ABSTRACT
Purpose: To evaluate the safety and efficacy of gevokizumab for the treatment of Behçet’s disease uveitis in a prospective, open-label, randomized phase 2 trial.
Methods: Behçet’s disease patients with new acute ocular exacerbation or at risk of exacerbation received 30 or 60 mg gevokizumab every 4 weeks intravenously or subcutaneously, on top of a stable regimen of immunosuppressives and corticosteroids (≤20 mg/day equivalent prednisolone). Patients withdrew in cases of ocular exacerbation.
Results: A total of 21 patients were included (17 acute and 4 at-risk; mean duration of uveitis 45.6 ± 37.4 months). There were no serious adverse events related to gevokizumab. Recorded adverse events were mostly associated with exacerbation of uveitis or its complications. Response was evaluated for 14 acute patients and all showed rapid control of acute ocular exacerbation, mostly within 1 week, without any increase in corticosteroid dosage.
Conclusions: Gevokizumab was well tolerated and rapidly controlled acute ocular exacerbations of Behçet’s disease uveitis without the need for high-dose corticosteroid.
INTRODUCTION
Behçet’s disease is a multisystem inflammatory disorder of unknown etiology.Citation1,Citation2 The disease has a chronic course, which is characterized by recurrent acute exacerbations of manifestations including oral aphthous ulcers, genital ulcers, skin lesions, and uveitis, as well as lesions affecting the large vessels, gastrointestinal tract, and central nervous and respiratory systems. About half of patients have eye lesions, which usually appear after the onset of recurrent oral ulcers, but may constitute the first manifestation of Behçet’s disease in approximately 10% of cases. Ocular inflammation is characterized by a nongranulomatous panuveitis with retinal vasculitis and leads to blindness in approximately 25% of cases.Citation3 First-line therapy generally involves corticosteroids and immunosuppressive drugs such as azathioprine and cyclosporine, which have limitations with respect to tolerability and long-term efficacy. For patients refractory to conventional immunosuppressive therapy, there are no randomized controlled trials documenting the efficacy of any treatment, though interferon-alpha or anti–tumor necrosis factor (TNF) agents are recommended on the basis of favorable observations in case series.Citation4
Gevokizumab is a recombinant humanized allosteric monoclonal antibody that binds to human interleukin (IL)–1β and thereby inhibits the activation of IL-1 receptors.Citation5-Citation7 IL-1 is known to have potent proinflammatory properties, and its role is currently being investigated in a number of inflammatory diseases. In a proof-of-concept study, the safety and efficacy of gevokizumab was tested in seven Behçet’s disease patients with posterior uveitis or panuveitis and/or retinal vasculitis refractory to azathioprine and/or cyclosporine treatment.Citation8 In this study, a single infusion of gevokizumab (0.3 mg/kg) resulted in a rapid and sustained reduction in intraocular inflammation, improvement in visual acuity, and reduction in anterior chamber cells and flare, vitreous haze, and retinal infiltrates, despite discontinuation of immunosuppressive drugs and without any increase in corticosteroid dosage.Citation8
In view of these observations, we performed the phase 2 study described here, recruiting a larger population to evaluate the safety of three different fixed-dose regimens of gevokizumab on top of a stable regimen of immunosuppressive drugs and corticosteroids in Behçet’s disease patients with uveitis. This exploratory study also provided an opportunity to confirm the pharmacokinetics, as well as the clinical and biological activity, of gevokizumab in Behçet’s disease.
METHODS
Study Design
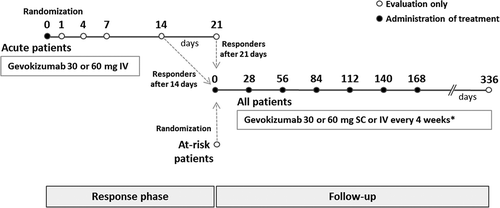
This prospective, open-label, randomized, parallel-group phase 2 trial was performed in seven centers in three countries, Turkey, South Korea, and Tunisia. The trial design () consisted of two periods. The initial period, a response phase, aimed to evaluate response to gevokizumab for up to 21 days in patients with a recent acute exacerbation of Behçet’s uveitis. Those who responded entered a follow-up period lasting until the next exacerbation or 336 days (follow-up beyond 168 days was optional). Patients that did not have acute exacerbation findings but were considered at risk of further exacerbation on the basis of retinal vascular leakage findings on fundus fluorescein angiography (FFA) were directly randomly assigned to the follow-up period. Local ethics committee approval was obtained in all countries, and all patients provided written informed consent prior to entry. The trial is registered in the Current Controlled Trials Database (ISRCTN15180871, www.controlled-trials.com).
Participants
Eligible patients were male or female patients aged ≥18 and ≤80 years and diagnosed with Behçet’s disease according to the International Study Group diagnostic criteria.Citation9 All had a history of Behçet’s disease uveitis involving the posterior segment of the eye and had been receiving a stable regimen of oral corticosteroids (equivalent to prednisolone 20 mg/day or less) and at least one of the standard immunosuppressive drugs (azathioprine 2.5 mg/kg/day or less, cyclosporine A 5 mg/kg/day or less, mycophenolate mofetil 3 g/day or less, or methotrexate up to the highest tolerable dose) for at least 4 weeks prior to entering the study. The patients continued to receive these drugs as a stable background treatment during the study.
