Abstract
This review summarizes the literature on the relation between the development of pleural plaques and non-malignant and malignant disease in cohorts exposed to asbestos and other fibers. The available evidence indicates that, absent any other pleural disease, the presence of pleural plaques does not result in respiratory symptoms or clinically significant impacts on lung function. For certain types of asbestos, the development of pleural plaques is statistically correlated with malignant disease, but the evidence is consistent with the hypothesis that pleural plaques without other pleural disease are a marker of exposure, rather than an independent risk factor. Pleural plaques have also developed in cohorts exposed to other fibers that have not proven to be carcinogenic. Risk analyses should be based on the avoidance of known adverse conditions, rather than pleural plaques per se.
Introduction
There is ample evidence that either occupational or environmental exposure to various types of asbestos is associated with an increase in the incidence of both malignant and non-malignant respiratory diseases (NMRD). The principal malignant diseases of concern are lung cancer and pleural mesothelioma. Non-malignant effects of asbestos exposure include respiratory symptoms, reductions in lung function, pleural plaques, pleural effusions, diffuse pleural thickening (DPT) and asbestosis (asbestos-related interstitial fibrosis). As pleural plaques can occur even after relatively low exposure, these are among the most common of the non-malignant effects (Antao et al., Citation2012; Chapman et al., Citation2003; Clarke et al., Citation2006; Crapo, Citation2005; Cugell & Kamp, Citation2004; Epstein, Citation1984; Evans & Gleeson, Citation2004; Hillerdal, Citation1981, Citation1994, Citation1997; Hourihane et al., Citation1966; IIAC, Citation2008; Jakobsson, Citation1993; Jakobsson et al., Citation1995; Jones, Citation1997; Myers, Citation2012; Roach et al., Citation2002; Sargent et al., Citation1977). Most researchers conclude that pleural plaques (alone) “are nearly always asymptomatic” (British Thoracic Society, Citation2011) and “are in themselves harmless and can be regarded as an objective sign of previous asbestos inhalation” (Hillerdal & Henderson, Citation1997). Although asbestos exposure is often implicated in the development of pleural plaques, exposure to other materials has also been reported to result in pleural plaques (Clarke et al., Citation2006; Lockey et al., Citation1996).
Recently, however, the US EPA (as part of a risk analysis for Libby Amphibole Asbestos [LAA]) (US EPA, Citation2014) selected pleural plaques as an adverse endpoint and calculated a reference dose (RfD) that might be used to develop cleanup levels at contaminated sites.Footnote1 And two recent publications (Pairon et al., Citation2013a, Citation2014) have suggested that pleural plaques might be an independent risk factor for malignant effects following asbestos exposure. Moreover, the US government has decided that Medicare will pay the costs of annual CT screenings for lung cancer for older Americans with long histories of heavy smoking, which (inter alia) is likely to result in the discovery of a substantial number of persons with pleural plaques. Subjects who test positive for plaques and their physicians will be interested in assessing the clinical implications. For these reasons, it is appropriate to review the available literature on the effects of pleural plaques in terms of possible linkages to respiratory symptoms, impaired lung function and malignant disease.Footnote2 As recent review articles have adequately covered respiratory symptoms and possible impairments to lung function (Kerper et al., Citation2014), this review focuses chiefly on malignant effects.
Background: what are pleural plaques and how are they detected?
Pleural plaques (also termed benign pleural plaques or localized pleural thickening [LPT]) are localized areas of pleural thickening with clearly demarcated edges. These are usually white or white-tan lesions of rubber-like consistency, but may become hard due to diffuse calcification. They are found in the lateral and lower half of the pleural cavity. Plaques are typically bilateral with well-defined borders. Plaques have been described by one physician (Crapo, Citation2005) as “a callus on the chest wall”.
The latency period for pleural plaques related to asbestos exposure is typically reported as 15–40 years (ACC Review, Citation2004; British Thoracic Society, Citation2011; Chapman et al., Citation2003; Hillerdal, Citation1981, Citation1994; Utell & Maxim, Citation2010). However, Larson et al. (Citation2010) reported a much reduced latency (median 8.6 years, range 1.4–14.7 years) for pleural plaques associated with exposure to LAA.
Pleural plaques may be diagnosed at autopsy, during thoracotomy,Footnote3 or with thoracoscopy,Footnote4 using magnetic resonance imaging (MRI), chest X-ray (CXR) or computed tomography (CT). MRI is much less sensitive than CT (Evans & Gleeson, Citation2004). Most frequently, pleural plaques are diagnosed using radiographic methods including CXR or CT. Use of CT scans increases both sensitivity and specificity compared to standard CXR (Al Jarad et al., Citation1991; Neri et al., Citation1994; O’Reilley et al., Citation2007; Roach et al., Citation2002) but results from these two methods are correlated (Suganuma et al., Citation2001). High-resolution computed tomography (HRCT) is superior to CT (Lynch et al., Citation1989). Diagnostic features are discussed in several publications (see, e.g. Copley et al. (Citation2001), Jones (Citation1997), Sargent et al. (Citation1977) or various papers of Hillerdal).
Interpretation of radiographic data may be difficult and requires special training: both false-positives and false-negatives result (Hillerdal, Citation1997, Citation2001; Clarke et al., Citation2006). The prevalence of plaques discovered at autopsy is typically greater than that determined using radiological methods (Hillerdal, Citation1997). A study by Gefter & Conant (Citation1988) affirmed the utility of CXR, but in comparing results of CXR and autopsies cautioned:
Autopsy series indicate that at least 60% of pleural plaques may be overlooked. Conversely, such series indicate that up to 20% of plaques are falsely diagnosed. The significance of visceral pleural thickening and the definition and positive predictive value of diffuse pleural thickening as they relate to asbestos exposure are unresolved issues. Data suggest that the CXR may fail to reflect significant asbestosis in 10% to 20% of cases.
Most of the earlier studies on pleural plaques used CXR, rather than CT, which complicates comparisons because of the possibility of undetected plaques in the CXR studies (Moolgavkar et al., Citation2014):
Research on pleural plaques is complicated by the fact that their diagnostic criteria have changed over time and are applied inconsistently across studies. As a result, findings may not be comparable across studies.
Other conditions that pose a challenge to correct diagnosis include rib fractures, subpleural fat, diffuse lung diseases and focal lung diseases (Clarke et al., Citation2006; Hillerdal, Citation2001; Jones, Citation1997; Larson et al., Citation2014).
Care must be taken to distinguish pleural plaques from other pleural abnormalities, such as diffuse pleural thickening [DPT] (Copley et al., Citation2001; Gevenois et al., Citation1998; Jeebun & Stenton, Citation2012; McLoud et al., Citation1985; Miles et al., Citation2008) or asbestosis, which have substantially different biological effects.Footnote5 In some publications the phrase “pleural disease” is used, which fails to distinguish between pleural plaques and DPT. It is also important to distinguish subjects or groups of subjects that may have only pleural plaques as distinct from those that might have plaques and also DPT or asbestosis.
Dose- or exposure-response and pleural plaques
Numerous studies have examined the dose- or exposure-response relation for pleural plaques (Bar-Shai et al., Citation2012; Boffetta, Citation1998; Clin et al., Citation2011; Eisenhawer et al., Citation2014; Ehrlich et al., Citation1992; Finkelstein & Vingilis, Citation1984; Hosoda et al., Citation2008; Jakobsson et al., Citation1995; Järvholm, Citation1992; Karjalainen et al., Citation1994; Lockey et al., Citation2015; Mastrangelo et al., Citation2009; McDonald et al., Citation1986; Moolgavkar et al., Citation2014; Paris et al., Citation2008, Citation2009; Rohs et al., Citation2008; Sandén & Järvholm, Citation1986; Shepherd et al., Citation1997; Soulat et al., Citation1999; Van Cleemput et al., Citation2001). Results differ among these studies,Footnote6 but exposure variables found relevant in one or more studies include time since first exposure (TSFE), age at first exposure (Jones, Citation1997), type (Hillerdal, Citation1997; Jones, Citation1997; Sandén & Järvholm, Citation1986) and dimensions of fiber (Hillerdal, Citation1997; Jones, Citation1997), lung fiber burden (Karjalainen et al., Citation1994; Roberts, Citation1971), duration of exposure, intensity of estimated asbestos exposure and cumulative exposure (measured in various ways). Smoking has been found to be an important factor in the development of parenchymal fibrosis and lung cancer, but many researchers have reported that this is not true for pleural lesions [see Hillerdal (Citation1997) and references threrein]. Moolgavkar et al. (Citation2014) offer the following comments on the relationship between smoking and pleural plaques:
The extent to which smoking history may confound the association between asbestos exposure and the prevalence of pleural plaques is unclear, given that the relationship between smoking and pleural plaques has not been studied thoroughly. Studies of this relationship have generally classified smoking crudely as ever vs. never, or current vs. former vs. never-smoking status; we identified no studies that evaluated detailed smoking exposure in terms of pack-years or other quantitative measures. Moreover, many studies did not distinguish between LPT and DPT on chest radiographs. Perhaps, due in part to this misclassification of both the exposure and the outcome, as well as confounding and typically modest sample sizes, findings have been mixed.
