Abstract
Background: Rituximab is a widely used biologic agent, which has shown favourable results in the treatment of vasculitis. But immunosuppressive treatment also bears the risk of severe complications.
Methods: A patient with rheumatoid arthritis, progressive scleromalacia, and acute retinal necrosis on therapy with rituximab is reported.
Results: For the first time, a correlation between rituximab and acute retinal necrosis in a patient with progressive rheumatoid scleromalacia is shown.
Conclusions: Although rituximab is a promising biologic agent for the treatment of autoimmune diseases, it bears the risk of reactivation of viral infections, including the onset of acute retinal necrosis.
Rituximab is a B-cell–depleting anti-CD20 monoclonal antibody that is increasingly used to treat autoimmune disorders and B-cell non-Hodgkin lymphoma. It has become an important therapeutic option for the treatment of vasculitic complications in rheumatoid arthritis and ocular inflammation such as refractory scleritis, ocular involvement in mucous membrane pemphigoid, uveitis, and peripheral ulcerative keratitis.Citation1,Citation2 As with other immunosuppressive agents, there is the risk of opportunistic infections or reactivations, including herpes viruses.Citation3,Citation4 Here we report a potential association between rituximab and necrotizing herpetic retinitis.
Clinical Case
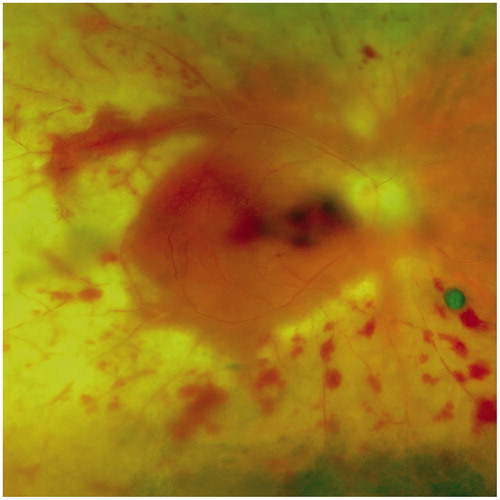
A presently 47-year-old female patient with diagnosis of rheumatoid arthritis since early adolescence has been suffering from rheumatoid nodules and erosive arthritis. Testing for rheumatoid factor and antinuclear antibodies was positive. At the age of 36, nodular scleritis developed in both eyes. Three years after onset, the recurrent scleritis became necrotizing in her right eye (). Posterior involvement with macular edema as manifestation of posterior scleritis in this eye led to visual impairment. At this stage, the patient consented to an immunosuppressive therapy: adalimumab (Humira) (40 mg subcutaneous every 2 weeks) and prednisone (initially 80 mg/24 h, then tapered) were applied for 48 months. Despite complete regression of macular edema and visual recovery (from 6/36 to 6/9), a progression of scleral destruction was noted (). A change of therapy from adalimumab to cyclophosphamide (Endoxan) (100 mg/24 h) and prednisone (15 mg/24 h), which was given for 38 months, allowed control of inflammation and prevented further destruction of the sclera. To avoid cyclophosphamide-related side effects, the therapy was modified again to rituximab (MabThera) one cycle every 6 months (2 times 1000 mg intravenous 2 weeks apart) in combination with prednisone (10 mg/24 h). Cyclophosphamide was slowly tapered simultaneously. Two years later, 4 months after cessation of cyclophosphamide and the fourth cycle of rituximab, the patient presented with a sudden loss of vision (6/60) in her affected right eye. At slit-lamp examination, a mild inflammation in the anterior chamber and vitreous cavity was noted. Fundus examination revealed hemorrhagic occlusive vasculitis and confluent whitish areas of necrosis involving the full circumference of the retina. The posterior pole showed macular hemorrhage. Based on these clinical findings (), the diagnosis of an acute retinal necrosis (ARN) was suspected and rituximab was stopped immediately. ARN and cytomegalovirus retinitis were in the initial differential. This led to an empiric intravenous therapy with ganciclovir (5 mg/kg 2 times/24 h). The patient had daily ophthalmologic examinations for 10 days while she was admitted to hospital.
