocyanate-induced asthma. Adv Exp Med Biol 788:293–300
- Redlich CA. (2010). Skin exposure and asthma: is there a connection? Proc Am Thorac Soc 7:134–7
- Redlich CA, Stowe MH, Coren BA, et al. (2002). Diisocyanate-exposed auto body shop workers: a one-year follow-up. Am J Ind Med 42:511–18
- Remiarz RJ, Argawal JK, Quant FR, SEM GJ. (1983). A real-time aerodynamic particle size analyzer. In: Marple VA, Liu BYH (eds.) Aerosols in the mining and industrial work environments. Ann Arbor, MI: Science Publ. Inc, 879–95
- Schroeter JD, Kimbell JS, Asgharian B, et al. (2013). Inhalation dosimetry of hexamethylene diisocyanate vapor in the rat and human respiratory tracts. Inhal Toxicol 25:168–77
- Shiba T, Maruyama T, Kurohane K, et al. (2009). TRPA1 and TRPV1 activation is a novel adjuvant effect mechanism in contact hypersensitivity. J Neuroimmunol 207:66–74
- Shiotsuka RN, Stuart BP, Charles JM, et al. (2010). Chronic inhalation exposure of Fischer 344 rats to 1,6-hexamethylene diisocyanate did not reveal a carcinogenic potential. Inhal Toxicol 22:875–87
- Shiotsuka RN, Stuart BP, Sangha GK, et al. (2006). Subacute inhalation exposure of rats to 1,6-hexamethylene diisocyanate. Inhal Toxicol 18:659–65
- Thomasen JM, Fent KW, Nylander-French LA. (2011). Development of a sampling patch to measure dermal exposures to monomeric and polymeric 1,6-hexamethylene diisocyanate: a pilot study. J Occup Environ Hyg 8:709–17
- Thorne PS, Hillebrand JA, Lewis GR, Karol MH. (1987). Contact sensitivity by diisocyanates: potencies and cross-reactivities. Toxicol Appl Pharmacol 87:155–65
- Tornling G, Alexandersson R, Hedenstierna G, Plato N. (1990). Decreased lung function and exposure to diisocyanates (HDI and HDI-BT) in car repair painters: observations on re-examination 6 years after initial study. Am J Ind Med 17:299–310
- Vandenplas O. (2011). Occupational asthma: etiologies and risk factors. Allergy Asthma Immunol Res 3:157–67
- Vandenplas O, Cartier A, Lesage J, et al. (1993a). Prepolymers of hexamethylene diisocyanate as a cause of occupational asthma. J Allergy Clin Immunol 91:850–61
- Vandenplas O, Cartier A, Ghezzo H, et al. (1993b). Response to isocyanates: effect of concentration, duration of exposure, and dose. Am Rev Respir Dis 147:1287–90
- Vandenplas O, Malo J-L, Saetta M, et al. (1993c). Occupational asthma and extrinsic alveolitis due to isocyanates: current status and perspectives. Br J Ind Med 50:213–28
- Vanoirbeek JA, De Vooght V, Vanhooren HM, et al. (2007). How long do the systemic and ventilatory responses to toluene diisocyanate persist in dermally sensitized mice? J Allergy Clin Immunol 121:456–63
- Villeneuve DL, Crump D, Garcia-Reyero N, et al. (2014). Adverse outcome pathway (AOP) development I: strategies and principles. Toxicol Sci 142:312–20