With regards to exposure specifically, several of the candidate determinants of exposure have been shown to be correlated with the prevalence of pleural plaques – but results are not entirely consistent among studies – perhaps because exposure data are of varying quality. For example, in one series of analyses TSFE, mean fiber concentration and cumulative exposure were found to be significant (Paris et al., Citation2008, Citation2009). Various mathematical models have been suggested to describe the relationship between exposure and the prevalence of pleural plaques (Finkelstein & Vingilis, Citation1984; Järvholm, Citation1992; McDonald et al., Citation1986; Paris et al., Citation2008, Citation2009).
The logistic model employed by Paris et al. (Citation2008), for example, relates the probability that an individual worker i (among a population of n workers; i = 1, n) develops a pleural plaque, Pi, when exposed at levels Xij, j = 1, r, by means of the relation:
(1)
where the empirical parameters β0 and βj ( j = 1, r) are estimated by statistical fitting procedures (Hosmer & Lemeshow, Citation2000; Kleinbaum & Klein, Citation2002), such as maximizing the log-likelihood function. (Statistical issues and difficulties in fitting such relationships are discussed in a later section.)
Continuing the example, Paris et al. (Citation2008) reported results of a study of 1011 heavily asbestos-exposed volunteers (mainly asbestos textile and friction materials fabrication, insulation and energy production) over 50 years of age (both working and retired) who participated in a screening program in Normandy, France. The presence or absence of plaques among members of this cohort was determined using HRCT. Occupational histories were collected from participants and used to estimate the TSFE and mean and cumulative exposures for each participant were estimated based on occupational hygiene measurements and a job-exposure matrix.
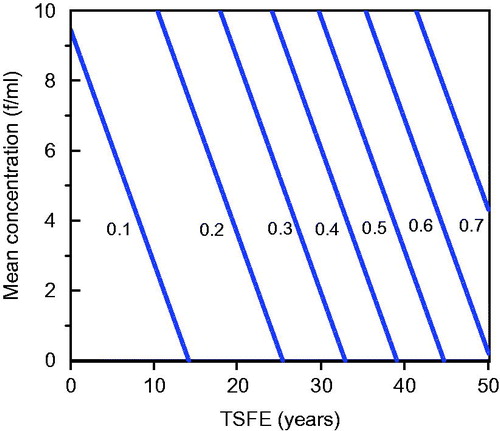
These investigators fitted two logistic models to estimate the frequency of plaques, one including TSFE (years) and mean exposure (in fibers per milliliter f/ml) as the exposure variables and a second including TSFE and cumulative exposure (in f-year/ml). Both models resulted in statistically significant fits (p < 0.001). The best fit values for the coefficients of the first model were β0 = −3.22, β1 = 0.072 and β2 = 0.108 and those for the second model were β0 = −2.83, β1 = 0.065 and β2 = 0.0035. shows the estimated relation between the prevalence of plaques (fraction), TSFE (years from 0 to 50) and mean estimated asbestos exposure f/ml (0 through 10 f/ml).
Figure 1. Best-fit equation relating the prevalence of pleural plaques, TSFE and mean exposure (f/ml) as determined by Paris et al. (Citation2008).
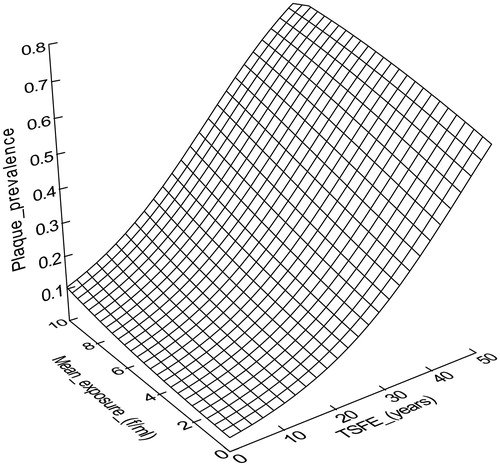
The best fit model developed by Paris et al. (Citation2008) when TSFE and mean exposure were included as candidate exposure variables indicated that TSFE had a greater contribution to plaque formation than mean exposure.Footnote7 This is most easily seen by examining contour lines of constant plaque probability or prevalence, which are shown in . As can be seen from although both exposure variables were found to be statistically significant determinants of plaque prevalence, TSFE has greater leverage.Footnote8 The contour lines in are nearly perpendicular to the X axis, which would be the case if plaque probability were independent of mean exposure – which we find counterintuitive. It is possible that this result is an artifact of the quality of the exposure data. (It is also relevant to note that one characteristic of the model is that it predicts that plaques will develop even at zero exposure, provided that TSFE is sufficiently great, a logical impossibility.) Rohs et al. (Citation2008) found that pleural plaques (detected by CXR) were strongly dependent upon cumulative exposure for LAA, which is more in accord with expectation. More recently Lockey et al. (Citation2015) used HRCT/CT in a similar analysis of LAA and found that pleural changes (including both localized and diffuse pleural thickening) increased with cumulative exposure and employment duration.
Pleural plaques and respiratory symptoms
With very few exceptions (Cramond & Casserly, Citation2006; Hilt et al., Citation1987; Mukherjee et al., Citation2000; Myers, Citation2012) investigators have concluded that most subjects with pleural plaques are nearly always asymptomatic (Ameille & Letourneux, Citation1999; Broderick et al., Citation1992; Christen et al., Citation1997; Crane et al., Citation2005; Crapo, Citation2005; Dweik & Mazzone, Citation2010; Fishwick & Barber, Citation2014; Hillerdal, Citation1997, Citation2001; Hillerdal & Henderson, Citation1997; IIAC, Citation2008; Järvholm & Larsson, Citation1988; Jones, Citation1997; Meirelles et al., Citation2005; Mohr, Citation2012; Montes et al., Citation2005; Park et al., Citation2011; Roach et al., Citation2002; Rubin, Citation1986; Utell & Maxim, Citation2010). For example:
Broderick et al. (Citation1992) studied an asbestos exposed population of sheet metal workers and examined the relationship between circumscribed plaque and four symptoms; dyspnea upon hurrying or walking up a slight hill (DOE), dyspnea worse than peers while walking (DSP), chest pain and cough. They found elevated odds ratios for DOE, DSP and chest pain, which were statistically significant only for DOE. However, after controlling for confounders (age, pack-years and the presence of asthma and angina) these odds ratios were no longer statistically significant.
Järvholm & Larsson (Citation1988) administered a questionnaire to asbestos-exposed workers participating in a health-screening program. One hundred thirty subjects who were found to have pleural plaques were compared with 1103 control subjects who had no plaques. No difference in the occurrence of thoracic pain was found between the two groups. Dyspnea was more common among subjects with pleural plaques. The increased number of complaints of dyspnea among subjects with pleural plaques could not be explained by differences in age or in smoking habits, but the authors suggested that dyspnea in patients with pleural plaques is caused by sub-roentgenologic fibrosis, not by plaques.
An exception to the finding that persons with pleural plaques are symptom free was reported by Mukherjee et al. (Citation2000) who studied subjects undergoing medical surveillance due to prior asbestos exposure at Wittenoom, Australia. They found that there was a significant association between angina pain and the presence of pleural and parenchymal abnormalities. Another exception was reported by Hilt et al. (Citation1987) who studied a cohort of asbestos exposed persons and found that among 200 subjects less than 70 years of age who had pleural plaques only, a statistically significant increase was observed in the prevalence of breathlessness grade 1 (light) after adjustment for smoking compared to an external reference population.
The most recent study that addresses chest pain in asbestos exposed workers with pleural plaques is that of Park et al. (Citation2011). These investigators studied 621 subjects who attended the Workers’ Compensation (Dust Diseases) Board of New South Wales, Australia for a routine examination as part of an application for compensation, or as follow-up of a diagnosed occupational disease. Some 160 of these subjects had pleural plaques. The endpoints studied included dyspnea, chest pain and cough. Those with pleural plaques alone experienced these symptoms. However, the adjusted odds ratios (plaques versus no plaques) corrected for such variables as age, smoking status (pack-years of smoking) and body mass index (BMI), were not significantly elevated.
In the study of symptoms (as with other possible endpoints of concern), it is important to distinguish pleural plaques from other pleural findings, including pleural effusions and DPT. Recognizing that there are many causes of chest pain (Brims et al., Citation2010) several investigators have concluded that chest pain is associated with pleural effusions (Becklake et al., Citation2007; Chapman et al., Citation2003; Crane et al., Citation2005; Cugell & Kamp, Citation2004; Dweik & Mazzone, Citation2010; Miles et al., Citation2008; Montes et al., Citation2005; Myers, Citation2012; Robinson & Musk, Citation1981) and DPT has also been associated with chest pain (Broderick et al., Citation1992; Crane et al., Citation2005; Miles et al., Citation2008). Moreover, some studies (Allen et al., Citation2011) examine groups of subjects with several different pleural diseases, so that it is not possible to evaluate the individual effects of each.
