Abstract
Purpose: To describe a case of cytomegalovirus (CMV) viremia and colitis in a patient on mycophenolate mofetil (MMF) monotherapy for birdshot chorioretinopathy.
Design: Case report.
Methods: Retrospective chart review.
Results: Treatment with MMF 1.5 g twice daily for 5 years led to leucopenia and a CD4 count of 299, which resulted in active CMV infection.
Conclusions: Treatment with MMF alone may put otherwise immune-competent individuals at risk for opportunistic CMV infection. Greater awareness of this association may allow for better monitoring, earlier detection, and treatment of future cases.
Cytomegalovirus (CMV) sero-positivity is present in 50–100% of adults worldwide.Citation1 In healthy individuals, primary infection is most often asymptomatic, but when manifest causes an infectious mononucleosis-like illness with fever, atypical lymphocytosis, and hepatomegaly/hepatitis, all of which usually resolve without sequelae. Afterward, the virus remains latent within leukocytes. Should the host become immunosuppressed, reactivation may occur. The three most commonly affected groups include patients with AIDS, people with allogeneic bone marrow transplants, and recipients of solid organ transplants (heart, liver, kidney). In all of these settings, CMV can produce serious, life-threatening disease by affecting the lungs, retina, and gastrointestinal tract.
Mycophenolate mofetil (MMF) is an effective corticosteroid-sparing agent in the management of birdshot chorioretinopathy.Citation2 Over the last decade, some studies have indicated that patients treated with MMF are particularly susceptible to cytomegalovirus (CMV) infection; these reports are most prevalent within the organ transplant literature.Citation3–5 Though this association seems likely, the picture is confounded because all transplant patients are on other immunosuppressives (i.e., cyclosporine, azathioprine, tacrolimus, or corticosteroids) in addition to MMF. There is one case report of CMV colitis in the setting of recent Salmonella infection in a patient with Wegener’s granulomatosis treated with MMF and low-dose prednisone.Citation6 However, to our knowledge, there are no reports in the literature of active CMV infection in the setting of MMF therapy used alone to treat ocular inflammation. We present an unusual case of CMV colitis in a patient on mycophenolate mofetil monotherapy for birdshot chorioretinopathy.
CASE
In January 2002, a 34-year-old Caucasian male presented to his ophthalmologist with progressively blurry vision in the left eye over 1 week. The exam was significant for vitritis bilaterally, optic disc swelling, and peripapillary hemorrhage consistent with a diagnosis of idiopathic posterior uveitis. The patient was started on oral prednisone 80 mg per day, which was subsequently tapered to 10 mg over 18 months. Optic disc swelling never completely resolved and by August 2003 the patient had mild changes in visual field testing. Given these findings, a decision was made to increase the prednisone to 60 mg daily and start MMF therapy at 1 g twice daily. By January 2004, he had been tapered to only 10 mg of oral prednisone daily and disc edema had completely resolved, but rare cells were found in the vitreous of both eyes. For this, his MMF was increased to 1.5 g twice daily. Between January 2004 and April 2005, indirect ophthalmoscopy revealed an increasing number of creamy, ovoid spots in the periphery and along the vascular arcades. Although an HLA-A29 result was not available, findings were felt to be most consistent with birdshot chorioretinopathy (). He was maintained on MMF 1.5 g twice daily. His white blood cell (WBC) count remained consistently between 4400 and 5600 and aspartate amino-transferase (AST) and alanine amino-transferase (ALT) were always within normal limits. His prednisone had been tapered off completely by May 2005.
FIGURE 1 Fundus photographs taken in November 2010 showing classic white ovoid hypopigmented spots classic for birdshot retinopathy.
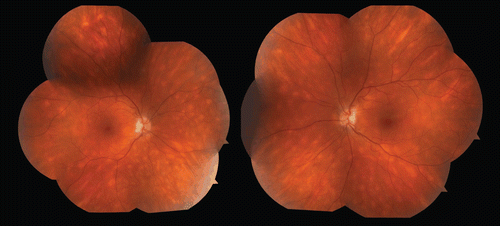
In April of 2010, nearly five years after starting MMF monotherapy 1.5 g twice daily, the patient presented to his primary care physician with severe abdominal pain and diarrhea. He also reported 2–3 months of increasing malaise, daily fevers, and a 22-lb (10-kg) weight loss. He had taken himself off MMF 1 week prior to presentation. He was treated empirically with ciprofloxacin and metronidazole for presumed Clostridium difficile infection, but noted no improvement 2 weeks later. He then presented to an outside emergency department with similar complaints. Temperature on admission was 39°C and exam was notable for oral thrush and mild right-upper- and left-upper-quadrant abdominal tenderness. Computed tomography (CT) of the abdomen and pelvis revealed inflammatory changes of the distal sigmoid and rectum consistent with colitis and he was empirically restarted on ciprofloxacin and metronidazole. Initial laboratory evaluation was significant for WBC count of 2.9, AST 74, ALT 145, and albumin of 3.1. Over the course of his hospital stay, his transaminitis worsened and infectious disease and gastroenterology services were consulted. Workup included a hepatitis panel, human immunodeficiency virus (HIV) test, Epstein-Barr virus (EBV) titer, and Clostridium difficile stool culture, all of which were negative, but his CD4 count was found to be 299. The MMF was discontinued, and a colon biopsy, performed via colonoscopy, was significant for “large intranuclear smudgy appearing inclusions highly suggestive of cytomegalovirus inclusions.” Immunostains were positive, thus confirming the diagnosis of active CMV infection within the colon. A peripherally inserted central catheter (PICC) line was placed and the patient was started on iv ganciclovir. The patient was discharged from the hospital shortly thereafter.