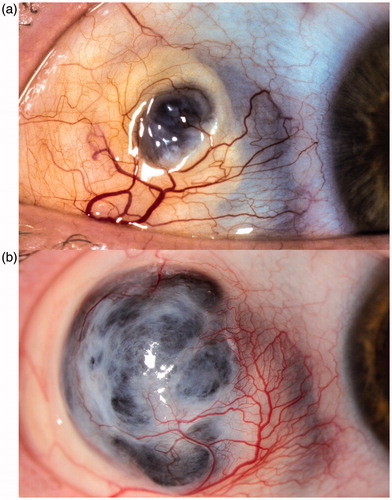
FIGURE 1. Scleromalacia located in the upper area of the temporal bulbar quadrant. (a). Note the loss of scleral tissue centrally and a change of transparency in the adjacent tissue, visible as a bluish discoloration. (b). The same eye is shown 8 years later with marked progression of the scleromalacia and a bulging staphyloma.
Diagnostics and Intervention
Laboratory investigations were unremarkable except for an isolated lymphopenia (absolute lymphocyte count of 0.27 × 109/L), which had already been noted 4 weeks before the acute ocular symptoms. An anterior chamber tap was performed and PCR testing of the anterior chamber fluid was positive for herpes simplex virus type 1 (HSV-1). To target HSV, the therapy was changed to intravenous aciclovir (10 mg/kg 3 times/24 h) and prednisone 60 mg/24 h. On this regimen the retinal necrosis showed no further progression and aciclovir was subsequently oralized (500 mg 5 times/24 h) and given for 8 weeks after the onset of ARN. Valaciclovir (500 mg 2 times/24 h) then replaced aciclovir for prevention of relapse. Prednisone was tapered to 7.5 mg/24 h during 8 months. No other immunosuppressive therapy was started. Nine months after the onset of ARN the scleromalacia appeared stable and the retina remained attached. No significant visual recovery was noted. The left eye stayed unaffected.
Discussion
This is the first report on acute retinal necrosis in an eye with scleromalacia. Scleromalacia describes necrotizing scleritis without clinical inflammation.Citation5 It is a manifestation of inflammatory small vessel disease, typically seen in patients with long-standing rheumatoid arthritis. A compromised immune system is a known risk factor for acute retinal necrosis.Citation6,Citation7 Therefore, patients with autoimmune disorders are at risk while on anti-inflammatory medication. Various immunosuppressive drugs have been linked with the reactivation of viral infections. They have also been reported for rituximab, including reactivations of polyomaviruses and viruses of the herpes group (cytomegalovirus and Epstein-Barr virus).Citation3,Citation4 But there is no previous report on reactivation of HSV-1 or on retinal necrosis.
The sequence of therapy change in our case strongly suggests the relation between rituximab and the onset of acute retinal necrosis due to HSV-1. It is also conceivable that the previous cyclophosphamide has put our patient additionally at risk for herpes reactivation.
Monitoring of hematologic parameters and clinical evaluation for infection are important for all immunosuppressive medications, including rituximab, a promising and relatively new biologic agent.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Patient consent: The patient has consented to the submission of the case report to the journal.
References
- Miserocchi E, Pontikaki I, Modorati G, et al. Anti-CD 20 monoclonal antibody (rituximab) treatment for inflammatory eye diseases. Autoimmun Rev. 2011;11:35–39
- Huerva V, Sanchez MC, Traveset A, et al. Rituximab for peripheral ulcerative keratitis with Wegener granulomatosis. Cornea. 2010;29:708–710
- Kroll JL, Beam C, Li S, et al. Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol. 2013;57:115–119
- Aksoy S, Harputluoglu H, Kilickap S, et al. Rituximab-related viral infections in lymphoma patients. Leuk Lymphoma. 2007;48:1307–1312
- Watson PG, Hayreh SS. Scleritis and episcleritis. Br J Ophthalmol. 1976;60:163–191
- Altamirano D, Rochat C, Claeys M, Herbort CP. Acute retinal necrosis: a result of immune dysfunction. Ophthalmologica. 1994;208:49–53
- Rochat C, Polla BS, Herbort CP. Immunological profiles in patients with acute retinal necrosis. Graefes Arch Clin Exp Ophthalmol. 1996;234:547–552