Pleural plaques and impairments to lung function
The relation between plaques and possible impairment of lung function has been studied extensively (Clark et al., Citation2014; Jones et al., Citation1988; Kerper et al., Citation2014; Kopylev et al., Citation2014; Lockey et al., Citation2015; Moolgavkar et al., Citation2014; Ohlson et al., Citation1985; Sood & Gee, Citation1997; Weill et al., Citation2011; Wilken et al., Citation2011, Zu et al., Citation2015 and contained references). Among the three most recent reviews:
Moolgavkar et al. (Citation2014) reviewed various studies on the relation between pleural plaques and lung function considered by EPA in their analysis of LAA and concluded:
In summary, in the six cross-sectional studies of non-Libby populations that evaluated the association between pleural plaques and pulmonary function with adjustment for asbestos exposure (Broderick et al., Citation1992; Clin et al., Citation2011; Lilis et al., Citation1991; Miller et al., Citation1994; Oliver et al., Citation1988; Wang et al., Citation2001), results varied depending on the measure of lung function, with no consistent patterns of association with spirometric or symptom-based outcomes. Moreover, two studies did not distinguish LPT from DPT (Miller et al., Citation1994; Wang et al., Citation2001), only one used high-resolution computed tomography to detect LPT (Clin et al., Citation2011) and all six were susceptible to residual confounding by asbestos exposure and occult parenchymal fibrosis.
Thus, Moolgavkar et al. (Citation2014) argued that these studies did not resolve the question of whether pleural plaques met the definition of an adverse effect on pulmonary function.
Kopylev et al. (Citation2014) reviewed the literature and concluded that the presence of pleural plaques is associated with small, but statistically significant differences in forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1).
Kerper et al. (Citation2014) reviewed 16 CT and 36 CXR studies and selected six CT and 16 CXR studies as higher quality based on a “risk-of-bias” analysis. They found that half of the higher quality studies reported small but statistically significant mean lung function decrements associated with plaques. None of the differences were clinically significant, however. Many studies had limitations, such as inappropriate controls and/or a total or partial lack of adjustment for confounders. There was little consistency in the direction of effect for the most commonly reported measurements. Kerper et al. (Citation2014) found that pleural plaques were not associated with changes in lung function over time in longitudinal studies. These investigators concluded that the weight of evidence indicated that pleural plaques do not have clinically significant impacts on lung function and that the observed association is most likely due to unidentified pleural thickening or other factors.
Lockey et al. (Citation2015) recently reported results of a careful study of workers employed at a plant that expanded Libby Vermiculite contaminated with LAA in Marysville, Ohio. They identified pleural plaques in the cohort using HRCT/CT and also measured FVC and FEV1 values. They found that, adjusting for pack years of smoking and body mass index, members of the exposed cohort with plaques had lower predicted pulmonary function values compared to those with normal HRCT/CTs by 5.4 and 3.3% in percent predicted FVC (p < 0.05) and FEV1 (p = 0.14), respectively. Though proportionately small, these decrements might be relevant for subjects with compromised lung function, but would not be judged clinically significant by most standards. It is possible that decrements of this magnitude might give rise to symptoms – Brodkin et al. (Citation1993) reported that cough, phlegm and chronic bronchitis were associated with a 2–8% reduction (p < 0.001) in predicted values for FVC and FEV1 in a study of 816 asbestos exposed workers.
Overall there appears to be fairly broad agreement that possible impacts on lung function, even if statistically significant, are small. This is consistent with the view of Jones (Citation1997), who wrote:
In summary, there has yet to be a persuasive demonstration that plaques can cause important functional effects, those that might interfere with the ability to meet the demands of physical exertions.
However, even if it is judged that the lung function impairments are material, it makes sense to base risk analyses on the relevant toxic endpoint(s), such as FVC or FEV1, rather than pleural plaques, which are at best a surrogate for exposure.
Relation between plaques and malignant disease
If pleural plaques are asymptomatic and do not lead to clinically significant decrements on lung function (at least for most exposed individuals), the case for using pleural plaques as an endpoint for risk analyses rests on possible linkages between pleural plaques and malignant effects.
Are pleural plaques premalignant?
There is broad agreement that pleural plaques are not “premalignant” (ACC, Citation2004; Banks & Dedhia, Citation2011; British Thoracic Society, Citation2011; Chapman et al., Citation2014; Collins & Stern, Citation2015; Donaldson et al., Citation2010; Edelman, Citation1988; Gevenois & De Vuyst, Citation2006; IIAC, Citation2008; Kratzke, Citation2013; Miles et al., Citation2008; Miller et al., Citation1996; Myers, Citation2012; Nishimura & Broaddus, Citation1998; Rey et al., Citation1993; Rubin, Citation1986; Rudd, Citation1996; Schwartz, Citation1994; van Gelder et al., Citation1989; Walker et al., Citation2012), that is, they do not become cancerous over time. As noted by IIAC (Citation2008):
Despite an initial case report that raised the concern (Lewinsohn, Citation1974), it is now well established that pleural plaques do not in themselves become malignant. Neither are they a cause of cancer of the pleura or at other sites, such as the lung.
Pleural plaques as markers of exposure
Pleural plaques are widely referred to (Attanoos & Gibbs, Citation2009; Dalphin, Citation2011; Dweik & Mazzone, Citation2010; Edelman, Citation1988; Gevenois & De Vuyst, Citation2006; Fishwick & Barber, Citation2014; Hourihane et al., Citation1966; IIAC, Citation2008; Moolgavkar et al., Citation2014; Nielsen et al., Citation2014) as “markers of asbestos exposureFootnote9” or an index or “signpost” (Sargent et al., Citation1977) of exposure meaning that (other things being equal) an asbestos-exposed person that develops a pleural plaque is likely to have had greater asbestos exposure than someone without a plaque.Footnote10 Thus, even if the development of pleural plaques is an entirely independent process from the development of malignant effects, such as lung cancer or mesothelioma, it is likely that there will be a correlation between pleural plaques and other serious endpoints, including lung cancer or mesothelioma. Several studies (Bianchi et al., Citation1997; Cullen et al., Citation2005; Cvitanović et al., Citation2003; Edge, Citation1976; Fletcher, Citation1972; Hillerdal, Citation1994; Karjalainen et al., Citation1999; Liddell & McDonald, Citation1980; Mollo et al., Citation1984; Sandén et al., Citation1992; Sheers, Citation1979) have shown that subjects with pleural plaques have a greater likelihood of developing either lung cancer or mesothelioma than those who do not. However, not all studies have shown a correlation between plaques and lung cancer or mesothelioma (Hughes & Weill, Citation1991; Kiviluoto et al., Citation1979; Koskinen et al., Citation2002; Lotti, Citation2010; Partanen et al., Citation1992; Sandén & Järvholm, Citation1991 Footnote11; Thiringer et al., Citation1980; Wain et al., Citation1984).
A finding that a higher proportion of subjects with pleural plaques have either lung cancer or mesothelioma compared to those who do not may reflect that fact that there is a dose-response for plaques and subjects with plaques may simply have had greater exposure than those without plaques. For example, Hillerdal (Citation1994) examined subjects with and without pleural plaques, but computed the expected number of lung cancers based on the age- and year-specific expected incidence from the official cancer registry of Sweden, which includes many individuals that were not exposed to asbestos. In short, the finding that pleural plaques are correlated with lung cancer or mesothelioma does not in and of itself indicate that plaques are an independent risk factor for the development of either disease.
The central issue is stated clearly by Goldberg (Citation2005):
Both benign and malignant diseases have been associated with asbestos exposure. To understand the mechanisms of asbestos induced cancers, we should understand whether the strong association between benign and malignant asbestos related diseases only reflects the common cause – asbestos – without being involved in the same pathological process, or whether benign diseases are a preliminary step towards cancer. If benign pleural and lung diseases are on the pathways towards cancer, an additional specific question is whether this is a necessary step, or if benign pleural and lung diseases only increase the risk of developing a cancer by interacting with other factors, such as tobacco smoke.
Stated somewhat differently, the operative question is whether two equivalently exposed individuals (i.e. having the same asbestos exposure), one with a pleural plaque and another without) would have the same or different probabilities of developing lung cancer or mesothelioma? If the equivalently exposed person with the plaque has a greater probability of developing either lung cancer or mesothelioma, then pleural plaques are legitimately termed an independent risk factor for these diseases. This is an important question to answer because if plaques are merely markers of exposure, then it is inappropriate to define these as an endpoint for risk analysis of malignant effects.
This is also a difficult question to answer empirically because asbestos exposure and pleural plaque formation occur together (are co-linear) and disentangling possibly separate (but correlated) effects presents a challenge. Failure to adjust for confounders may lead to false conclusions. However, adjusting (correcting) for asbestos exposure is not a simple task because (depending upon whether CXR or CT was used) plaques may not be detected and (perhaps more important) exposure data may be missing or inaccurate – there are numerous such examples.,Footnote13 One possible consequence of missing or inaccurate exposure data is that pleural plaques might actually be a “better indicator” of exposure than other available measures – which could lead an investigator to conclude incorrectly that plaques are an independent risk factor.
Even if complete and accurate, the various exposure-related measures are likely to be correlated.Footnote14 For example, in a prospective study TSFE, age, duration of exposure and cumulative exposure of cohort members are correlated. In a retrospective study (after exposure has ceased), correlations may still persist. And, in any event, the exposure measures are likely to be correlated with the prevalence of plaques. Multicollinearity is an issue in regression models with several undesirable effects including; (1) small changes in the data may produce wide swings in the parameter estimates [e.g. the βs in Equation (Equation1(1) )], (2) β coefficients may have very high standard errors and low significance levels even though they are jointly significant and (3) β coefficients may even have the “wrong” sign or implausible magnitude. Given the difficulties of correcting for asbestos exposure (and possibly other confounders) it is not surprising that there is some controversy about the issue of whether or not pleural plaques are an independent risk factor for lung cancer or mesothelioma. For a well written general discussion of statistical issues relevant to the study of independent risk factors, refer Brotman et al. (Citation2005).
