Abstract
Purpose: To report a case of occlusive retinal vasculopathy following varicella zoster infection in an immunocompetent adult.
Design: Observational case report.
Methods: A patient with defective vision following chickenpox was evaluated with fluorescein angiography, spectral domain optical coherence tomography and fundus auto fluorescence.
Results: Fundus showed multiple cotton wool spots and a well-demarcated zone of retinal ischemia in the posterior pole with normal optic disc without any evidence of anterior or posterior uveitis. Fluorescein angiography, spectral domain optical coherence tomography and fundus auto fluorescence findings revealed occlusive vasculopathy as the cause of defective vision.
Conclusions: We report a hitherto undescribed case of purely occlusive vasculopathy following varicella zoster infection without features of vasculitis or anterior and posterior uveitis in an immunocompetent individual.
The two main stereotypical subgroups of herpetic uveitis are chronic anterior uveitis and acute herpetic retinitis.Citation1 The classic and the most common presentation of varicella zoster (VZ)-related retinopathy is acute retinal necrosis (ARN) in immunocompetent patients and progressive outer retinal necrosis (PORN) in immunocompromised patients. Atypical manifestations of ARN and the nonnecrotizing type of atypical posterior uveitis are being reported with increasing frequency. Reported retinal vascular involvement in VZV infection include vasculitis, transient retinal arteriolitis, occlusive vasculopathy and recurrent multiple branch retinal artery occlusions, all occurring in eyes with signs of inflammation.
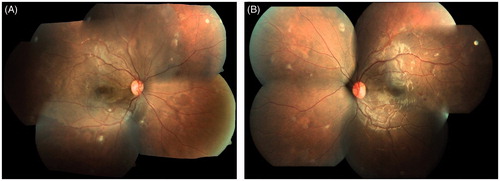
An 18-year-old female presented to us with defective vision, 3 months after chickenpox. Five days after the onset of cutaneous eruptions the patient developed unsteadiness and reportedly became disoriented. Magnetic resonance imaging showed pituitary microadenoma with hemorrhage. The rest of the brain study was normal. Visual evoked potential was also normal. With a diagnosis of post varizella zoster encephalitis and cerebellitis, she was given a course of intravenous methyl prednisolone and intravenous acyclovir by the neurologist. When she regained orientation the next day after iv acyclovir and methyl prednisolone she noticed defective vision. On her initial visit to us 3 months after the onset of chickenpox with a history of blurring of vision of both eyes she had healed chickenpox scars. On examination her best corrected visual acuity in the right eye was 3/60, N36 and left eye was 6/18, N8. Anterior segment and intraocular pressure were normal in both eyes. Dilated fundus examination of both eyes showed similar features (). The media was clear. There was a well-demarcated area of retinal whitening in the posterior pole in both eyes. There were multiple cotton wool spots all over the fundus. The optic disc and retinal vessels were normal. There were a few hemorrhages in the posterior pole in the left eye.
Figure 1. Fundus photographs showing well-demarcated area of retinal whitening in the posterior pole with multiple cotton wool spots in both eyes and retinal hemorrhage in the left eye.
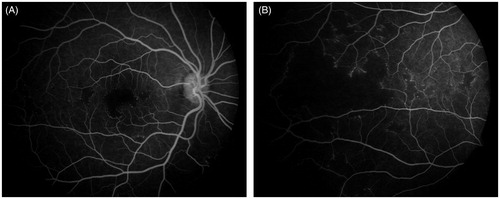
Interestingly fluorescein angiography features were also similar in both the eyes (). The foveal avascular zone was clearly increased and there were multiple areas of patchy capillary dropouts in the periphery and posterior pole with numerous micro aneurysms at their edges. Strikingly there was no staining or leakage from the retinal vessels at any phase of angiogram. The optic disc was also normal and there was no cystoid macular edema.
Figure 2. Fluorescein angiography pictures of right eye showing increased foveal avascular zone and multiple areas of patchy capillary dropouts in the periphery and posterior pole.