Two weeks after discharge, the patient was transitioned to valganciclovir 900 mg orally per day. Follow-up serum CMV DNA quantification by polymerase chain reaction (PCR) revealed 446 copies/µL, which increased to 690 copies/µL 2 weeks later. Valganciclovir was increased to 900 mg orally bid, and a third CMV DNA quantification was undetectable. Valganciclovir was stopped and MMF was restarted at 500 mg orally bid 1 week later.
On follow-up in November of 2010, the patient’s CD4 count was again noted to be depressed at 411. The MMF was discontinued and the patient has remained asymptomatic and without progression of his birdshot retinopathy since.
COMMENT
Our case is consistent with a finding the transplant literature already suggests—that MMF increases the risk for active CMV infection. Previous studies have supported this association only when MMF is used in combination with steroids or other steroid sparing agents. This example suggests that MMF by itself has the immunomodulatory power to allow for CMV reactivation colitis and viremia. If true, this finding has important implications for patients put on immunosuppressant monotherapy with MMF, such as those with birdshot chorioretinopathy, Behçet disease, sarcoidosis, Vogt-Koyanagi-Harada disease, or idiopathic chronic uveitis. Recommending routine CD4 counts based on a single case report would be unreasonable; however, the association between MMF therapy, CD4 suppression, and active CMV might prompt checking cell counts or CMV serologies on a case-by-case basis in the acutely ill patient, given the potential morbidity and mortality of systemic infection.
Active CMV is classically thought of as an opportunistic infection in HIV patients with CD4 counts under 50; however, our patient had a CD4 count of 299. We cannot conclude with certainty that the active CMV infection observed here was a direct result of the low CD4 count and not some other unknown immunologic defect. However, the discrepancy between the well-established threshold for suspected CMV infection and our patient’s cell count suggests slightly different pathogeneses. HIV may permit active CMV infection through direct depletion of T-helper cells, while MMF may allow for infection by specifically targeting activated T lymphocytes for apoptosis, thereby eliminating clones of cells responding to the CMV antigens.Citation7
It is unclear whether lower doses of MMF would also result in CD4 count suppression and active CMV infection. Additionally, generalizations cannot be drawn from a single case regarding the risk of active CMV on MMF therapy. The complication is rare and remains mostly unknown by ophthalmologists; greater awareness of the association between MMF and possible CMV infection may allow earlier detection and intervention in future cases.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease, 7th ed. Philadelphia: Elsevier Saunders; 2005.
- Kiss S, et al. Long-term follow-up of patients with birdshot retinochoroidopathy treated with corticosteroid-sparing systemic immunomodulatory therapy. Ophthalmology. 2005; 112(6): 1066–1071.
- Basic-Jukic N, et al. Does mycophenolate mofetil increase the incidence of cytomegalovirus disease compared with azathioprine after cadaveric kidney transplantation? Transplant Proc. 2005; 37(2): 850–851.
- Moreso F, et al. Incidence of leukopenia and cytomegalovirus disease in kidney transplants treated with mycophenolate mofetil combined with low cyclosporine and steroid doses. Clin Transplant. 1998; 12(3): 198–205.
- Sarmiento JM, et al. Mycophenolate mofetil increases cytomegalovirus invasive organ disease in renal transplant patients. Clin Transplant. 2000; 14(2): 136–138.
- Woywodt A, et al. Cytomegalovirus colitis during mycophenolate mofetil therapy for Wegener’s granulomatosis. Am J Nephrol. 2000; 20(6): 468–472.
- Allison AC, Eugui EM. Mycophenolate mofetil and its mechanisms of action. Immunopharmacology. 2000; 47(2-3): 85–118.