Studies that have attempted to correct for confounders
Difficult or not, it is necessary to correct for confounders in order to understand whether or not plaques are an independent risk factor in the development of lung cancer or mesothelioma. summarizes results of several studies that have attempted to correct for confounders.
Table 1. Studies that have attempted to correct for confounders in assessing the relation between pleural plaques and malignant effects.
With the exception of the recent Pairon et al. studies, these results are consistent with the hypothesis that pleural plaques are simply a marker of exposure and not an independent risk factor. Both the Pairon et al. studies are suggestive and should not be dismissed out of hand. However, these are not without limitations, several of which are carefully discussed in the articles. Moolgavkar et al. (Citation2014) also reviewed the Pairon et al. (Citation2013a) mesothelioma study and offered the following comments:
However, the study was small, with only 17 mesothelioma cases, and measures of exposure were crude. More importantly, in joint analyses with pleural plaques and various measures of asbestos exposures as predictors of mesothelioma, the pleural plaques were significantly associated with mesothelioma, but the traditional measures of asbestos exposure, such as duration of exposure, cumulative exposure index and time since first exposure, were not. The appropriate conclusion from these analyses is that, in this particular study, pleural plaques were better measures of asbestos exposure than the traditional measures, which were highly imprecise. Therefore, while this study is suggestive, it provides at best very weak evidence that pleural plaques are independent predictors of mesothelioma.
Although the number of lung cancer deaths in the Pairon et al. (Citation2014) study was greater, this study suffers from the same difficulty as the earlier study on mesothelioma in that neither of the exposure measures was significantly related to hazard ratio.
After considering the results of the Pairon et al. (Citation2013a) study, Moolgavkar et al. (Citation2014) wrote:
Taken together, the totality of the evidence suggests strongly that pleural plaques are markers of exposure with little or no clinical significance. Thus, pleural plaques do not appear to satisfy EPA’s own definition of an adverse condition.
Conclusions of review articles
summarizes the conclusions of various scientists who have reviewed the applicable experimental/epidemiological studies. There appears to be relatively broad agreement with the hypothesis that pleural plaques are correlated with lung cancer or mesothelioma, but not an independent risk factor. Some of the most recent reviews shown in were written by co-authors of the most recent Pairon et al. papers but appear to differ from the tentative conclusions of these investigators.
Table 2. Conclusions of various review articles regarding whether pleural plaques are an independent risk factor for either lung cancer or mesothelioma.
Findings of external review groups/agencies
Evidence for and against the hypothesis that pleural plaques are an independent risk factor for lung cancer or mesothelioma has been considered by external review groups/agencies as well as independent researchers. For example:
An International Expert Meeting on Asbestos, Asbestosis and Cancer was convened in Helsinki on 20–22 January 1997 to discuss disorders of the lung and pleura in association with asbestos and to agree upon state-of-the-art criteria for their diagnosis and attribution with respect to asbestos (Tossavainen, Citation1997). This group noted: “Pleural plaques are usually asymptomatic, and without clinically important findings”. The group also noted:
Pleural plaques are an indicator of exposure to asbestos fibers. Because pleural plaques may be associated with low levels of asbestos exposure, the attribution of lung cancer to asbestos exposure must be supported by an occupational history of substantial asbestos exposure or measures of asbestos fiber burden.
The American Thoracic Society (Citation2004) offered the following conclusion regarding plaques and risk of lung cancer or mesothelioma:
The presence of plaques is associated with a greater risk of mesothelioma and of lung cancer compared with subjects with comparable histories of asbestos exposure who do not have plaques (Hillerdal, Citation1994; Hillerdal & Henderson, Citation1997). This is thought to be due to greater exposure or retained body burden, not malignant degeneration. Therefore, the presence of pleural plaques should be interpreted as a marker for elevated risk of malignancy, which may be higher than the occupational history alone might suggest. [References inserted in place of reference numbers.]
However, as noted by Moolgavkar et al., (Citation2014), the actual claims made in the ATS statement are not consistent with the statements made in the references (see below). Weill & Weill (Citation2005) suggested additional reasons why the ATS statement is flawed, including the failure to consider all relevant evidence (including Jones et al., Citation1996; Hughes & Weill, Citation1991; Weiss, Citation1993) in addressing the impact of plaques.
The New Zealand Accident Compensation Corporation (ACC Review, Citation2004) examined asbestos related diseases and concluded: “Pleural plaques require no management or surveillance”.
The UK Industrial Injuries Advisory Council (IIAC, Citation2008) [IIAC is an advisory non-departmental public body, sponsored by the UK Department for Work and Pensions] reviewed the available evidence and wrote:
Higher quality cohort studies, which allow for exposure history and (for lung cancer) smoking habits, suggest that the increases are a consequence of the degree of exposure to asbestos and that the presence of pleural plaques does not, of itself, independently affect risk levels. In other words, any increase in risk in those with pleural plaques arises because they have been exposed to asbestos, not because they have pleural plaques. Since plaques can arise from low as well as high levels of exposure, and are imperfectly diagnosed by most research inquiries, the predictive information about future risks is limited and imprecise, a more useful indicator being the employment history.
Banks et al. (Citation2009) reported the results of a Delphi process to develop the American College of Chest Physicians Consensus Statement among selected experts on the Respiratory Health Effects of Asbestos. Agreement was reached on many statements, such as “Workers with asbestos exposure and pleural plaques or diffuse pleural thickening (in the absence of fibrosis) are at increased risk of mesothelioma”. However, there was no consensus on the statement: “Workers with asbestos-induced pleural abnormalities are at increased risk for lung cancer compared to workers with similar exposure without these pleural abnormalities”. [Emphasis added.]
Worksafe BC (Citation2009) addressed (among other things) the linkage between pleural plaques and malignant effects. They concluded that the presence of pleural thickening or plaques, as diagnosed by plain film radiography, is associated with an increased risk of lung cancer, but did not clearly distinguish between pleural thickening and pleural plaques. A meta-analysis of four studies reported in this publication gives an RR of 1.36 [CI = 1–1.84] for lung cancer. Among the reviewers of this study, Dr. Sverre Vedal offered the following comment:
… while pleural disease does confer risk of lung cancer, it does so only to the extent that it reflects exposure. The utility of pleural disease, then, is that it documents that some exposure occurred and may be useful in that regard in situations where exposure may be in doubt. Beyond that, it cannot be used to refine an estimate of lung cancer risk.
The European Respiratory Society and the European Society of Thoracic Surgeons developed guidelines for the management of malignant pleural mesothelioma [MPM] (Scherpereel et al., Citation2010). Addressing the possible correlation between plaques and MPM the group reported:
MPM may be observed in exposed individuals without any other asbestos-related disease (lung or pleural fibrosis). In most cases, pleural plaques are a sign of asbestos exposure in the past, and it has been reported that they are associated with a greater risk of mesothelioma. Indeed, it is expected that mesothelioma is more frequent in subjects having had pleural plaques than in the general population because both diseases are strongly associated with asbestos exposure. Such association has been reported in some necropsy or cohort studies. In contrast, other cohort studies did not report such an association. In a cancer prevention program at the crocidolite mining and milling town of Wittenoom (Australia), pleural thickening was not associated with an increased risk of pleural mesothelioma after adjusting for time since first exposure, cumulative exposure and age at the start of the program. The same authors reported an excess of peritoneal mesothelioma in this population. Therefore, overall there is no clear evidence that pleural plaques alone increase the risk of pleural mesothelioma.
The British Thoracic Society (Citation2011) published a pamphlet titled “Pleural plaques, Information for Health Care Professionals” that stated (under key points):
The cause of pleural plaques is exposure to asbestos fibres, most commonly in an occupational setting.
Pleural plaques are benign and are the commonest manifestation of past exposure to asbestos.
Plaques only indicate that there has been exposure to asbestos. The risk of other asbestos-related conditions is best quantified according to the latency period, duration of exposure, level of exposure and cumulative exposure.
Pleural plaques are nearly always asymptomatic.
In asymptomatic patients with pleural plaques, further investigations are not indicated and may be harmful.
With the exception of the 2004 ATS review, these opinions of advisory bodies are broadly consistent with the statement that pleural plaques are a marker of asbestos exposure. To be sure, an individual with a pleural plaque may be at greater risk for developing lung cancer or mesothelioma, but this is due to the asbestos exposure, not the pleural plaque. As noted above, the ATS review cited two references (Hillerdal, Citation1994; Hillerdal & Henderson, Citation1997) to support their finding. However, as noted by Moolgavkar et al. (Citation2014), this is a misreading of the references. Hillerdal (Citation1994) concluded:
The finding of definite pleural plaques at chest roentgenogram indicates exposure to asbestos at a level which is of clinical importance. There is a definitely increased risk for mesothelioma, and the data suggest that there is possibly also an increased risk for bronchial carcinoma, though not very great.
Hillerdal & Henderson (Citation1997) actually wrote:
Pleural plaques are the most common radiological finding in persons exposed to asbestos. They are in themselves harmless and can be regarded as an objective sign of previous asbestos inhalation.
And, in any event, Hillerdal (Citation1997) later wrote:
Plaques are in themselves harmless, they may be regarded as an objective sign of previous asbestos inhalation, and it is this exposure that is of possible importance for future health.