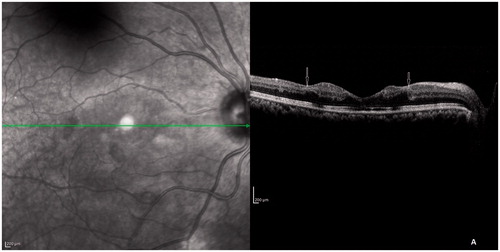
Spectral domain–optical coherence tomography scan of both eyes () through the fovea showed thickening of inner retinal layers corresponding to the area of capillary dropout around the fovea with shadowing of outer retinal layers due to the thickened inner layers. The external limiting membrane (ELM), inner segment–outer segment (IS-OS) junction and cone outer segment tip (COST) line were disrupted at the fovea. There was a clear demarcation line on OCT at the edges of capillary dropout around the fovea as seen on fluorescein angiography.
Figure 3. SD-OCT scan through fovea showing thickening of inner retinal layers and shadowing of outer retinal layers with a clear demarcation at the margin of widened foveal avascular zone (arrows). The ELM, IS-OS junction, and COST line were disrupted at the fovea.
Fundus autofluorescence of both eyes showed decreased autofluorescence at the macula, which was more pronounced in the left eye. This is due to the blockage of normal RPE autofluorescence by the thickened swollen inner retinal layers due to ischemia.Citation2 A thorough vasculitic workup (ANA, RF, dsDNA, Mantoux tests, VDRL, angiotensin converting enzyme, cANCA, pANCA, toxoplasma IgG and IgM) proved negative.
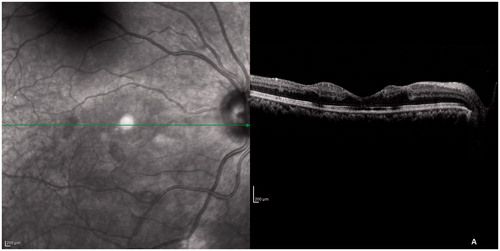
With a diagnosis of chickenpox-related retinal occlusive vasculopathy she was given oral acyclovir 600 mg 5 times daily for 2 weeks and then 400 mg 2 times daily for another 4 weeks. Oral prednisolone was started at 1 mg/kg body weight and was tapered and stopped over a period of 4 weeks. When she came to us after 6 weeks her BCVA in both eyes was 6/18, N6. The retinal whitening remained the same even after 6 weeks but the cotton wool spots had reduced in number (though not completely disappeared). FA was repeated then, which had the same features as the initial one. On SD-OCT (), inner retinal layers looked the same. However, the outer retinal layers (ELM, IS-OS junction, and the COST line) had started regaining their original structure. The improvement in VA is most probably due to this because the area of capillary nonperfusion on FA remained the same even after 6 weeks. After 5 months her BCVA in both eyes improved to 6/9, N6 and she had a near normal fundus except for mild residual retinal whitening at the posterior pole.
Figure 4. SD-OCT after 6 weeks. The ELM, IS-OS junction, and COST line started regaining their normal architecture but the inner retinal layers looked the same.
The hallmark of acute herpetic retinitis is full-thickness retinal necrosis. Though uncommon, there are reports of nonnecrotizing varicella-related retinopathies and vasculitis. Wickeremasinghe et al.Citation1 reported 4 patients with transient retinal arteriolitis occurring as a complication of typical chronic herpetic anterior uveitis. None of these patients had significant leakage or vascular occlusion on FA. PCR for VZ was positive in 2 of their patients. Bodhagi et al.Citation3 described 5 patients who were either steroid dependent or steroid resistant, with posterior uveitis along with “birdshot” like choroiditis or retinal vasculitis. None of these patients showed staining of vessels on FA. Three of these patients were PCR positive for VZ virus. In these patients use of antiviral drugs controlled the ocular inflammation and allowed tapering of corticosteroids. Kuo et al.Citation4 reported a case of retinal vasculitis 2 weeks following chickenpox who also had anterior uveitis and vitreous cells. FA of this patient showed staining of vessel without much leakage or occlusion. Wensing et al.Citation5 described 7 patients presenting with vitritis, vasculitis, and/or papillitis or as pan uveitis without any retinal lesions following VZV infection. Zamora et al.Citation6 had reported earlier, a case of multiple recurrent branch retinal artery occlusions and mild vitritis in a patient with PCR-confirmed varicella zoster infection. Monifer et al.Citation7 had reported choroiditis occurring as part of acute systemic varicella infection in a child. There are also other case reports of atypical variants of ARN and all of them had features of uveitis.Citation8–10
In contrast, in our patient multiple cotton wool spots all over the fundus and retinal whitening at the posterior pole were the characteristic features. She had no features of anterior or posterior uveitis. There was no evidence of vascular sheathing, disc edema, or cystoid macular edema. FA demonstrated capillary dropouts at the macula and periphery without any staining or leakage from the vessel wall. Unfortunately, the patient did not have an ophthalmic examination in the first 3 months post varizella zoster infection and it is possible that the patient might have had vasculitis initially. However, at presentation the patient did not have any evidence of old anterior or posterior uveitis (like pigmented keratic precipitates, posterior synechae, iris atrophy, pigments in the vitreous or fibrosis around vessel walls) and the angiogram did not show features of old vasculitis like staining or CME. We did not do PCR of ocular fluids for VZV but the temporal association of VZ infection and onset of defective vision and presence of healed chickenpox scars point to the etiology of VZV.