In short, the findings of expert review groups are broadly consistent with those of most individual scientists.
Is the relation between plaques and malignant effects consistent across fibers?
Somewhat separate from the question of whether or not pleural plaques are an independent risk factor for malignant disease is the issue of whether or not the relationship between the development of plaques and malignant disease is consistent across all fiber types. Although plaques are typically taken to be a marker of various types of asbestos exposure, researchers have suggested (Clarke et al., Citation2006) that plaques may be associated with exposure to other fibers and materials. For example:
Koskinen et al. (Citation1997) reported results of a study of workers (mean exposure 25 years) in a Finnish limestone-wollastonite mine and mill. Among the 49 workers included in the study, nine workers (18%) were found to have pleural plaques of whom five were reported to have had some prior asbestos exposure. Yet, workers at the Finnish limestone-wollastonite quarry were also subjects of a mortality study (Huuskonen & Tossavainen, Citation1996; Huuskonen et al., Citation1983). The mortality study covered the period 1923–1980 and included 238 workers (192 men and 46 women) who had been on the factory's payroll for at least 1 year. The calculated standard mortality ratios for all neoplasms and cancer of the lung and bronchus for both men and women were less than unity, which fails to indicate that wollastonite is carcinogenic. Wollastonite toxicity has been the subject of review articles (Maxim & McConnell, Citation2005; Maxim et al., Citation2014) that indicate that this fiber is unlikely to be carcinogenic.
Studies have suggested that exposure to mineral wool may be associated with the development of pleural plaques (Sandén & Järvholm, Citation1986; Järvholm et al., Citation1995). The 1995 study by Järvholm of workers in a factory producing rock wool indicated that there was an increasing relationship between the age of workers and pleural plaques in this cohort compared to the occurrence of pleural plaques in non-exposed referents, although the differences were not statistically significant. Sandén & Järvholm (Citation1986) determined that for all worker groups (exposed to what they identified as man-made mineral fibers – probably mineral wool) with at least 20 years of employment at shipyards the rate ratio for prevalence of pleural plaques was 1.4 [CI = 1.1–1.8], although several epidemiological studies (Boffetta et al., Citation2014; Greim et al., Citation2014) indicate that exposure to mineral wool is not associated with an increase in rates of lung cancer or mesothelioma.
Studies (Lockey et al., Citation1996) have indicated that exposure to refractory ceramic fibers is associated with an increase in pleural plaques, but the available evidence (Greim et al., Citation2014; LeMasters et al., Citation2003) suggests that exposure to RCF is not associated with any increase in lung cancer or mesothelioma.
The available evidence fails to indicate that there is a consistent relationship between pleural plaques and either lung cancer or mesothelioma in cohorts that have developed pleural plaques when occupationally exposed to these other fibers.
Summary
The available evidence is consistent with the hypothesis that the development of pleural plaques is a marker of exposure to several fibers, particularly asbestos. Absent other non-malignant pleural disease, pleural plaques are not associated with respiratory symptoms or (in most individuals) clinically significant impairments to lung function. In cohorts occupationally exposed to asbestos there is a correlation between the presence of pleural plaques and malignant effects (lung cancer and mesothelioma), but the evidence indicates that this relationship is a consequence of the degree of exposure to asbestos and that the presence of pleural plaques does not, of itself, independently affect risk levels. The recent studies of Pairon and colleagues suggest that plaques might be an independent risk factor and further research on this topic is appropriate, but these results cannot be regarded as definitive. In any event, the choice of pleural plaques as an adverse condition for risk analysis purposes is not justified by the available evidence. When risk estimates need to be made for various malignant or non-malignant diseases with clinical effects, a more direct and preferable course of action is to develop exposure limits based on data relating exposure and the clinically adverse health effects of concern.
Acknowledgements
We acknowledge the constructive comments of the anonymous reviewers of this manuscript in draft. Their comments were useful and improved the quality of this work.
Declaration of interest
This research was sponsored by Unifrax 1 LLC, a major producer of several synthetic high temperature fibers. The findings and opinions expressed herein are those of the authors alone, however, and do not necessarily reflect the views of the sponsor.
Notes
1In a critical review, Moolgavkar et al. (Citation2014) argued that the EPA analysis sets a poor precedent and, moreover, the technical features of the BMD analysis to establish the RfC were flawed. Zu et al. (Citation2015) also concluded that the EPA choice of pleural plaques as an endpoint was flawed.
2We used various search engines and databases (e.g. PubMed, Google Scholar) to locate relevant articles.
3Via a surgical incision in the chest wall.
4Via use of a thin, lighted tube (called an endoscope) to examine the inside of the chest.
5For example, there is evidence that asbestosis (while not the sole cause of certain respiratory cancers) is an independent risk factor for malignant diseases and, moreover (Moolgavkar et al., Citation2014), may result in fatality absent malignancy. Asbestosis is a legitimate endpoint for risk analysis.
6For example, Bar-Shai et al. (Citation2012) found no correlation between duration of exposure and extent of pleural plaques.
7The same is true for the second model of Paris et al. (Citation2008) wherein cumulative exposure (f-year/ml) is substituted for mean exposure.
8This finding is not unprecedented. Ehrlich et al. (Citation1992) found, in a study (short-term, high-exposure) of employees in a plant in Paterson, NJ, that time since first employment was a useful predictor of pleural abnormalities, whereas cumulative exposure was not. Jakobsson et al. (Citation1995) also highlighted the importance of time, even after brief exposures.
9This is presumably meant as a statistical fact; pleural plaques are also associated with exposure to other materials. As noted by Sargent et al. (Citation1978): “Although asbestos dust inhalation is not the sole cause of pleural plaques, it is certainly the most common”.
10Some investigators [see, e.g. Thomas et al. (Citation2011) in a study of Australians exposed to secondary product rather than mining or milling] have noted that pleural plaques were more extensive in those reporting past asbestos exposure and more frequent in those with higher asbestos body counts, but the observed strength of the association does not support their use as a marker of risk for asbestos-related disease.
11The study of Sandén et al. (Citation1992) was negative in terms of the relation between plaques and lung cancer, but positive for mesothelioma. They used one subgroup of workers with 20+ years of asbestos exposure for this comparison, but did not explicitly correct for possible exposure differences among this cohort.
12In one study (Järvholm & Sandén, Citation1987) asbestos exposures were estimated by the exposed male shipyard workers and by a panel of experts whose judgment was based only on occupational title. The analysis was restricted to men with at least 20 years of exposure. These investigators found that there was a much closer correlation between the occurrence of pleural plaques and the men’s own estimates of exposure than between the occurrence and the experts’ estimates. This is a cautionary tale as many studies use job titles for estimating exposure.
13Another example of lack of precision in exposure estimates is reported by Finkelstein & Vingilis (Citation1984) who carefully calculated cumulative exposure of various members of a cohort of asbestos-cement workers (f-year/ml) and noted that these estimates were estimated to be accurate within a factor of 3–5. Many studies have not reported estimates of the precision of the exposure estimates.
14See, for example, remarks contained in Welch et al. (Citation2007).
References
- Accident Compensation Corporation (ACC) Review. (2004). ACC Review: Asbestos-related disease. ACC 11:2
- Al Jarad N, Poulakis N, Pearson MC, et al. (1991). Assessment of asbestos-induced pleural disease by computed tomography – correlation with chest radiograph and lung function. Respir Med 85:203–8
- Allen RKA, Cramond T, Lennon D, Waterhouse M. (2011). A retrospective study of chest pain in benign asbestos pleural disease. Pain Med 12:1303–8
- Ameille J. (2012). The different pleuro-pulmonary pathologies related to asbestos: definitions, epidemiology and evolution [Article in French]. Rev Mal Respir 29:1035–46
- Ameille J, Brochard P, Letourneux M, et al. (2011). Asbestos-related cancer risk in patients with asbestosis or pleural plaques. Rev Mal Respir 28:e11–17
- Ameille J, Letourneux M. (1999). Nonmalignant asbestos-related diseases [Article in French]. Rev Mal Respir 16:S25–33
- American Thoracic Society (ATS). (2004). Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 170:691–715
- Antao VC, Larson TC, Horton DK. (2012). Libby vermiculite exposure and risk of developing asbestos-related lung and pleural diseases. Curr Opin Pulm Med 18:161–7
- Attanoos RL, Gibbs AR. (2009). An approach to industrial post-mortems. Histopath 54:134–42
- Banks D, Dedhia H. (2011). Chapter 110: the health risks of asbestos exposure inhalation. In: Jindal S, Shankar PS, Raoof S, et al. (eds.) Textbook of pulmonary and critical care medicine. Vol. 1 and 2. New Delhi: Jaypee Brothers Medical Publishers P(Ltd), 1351–66
- Banks DE, Shi R, McLarty J, et al. (2009). American College of Chest Physicians consensus statement on the respiratory health effects of asbestos: results of a Delphi study. Chest 135:1619–27
- Barbot R, Marquand C Le, Pairon J-C, et al. (2012). Do pleural plaques carry an excess of risk of lung cancer in asbestos-exposed workers? European Society of Radiology ECR Congress 2012, Poster B-0026. Available from: http://posterng.netkey.at/esr/viewing/index.php?module=viewing_poster&pi=110571 [last accessed 18 Feb 2015]
- Bar-Shai A, Tiran B, Topilsky M, et al. (2012). Continued progression of asbestos-related respiratory disease after more than 15 years of non-exposure. IMAJ 14:560–5
- Becklake M, Bagatin E, Neder J. (2007). Asbestos-related diseases of the lungs and pleura: uses, trends and management over the last century. Int J Tuberc Lung Dis 11:356–69
- Bianchi C, Brollo A, Ramani L, Zuch C. (1997). Pleural plaques as risk indicators for malignant pleural mesothelioma: a necropsy-based study. Am J Ind Med 32:445–9
- Boffetta P. (1998). Health effects of asbestos exposure in humans: a quantitative assessment. Med Lav 89:471–80
- Boffetta P, Donaldson K, Moolgavkar S, Mandel JS. (2014). A systematic review of occupational exposure to synthetic vitreous fibers and mesothelioma. Crit Rev Toxicol 44:436–49
- Brims FJH, Davies HE, Lee YCG. (2010). Respiratory chest pain: diagnosis and treatment. Med Clin North Am 94:217–32
- British Thoracic Society. (2011). Pleural plaques information for health care professionals. London, UK: British Thoracic Society, 11 pp. Available from: https://www.brit-thoracic.org.uk/document-library/clinical-information/mesothelioma/pleural-plaques-information-for-patients/ [last accessed 18 Feb 2015]
- Broderick A, Fuortes LJ, Merchant JA, et al. (1992). Pleural determinants of restrictive lung function and respiratory symptoms in an asbestos-exposed population. Chest 101:684–91
- Brodkin CA, Barnhart S, Anderson G, et al. (1993). Correlation between respiratory symptoms and pulmonary function in asbestos-exposed workers. Am Rev Respir Dis 148:32–7
- Brotman DJ, Walker E, Lauer MS, O’Brien RG. (2005). In search of fewer independent risk factors. Arch Intern Med 165:138–45
- Chapman S, Robinson G, Stradling J, et al. (eds.) (2014). Chapter 17: Asbestos and the lung. In: Oxford handbook of respiratory medicine. Oxford: Oxford University Press, 111–24
- Chapman SJ, Cookson WOC, Musk AW, Lee YCG. (2003). Benign asbestos pleural diseases. Curr Opin Pulm Med 9:266–71
- Christen B, Wegmann W, Vogt P. (1997). Clinical pathology and histology of pleural plaques. Indoor Built Environ 6:79–85
- Clark KA, Flynn JJ, Goodman JE, et al. (2014). Pleural plaques and their effect on lung function in Libby Vermiculite Miners. Chest 146:786–94
- Clarke CC, Mowat FS, Kelsh MA, Roberts MA. (2006). Pleural plaques : a review of diagnostic issues and possible nonasbestos factors. Arch Environ Occup Health 61:183–92
- Clin B, Paris C, Ameille J, et al. (2011). Do asbestos-related pleural plaques on HRCT scans cause restrictive impairment in the absence of pulmonary fibrosis? Thorax 66:985–91
- Collins J, Stern E. (2015). Chest radiology – the essentials. 3rd ed. Philadelphia: Wolters Kluwer Health, 384
- Copley SJ, Wells AU, Rubens MB, et al. (2001). Functional consequences of pleural disease evaluated with chest radiography and CT. Radiology 220:237–43
- Cramond T, Casserly M. (2006). Pain and asbestos related pleural plaques. Pain Med 7:467–8
- Crane M, Alain P, Letourneux M. (2005). Chapter 7: pleural diseases. In: Kusaka Y, Hering K, Parker J. (eds.) International classification of HRCT for occupational and environmental respiratory diseases. Tokyo: Springer Verlag, 73–92
- Crapo J. (2005). A fair and efficient system to resolve claims of victims for bodily injury caused by asbestos, and for other purposes. Written testimony (Dated April 23, 2005, 5 pp) for the U.S. Senate, 109th Congress Report, 1st Session, The Fairness In Asbestos Injury Resolution Act Of 2005, U. S. Government Printing Office, June 30, 2005. Washington, DC. Available from: http://www.judiciary.senate.gov/imo/media/doc/Crapo%20Testimony%20042605.pdf [last accessed 2 Apr 2015]
- Cugell DW, Kamp DW. (2004). Asbestos and the pleura. Chest 125:1103–17
- Cullen MR, Barnett MJ, Balmes JR, et al. (2005). Predictors of lung cancer among asbestos-exposed men in the β-carotene and retinol efficacy trial. Am J Epidemiol 161:260–70
- Cvitanović S, Znaor L, Konsa T, et al. (2003). Malignant and non-malignant asbestos-related pleural and lung disease: 10-year follow-up study. Croat Med J 44:618–25
- Dalphin J-C. (2011). Follow-up of subjects occupationally exposed to asbestos, what are the objectives, the benefits, and the possible risks? [Article in French]. Rev Mal Respir 28:1230–40
- Donaldson K, Murphy F, Duffin R, Poland C. (2010). Asbestos, carbon nanotubes and the pleural mesothelium: a review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol 7:5
- Dweik R, Mazzone P. (2010). Occupational lung disease. Cleveland Clinic Website. Available from: http://www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/Pulmonary/occupational-lung-disease/ [last accessed 10 Feb 2015]
- Edelman DA. (1988). Review article asbestos exposure, pleural plaques and the risk of lung cancer. Int Arch Occup Ind Health 60:389–93
- Edge JR. (1976). Asbestos related disease in Barrow-in-Furness. Environ Res 11:244–7
- Ehrlich R, Lilis R, Chan E, et al. (1992). Long term radiological effects of short term exposure to amosite asbestos among factory workers. Br J Ind Med 49:268–75
- Eisenhawer C, Felten MK, Tamm M, et al. (2014). Radiological surveillance of formerly asbestos-exposed power industry workers: rates and risk factors of benign changes on chest X-ray and MDCT. J Occup Med Toxicol 9:18
- Epstein DM. (1984). Pleural plaques: a marker for respiratory tract malignancy? Chest 86:660–1
- Evans A, Gleeson F. (2004). Radiology in pleural disease: state of the art. Respirology 9:300–12
- Finkelstein MM, Vingilis JJ. (1984). Radiographic abnormalities among asbestos-cement workers. An exposure-response study. Am Rev Respir Dis 129:17–22
- Fishwick D, Barber CM. (2014). Non-malignant asbestos-related diseases: a clinical view. Clin Med J R Coll Physicians London 14:68–71
- Fletcher DE. (1972). A mortality study of shipyard workers with pleural plaques. Br J Ind Med 29:142–5
- Gefter WB, Conant EF. (1988). Issues and controversies in the plain-film diagnosis of asbestos-related disorders in the chest. J Thorac Imaging 3:11–28
- Gevenois P, De Maertelaer V, Madani A, et al. (1998). Asbestosis, pleural plaques and diffuse pleural thickening: three distinct benign responses to asbestos exposure. Eur Respir J 11:1021–7
- Gevenois P, de Vuyst P. (2006). Chapter 8: non-malignant asbestos-related pleural disorders. In: Gevenois P, de Vuyst P. (eds.) Imaging of occupational and environmental disorders of the chest. Berlin: Springer-Verlag, 223–38
- Goldberg M. (2005). Are lung and pleural benign asbestos induced diseases a preliminary step in the pathogenic process of mesothelioma and lung cancer development? Occup Environ Med 62:663–4
- Greim H, Utell MJ, Maxim LD, Niebo R. (2014). Perspectives on refractory ceramic fiber (RCF) carcinogenicity: comparisons with other fibers. Inhal Toxicol 8378:789–810
- Harber P, Mohsenifar Z, Oren A, Lew M. (1987). Pleural plaques and asbestos-associated malignancy. J Occup Med 29:641–4