The exact mechanism of vascular occlusion remains speculative. When the patient was seen first (that is 3 months after chickenpox) she had retinal whitening, which remained the same even after 6 weeks, but the cotton wool spots had reduced in number (though not completely disappeared). When the patient was seen last after 5 months she had a near normal fundus except for mild residual retinal whitening at the posterior pole. The fluorescene angiography pictures were exactly same at the initial visit and after 6 weeks. In arterial occlusions (as in central retinal artery occlusions) the retinal whitening and FA pictures usually revert quickly. In our case the pathology may be different and a postviral immune-mediated reaction that produces longer lasting changes within the vessel wall may be responsible for the occlusive vasculopathy, which would have been present initially. In acute vascular occlusions reperfusion starts without prolonged delay and hence the whitening also disappears, but in our case changes in the vessel wall may be persisting and hence a low-grade ischemia and mild retinal whitening is lasting longer. The fluorescein angiography clearly shows the persistence of ischemia on repeat angiogram, which was done 6 weeks later. Bodhagi et al.Citation3 also suggested a similar mechanism for the vascular changes in post varicella zoster infection.
Here we report a case of chickenpox-related purely occlusive retinal vasculopathy without features of vasculitis, optic neuritis, anterior or posterior uveitis or cystoid macular edema. To the best of our knowledge nonnecrotizing purely occlusive vasculopathy without any other signs of ocular inflammation following chickenpox has not been reported earlier.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wickremasinghe SS, Stawell R, Lim L, et al. Non-necrotizing herpetic vasculitis (letter to the editor). Ophthalmology. 2009;116:361--362
- Mathew R, Papavasileiou E, Sivaprasad S. Autofluorescence and high definition optical coherence tomography of retinal artery occlusions. Clin Ophthalmol. 2010;4:1159–1163
- Bodaghi B, Rozenberg F, Cassoux N, et al. Nonnecrotizing herpetic retinopathies masquerading as severe posterior uveitis. Ophthalmology. 2003;110:1737–1743
- Kuo YH, Yip Y, Chen SN. Retinal vasculitis associated with chicken pox. Am J Ophthalmol. 2001;132:484–485
- Wensing B, Groot-Mijnes JD, Rothova A. Necrotising and nonnecrotising variants of herpetic uveitis with posterior segment involvement. Arch Ophthalmol. 2011;129:403–408
- Zamora RL, del Priore LV, Storch GA, et al. Multiple recurrent branch retinal artery occlusions associated with varicella zoster virus. Retina. 1996;16:399–404
- Monifer N, Wagner DG, Chrousos GA, et al. Paediatric varicella choroiditis. Br J Ophthalmol. 1998;82:1092–1093
- De Groot-Mijnes JDF, Rothova A, Van Loon AM, et al. Polymerase chain reaction and Goldmann-Witmer voefficient analysis arecomplimentary for diagnosis of infectious uveitis. Am J Ophthalmol. 2006;141:313–318
- Margolis R, Brasil OF, Lowder CY, et al. Multifocal posterior necrotising retinitis. Am J Ophthalmol. 2007;143:1003–1008
- Matsuo T, Nakayama T, Koyama T. A proposed mild type of retinal necrosis syndrome. Am J Ophthalmol. 1988;105:579–583