- Hillerdal G. (1981). Non-malignant asbestos pleural disease. Thorax 36:669–75
- Hillerdal G. (1994). Pleural plaques and risk for bronchial carcinoma and mesothelioma. A prospective study. Chest 105:144–50
- Hillerdal G. (1997). Pleural plaques: incidence and epidemiology, exposed workers and the general population a review. Indoor Built Environ 6:86–95
- Hillerdal G. (2001). Radiological changes as markers of environmental exposure and environmental risk of lung cancer and mesothelioma. USEPA 2001 Asbestos Health Effects Conference, 2001 May 1–10
- Hillerdal G, Henderson D. (1997). Asbestos, asbestosis, pleural plaques and lung cancer. Scand J Work Environ Health 23:93–103
- Hilt B, Lien JT, Lund-Larsen PG. (1987). Lung function and respiratory symptoms in subjects with asbestos-related disorders: a cross-sectional study. Am J Ind Med 11:517–28
- Hosmer D, Lemeshow S. (2000). Applied logistic regression. 2nd ed. New York: John Wiley & Sons, Inc., 383 p
- Hosoda Y, Hiraga Y, Sasagawa S. (2008). Railways and Asbestos in Japan (1928–1987) – epidemiology of pleural plaques, malignancies and Pneumoconioses. J Occup Health 50:297–307
- Hourihane DO, Lessof L, Richardson PC. (1966). Hyaline and calcified pleural plaques as an index of exposure to asbestos: a study of radiological and pathological features of 100 cases with a consideration of epidemiology. Br Med J 1:1069–74
- Hughes JM, Weill H. (1991). Asbestosis as a precursor of asbestos related lung cancer: results of a prospective mortality study. Occup Environ Med 48:229–33
- Huuskonen M, Tossavainen A. (1996). Health effects associated with wollastonite exposure. J Occup Health Saf Aust NZ 12:357–60
- Huuskonen MS, Jarvisalo J, Koskinen H, et al. (1983). Preliminary results from a cohort of workers exposed to wollastonite in a Finnish limestone quarry. Scand J Work Environ Health 9:169–75
- Industrial Injuries Advisory Council (IIAC). (2008). Position Paper 23 – Pleural plaques. London, UK: IIAC. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/328553/iiac-pp23.pdf [last accessed 18 Feb 2015]
- Jakobsson K. (1993). Colorectal cancer and non-malignant respiratory disease in asbestos cement and cement workers. Studies on mortality, cancer morbidity, and radiographical changes in lung parenchyma and pleura. Thesis, Department of Occupational and Environmental Medicine, University of Lund, Lund, Sweden, 59 p
- Jakobsson K, Strömberg U, Albin M, et al. (1995). Radiological changes in asbestos-cement workers. Occup Environ Med 52:20–7
- Järvholm B. (1992). Pleural plaques and exposure to asbestos: a mathematical model. Int J Epidemiol 21:1180–4
- Järvholm B, Hillerdal G, Jarliden K, et al. (1995). Occurrence of pleural plaques in workers with exposure to mineral wool. Int Arch Occup Environ Health 67:343–6
- Järvholm B, Larsson S. (1988). Do pleural plaques produce symptoms? A brief report. J Occup Med 30:345–7
- Järvholm B, Sandén A. (1987). Estimating asbestos exposure: a comparison of methods. J Occup Med 29:361–3
- Jeebun V, Stenton SC. (2012). The presentation and natural history of asbestos-induced diffuse pleural thickening. Occup Med 62:266–8
- Jones R. (1997). Pleural plaques: diagnostic problems and significance. Indoor Built Environ 6:106–13
- Jones RN, McLoud T, Rockoff SD. (1988). The radiographic pleural abnormalities in asbestos exposure: relationship to physiologic abnormalities. J Thorac Imaging 3:57–66
- Jones RN, Hughes JM, Weill H. (1996). Asbestos exposure, asbestosis, and asbestos-attributable lung cancer. Thorax 51:S9–15
- Kamp DW. (2009). Asbestos-induced lung diseases: an update. Transl Res 153:143–52
- Karjalainen A, Karhunen PJ, Lalu K, et al. (1994). Pleural plaques and exposure to mineral fibres in a male urban necropsy population. Occup Environ Med 51:456–60
- Karjalainen A, Pukkala E, Kauppinen T, Partanen T. (1999). Incidence of cancer among Finnish patients with asbestos-related pulmonary or pleural fibrosis. Cancer Causes Control 10:51–7
- Kerper LE, Lynch HN, Zu K, et al. (2014). Systematic review of pleural plaques and lung function. Inhal Toxicol 27:15–24
- Kiviluoto R, Meurman LO, Hakama M. (1979). Pleural plaques and neoplasia in Finland. Ann N Y Acad Sci 330:31–3
- Kleinbaum DG, Klein M. (2002). Logistic regression. 2nd ed. New York: Springer, 523 p
- Kopylev L, Christensen K, Brown J, Cooper G. (2014). A systematic review of the association between pleural plaques and changes in lung function. Occup Environ Med . [Epub ahead of print]. doi: 10.1136/oemed-2014-102468
- Koskinen HO, Nordman HL, Zitting AJ, et al. (1997). Fibrosis of the lung and pleura and long-term exposure to wollastonite. Scand J Work Environ Health 23:41–7
- Koskinen K, Pukkala E, Martikainen R, et al. (2002). Different measures of asbestos exposure in estimating risk of lung cancer and mesothelioma among construction workers. JOEM 44:1190–6
- Kratzke R. (2013). Detection of mesothelioma remains a conundrum [sic]. J Natl Cancer Inst 105:254–5
- Larson TC, Franzblau A, Lewin M, et al. (2014). Impact of body mass index on the detection of radiographic localized pleural thickening. Acad Radiol 21:3–10
- Larson TC, Meyer CA, Kapil V, et al. (2010). Workers with Libby amphibole exposure: retrospective identification and progression of radiographic changes. Radiology 255:924–33
- LeMasters GK, Lockey JE, Yiin JH, et al. (2003). Mortality of workers occupationally exposed to refractory ceramic fibers. JOEM 45:440–50
- Letourneux M. (1999). Risk assessment of benign asbestosis (dose-effect relationship, time-effect relationship, co-factors) [Article in French]. Rev Mal Respir 16:1270–7
- Lewinsohn HC. (1974). Early malignant changes in pleural plaques due to asbestos exposure: a case report. Br J Dis Chest 68:121–7
- Liddell FD, McDonald JC. (1980). Radiological findings as predictors of mortality in Quebec asbestos workers. Br J Ind Med 37:257–67
- Lilis R, Miller A, Godbold J, et al. (1991). The effect of asbestos-induced pleural fibrosis on pulmonary function: quantitative evaluation. Ann NY Acad Sci 643:162–8
- Lockey J, Lemasters G, Rice C, et al. (1996). Refractory ceramic fiber exposure and pleural plaques. Am J Respir Crit Care Med 154:1405–10
- Lockey JE, Dunning K, Hilbert TJ, et al. (2015). HRCT/CT and associated spirometric effects of low libby amphibole asbestos exposure. J Occup Environ Med 57:6–13
- Lotti M. (2010). Asbestos-related lung cancer [Article in Italian]. G Ital Med Lav Ergon 32:381–4
- Lynch DA, Gamsu G, Aberle DR. (1989). Conventional and high resolution computed tomography in the diagnosis of asbestos-related diseases. Radiographics 9:523–51
- Mastrangelo G, Ballarin MN, Bellini E, et al. (2009). Asbestos exposure and benign asbestos diseases in 772 formerly exposed workers: dose-response relationships. Am J Ind Med 52:596–602
- Maxim LD, McConnell EE. (2005). A review of the toxicology and epidemiology of wollastonite. Inhal Toxicol 17:451–66
- Maxim LD, Niebo R, Utell MJ, et al. (2014). Wollastonite toxicity: an update. Inhal Toxicol 26:95–112
- McDonald JC, Sebastien P, Armstrong B. (1986). Radiological survey of past and present vermiculite miners exposed to tremolite. Br J Ind Med 43:445–9
- McLoud TC, Woods BO, Carrington CB, et al. (1985). Diffuse pleural thickening in an asbestos-exposed population: prevalence and causes. Am J Roentgen 144:9–18
- Meirelles GSP, Kavakama JI, Jasinowodolinski D, et al. (2005). Asbestos-related pleural plaques: a literature review [Article in Portuguese]. Rev Portuguesa Pneumol 11:487–97
- Miles SE, Sandrini A, Johnson AR, Yates DH. (2008). Clinical consequences of asbestos-related diffuse pleural thickening: a review. J Occup Med Toxicol 3:20
- Miller A, Lilis R, Godbold J, et al. (1994). Spirometric impairments in long-term insulators: relationships to duration of exposure, smoking, and radiographic abnormalities. Chest 105:175–82
- Miller BH, Rosado-de-Christenson ML, Mason AC, et al. (1996). From the archives of the AFIP. Malignant pleural mesothelioma: radiologic-pathologic correlation. Radiographics 16:613–44
- Mohr L. (2012). Clinical background information and comments on recent scientific publications and the draft EPA report (August 2011) pertaining to libby amphibole asbestos, a report submitted to the Scientific Advisory Board, United States Environmental Protection Agency Medical University of South Carolina, Charleston, SC, 55 pp
- Mollo F, Andrion A, Colombo A, et al. (1984). Pleural plaques and risk of cancer in Turin, Northwestern Italy. An autopsy study. Cancer 54:1418–22
- Montes I, Abu Shams K, Alday E, et al. (2005). Guidelines on asbestos-related pleuropulmonary disease. Arch Bronconeumol 41:153–68
- Moolgavkar SH, Anderson EL, Chang ET, et al. (2014). A review and critique of U.S. EPA’s risk assessments for asbestos. Crit Rev Toxicol 44:499–522
- Mukherjee S, De Klerk N, Palmer LJ, et al. (2000). Chest pain in asbestos-exposed individuals with benign pleural and parenchymal disease. Am J Respir Crit Care Med 162:1807–11
- Myers R. (2012). Asbestos-related pleural disease. Curr Opin Pulm Med 18:377–81
- Neri S, Antonelli A, Falaschi F, et al. (1994). Findings from high resolution computed tomography of the lung and pleura of symptom free workers exposed to amosite who had normal chest radiographs and pulmonary function tests. Occup Environ Med 51:239–43
- Nielsen LS, Bælum J, Rasmussen J, et al. (2014). Occupational asbestos exposure and lung cancer – a systematic review of the literature. Arch Environ Occup Health 69:191–206
- Nishimura SL, Broaddus VC. (1998). Asbestos-induced pleural disease. Clin Chest Med 19:311–29
- Nurminen M, Tossavainen A. (1994a). Is there an association between pleural plaques and lung cancer without asbestosis? Scand J Work Environ Health 20:62–4
- Nurminen M, Tossavainen A. (1994b). Asbestos-related pleural plaques and lung cancer. Chest 106:648–9
- O’Reilley K, Mclaughlin A, Beckett W, Sime P. (2007). Asbestos-related lung disease. Am Fam Physician 75:683–8
- Ohlson CG, Bodin L, Rydman T, Hogstedt C. (1985). Ventilatory decrements in former asbestos cement workers: a four year follow up. Occup Environ Med 42:612–16
- Oliver LC, Eisen EA, Greene R, Sprince NL. (1988). Asbestos-related pleural plaques and lung function. Am J Ind Med 14:649–56
- Pairon J, Andujar P, Rinaldo M, et al. (2014). Asbestos exposure, pleural plaques, and the risk of death from lung cancer. Am J Respir Crit Care Med 190:1413–20
- Pairon J, Laurent F, Jacques A. (2013a). Pleural plaques and the risk of lung cancer in a French cohort of asbestos-exposed subjects. Eur Respir J 42(S57):1910
- Pairon J, Laurent F, Rinaldo M, et al. (2013b). Pleural plaques and the risk of pleural mesothelioma. J Natl Cancer Inst 105:293–301
- Paris C, Martin A, Letourneux M, Wild P. (2008). Modelling prevalence and incidence of fibrosis and pleural plaques in asbestos-exposed populations for screening and follow-up: a cross-sectional study. Environ Health 7:30
- Paris C, Thierry S, Brochard P, et al. (2009). Pleural plaques and asbestosis: dose- and time-response relationships based on HRCT data. Eur Respir J 34:72–9
- Park EK, Thomas PS, Wilson D, et al. (2011). Chest pain in asbestos and silica-exposed workers. Occup Med 61:178–83
- Partanen T, Nurminen M, Zitting A, et al. (1992). Localized pleural plaques and lung cancer. Am J Ind Med 22:185–92
- Reid A, de Klerk N, Ambrosini G, et al. (2005). The additional risk of malignant mesothelioma in former workers and residents of Wittenoom with benign pleural disease or asbestosis. Occup Environ Med 62:665–9
- Rey F, Boutin C, Steinbauer J, et al. (1993). Environmental pleural plaques in an asbestos exposed population of northeast Corsica. Eur Respir J 6:978–82
- Roach HD, Davies GJ, Attanoos R, et al. (2002). Asbestos: when the dust settles – an imaging review of asbestos-related disease. Radiographics 22:S167–84
- Roberts GH. (1971). The pathology of parietal pleural plaques. J Clin Path 24:348–53
- Robinson BW, Musk AW. (1981). Benign asbestos pleural effusion: diagnosis and course. Thorax 36:896–900
- Rohs AM, Lockey JE, Dunning KK, et al. (2008). Low-level fiber-induced radiographic changes caused by Libby vermiculite: a 25-year follow-up study. Am J Respir Crit Care Med 177:630–7
- Rom WN, Lockey JE. (1982). Diffuse malignant mesothelioma: a review. Western J Med 137:548–54
- Rubin AH. (1986). Common problems in asbestos-related pulmonary diseases. Am J Ind Med 10:555–62
- Rudd RM. (1996). New developments in asbestos-related pleural disease. Thorax 51:210–16
- Sandén A, Järvholm B. (1986). Pleural plaques, respiratory symptoms and respiratory function in shipyard workers exposed to man-made mineral fibres. Occup Med 36:86–9
- Sandén A, Järvholm B. (1991). A study of possible predictors of mesothelioma in shipyard workers exposed to asbestos. J Occup Med 33:770–3
- Sandén A, Järvholm B, Larsson S, Thiringer G. (1992). The risk of lung cancer and mesothelioma after cessation of asbestos exposure: a prospective cohort study of shipyard workers. Eur Respir J 5:281–5
- Sargent EN, Jacobson G, Gordonson JS. (1977). Pleural plaques: a signpost of asbestos dust inhalation. Semin Roentgenol 12:287–97
- Sargent EN, Gordonson J, Jacobson G, et al. (1978). Bilateral pleural thickening: a manifestation of asbestos dust exposure. Am J Roentgenol 131:579–85
- Schaer B, Michel Y. (2012). Pleural plaques, when and how to treat? [Article in French]. Rev Mal Suisse 26:1826–30
- Scherpereel A, Astoul P, Baas P, et al. (2010). Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 35:479–95
- Schwartz A. (1994). Chapter 4: the pathology of environmental lung disease. In: Witorsch P, Spagnolo S. (eds.) Air pollution and lung disease in adults. Boca Raton, FL: CRC Press, 97–128
- Sheers G. (1979). Asbestos-associated disease in employees of Devonport Dockyard. Ann N Y Acad Sci 330:281–7
- Shepherd JR, Hillerdal G, McLarty J. (1997). Progression of pleural and parenchymal disease on chest radiographs of workers exposed to amosite asbestos. Occup Environ Med 54:410–15
- Smith DD. (1994). Plaques, cancer, and confusion. Chest 105:8–9
- Sood A, Gee J. (1997). Asbestos-related pleural plaques and diffuse pleural thickening: functional consequences. Indoor Built Environ 6:114–18
- Soulat JM, Lauque D, Esquirol Y, et al. (1999). High-resolution computed tomography abnormalities in ex-insulators annually exposed to asbestos dust. Am J Ind Med 36:593–601
- Suganuma N, Kusaka Y, Hiraga Y, et al. (2001). Asbestos-related pleural abnormalities detected by chest X-ray: fair agreement with detection by computed tomography. J Occup Health 43:365–70
- Thiringer G, Blomqvist N, Brolin I, Mattson SB. (1980). Pleural plaques in chest X-rays of lung cancer patients and matched controls (preliminary results). Eur J Respir Dis Suppl 107:119–22
- Thomas JD, Mohammed O, Slaughter RE, et al. (2011). Relationships between pleural plaques, lung asbestos content and asbestos exposure in Australians with or at risk of lung cancer. J Thoracic Oncol 6:S9–10
- Tossavainen A. (1997). Asbestos, asbestosis, and cancer: the Helsinki criteria for diagnosis and attribution. Scand J Work Environ Health 23:311–16
- US Environmental Protection Agency (EPA). (2014). Toxicological review of Libby amphibole asbestos, EPA/635/R-11/002F. Washington, DC: US EPA, 685. Available from: http://www.epa.gov/iris/toxreviews/1026tr.pdf [last accessed 10 Feb 2015]
- Utell MJ, Maxim LD. (2010). Refractory ceramic fiber (RCF) toxicity and epidemiology: a review. Inhal Toxicol 22:500–21
- Van Cleemput J, de Raeve H, Verschakelen J, et al. (2001). Surface of localized pleural plaques quantitated by computed tomography scanning: no relation with cumulative asbestos exposure and no effect on lung function. Am J Respir Crit Care Med 163:705–10
- van Gelder T, Hoogsteden HC, Versnel MA, et al. (1989). Malignant pleural mesothelioma in the southwestern part of The Netherlands. Eur Respir J 2:981–4
- Wain SL, Roggli VL, Foster WL. (1984). Parietal pleural plaques, asbestos bodies, and neoplasia. A clinical, pathologic, and roentgenographic correlation of 25 consecutive cases. Chest 86:707–13
- Walker CM, Takasugi JE, Chung JH, et al. (2012). Tumorlike conditions of the pleura. Radiographics 32:971–85
- Wang XR, Yano E, Wang M, et al. (2001). Pulmonary function in long-term asbestos workers in China. J Occup Environ Med 43:623–9
- Weill D, Dhillon G, Freyder L, et al. (2011). Lung function, radiological changes and exposure: analysis of ATSDR data from Libby, MT, USA. Eur Respir J 38:376–83
- Weill D, Weill H. (2005). Diagnosis and initial management of nonmalignant diseases related to asbestos. Am J Respir Crit Care Med 171:527–30
- Weiss W. (1993). Asbestos-related pleural plaques and lung cancer. Chest 103:1854–9
- Weiss W. (1999). Asbestosis: a marker for the increased risk of lung cancer among workers exposed to asbestos. Chest 115:536–49
- Welch LS, Haile E, Dement J, Michaels D. (2007). Change in prevalence of asbestos-related disease among sheet metal workers 1986 to 2004. Chest 131:863–9
- Wilken D, Velasco Garrido M, Manuwald U, Baur X. (2011). Lung function in asbestos-exposed workers, a systematic review and meta-analysis. J Occup Med Toxicol 6:21. Available from: http://www.occup-med.com/content/6/1/21 [last accessed 18 Feb 2015]
- WorkSafe BC. (2009). Bronchogenic carcinoma (lung cancer) in asbestos-exposed workers. Vancouver, BC: WorkSafeBC, 178. Available from: http://www.worksafebc.com/regulation_and_policy/archived_information/policy_discussion_papers/pdf/bronchogeniccarcinomaaApendixB.pdf [last accessed 15 Feb 2015]
- Zu K, Ge T, Kerper L, et al. (2015). Evaluation of US EPA’s Noncancer Risk Assessment of Libby Amphibole Asbestos. Poster presentation #653 (abstract #770) at Society of Toxicology (SOT) 54th Annual Meeting and ToxExpo, 2015 March 22–26, San Diego, CA. Available from: http://www.toxicology.org/AI/Pub/Prog/2015Program.pdf [last accessed 23 Mar 2015]