A new ocular exacerbation (the entry criterion for acute patients) was defined as at least one of the following in the index eye: ≥2+ vitreous haze score using the Standardization of Uveitis Nomenclature (SUN)Citation10 with or without anterior chamber cells; a documented ≥15-letter ETDRS or two-line Snellen score decrease in best corrected visual acuity (BCVA)Citation11 attributed to ocular exacerbation associated with Behçet’s disease; or the presence of new active retinal infiltrates and/or acute retinal vasculitis (retinal findings were scored using the uveitis scoring system of BenEzra et al.).Citation12 The patients were required to have a BCVA of +1.7 logMAR or better (equivalent to Snellen 20/1000 or better) in both eyes prior to the qualifying exacerbation. In cases of bilateral simultaneous exacerbation, the investigators were instructed to note the index eye as the eye with more severe exacerbation.
At-risk patients were enrolled in the study if they had a history of one or more acute exacerbations in the previous 18 months and findings of retinal vascular leakage documented by an FFA score ≥6 using a standard method,Citation13 with central reading at the Department of Ophthalmology, Istanbul Faculty of Medicine. If both eyes had an FFA score ≥6, the eye with the most recent exacerbation was chosen as the index eye. They were also required to have BCVA of +1.0 logMAR or better (equivalent to Snellen 20/200 or better) in both eyes.
Main exclusion criteria included infectious uveitis or uveitis due to causes other than Behçet’s disease, severe cataract, and posterior synechiae. Patients with active tuberculosis or a history of tuberculosis disease were excluded by clinical examination, interferon-gamma release assay, and chest X ray, unless they had completed a full course of treatment for tuberculosis. In South Korea, according to local guidelines, patients with a positive interferon-gamma release assay test were excluded or withdrawn from the study if positive test results were available after randomization. In all other countries, patients with latent tuberculosis infection were allowed to be included if they agreed to receive prophylaxis treatment with isoniazid. Other exclusion criteria included immunodeficiency, active infectious diseases, pregnancy, breastfeeding, or possibility of becoming pregnant during the study, and significant laboratory abnormalities. Patients who had received a systemic biologic therapy in the previous 3 months, periocular corticosteroids within the previous 1 month, intravitreal corticosteroids within the previous 6 months, systemic alkylating agents within the previous year, or any injected or implantable corticosteroid-releasing device within the previous 3 years were also excluded.
Efforts were made to recruit a greater number of acute patients for better evaluation of the efficacy of gevokizumab in the acute setting. Acute patients were screened and enrolled within 72 hours after the beginning of ocular exacerbation. At-risk patients were screened within no more than 7 days prior to enrollment.
Interventions
For each group (acute and at-risk), patients were randomly assigned to one of three gevokizumab regimens at study entry via an interactive web response system. Gevokizumab was administered every 4 weeks either intravenously (IV) or subcutaneously (SC) by a trained health care professional. All acute patients initially received either 30 or 60 mg IV infusion of gevokizumab. At the time of response, they received a second IV infusion of gevokizumab (30 or 60 mg), followed by maintenance treatment with either 30 mg gevokizumab (IV or SC) or 60 mg gevokizumab (SC) (). At-risk patients entered the trial at day 0; after an initial IV infusion of 30 or 60 mg gevokizumab, they continued to receive the same treatment as that administered in the follow-up period for acute patients. Thus, for acute patients, the three regimens were: (1) 60 mg IV gevokizumab at entry, 30 mg IV at time of response, and then 30 mg SC every 4 weeks thereafter; (2) 30 mg IV gevokizumab at entry, 30 mg IV at time of response, and then 30 mg IV every 4 weeks thereafter; and (3) 60 mg IV gevokizumab at entry, 60 mg IV at time of response, and then 60 mg SC every 4 weeks thereafter. For at-risk patients, the three equivalent regimens were: (1) 60 mg IV gevokizumab at entry, and then 30 mg SC every 4 weeks thereafter; (2) 30 mg IV gevokizumab at every visit; and (3) 60 mg IV gevokizumab at entry, and then 60 mg SC every 4 weeks thereafter.
Evaluation of Response to Treatment and Reasons for Withdrawal
Response to gevokizumab treatment in acute patients was defined as any of the following improvements in the index eye on day 14 or 21 visits without any deterioration from the baseline in the other two parameters and no exacerbation in either eye: improved vitreous haze score by ≥2 units; ≥15-letter improvement in BCVA; or resolution of retinal infiltrates or acute signs of retinal vasculitis. Responding patients continued to receive gevokizumab as defined in the protocol for up to 336 days.
The protocol included strict instructions for withdrawal in acute patients if there was no response according to the above definition by day 21. In addition, acute patients were to be withdrawn at visits on day 4, 7, or 14 (i.e., before formal evaluation of response) if there was no sign of improvement in any of the anterior chamber, vitreous haze, or retinal findings. Both acute and at-risk patients were also to be withdrawn per protocol in case of any further occurrence of ocular exacerbation during the study, defined as any of the following in either eye: worsening vitreous haze by ≥2 units change with or without anterior chamber cells; ≥15-letter decrease in BCVA; or emergence of retinal infiltrates or acute retinal vasculitis detected by indirect ophthalmoscopy.
Outcomes
The primary objective was evaluation of safety of two doses of gevokizumab (30 or 60 mg, combined into three dosing regimens according to administration route) on top of immunosuppressive drugs, assessed by monitoring the occurrence of adverse events and any abnormal laboratory values (standard hematology and biochemistry tests, including liver and renal parameters), vital signs (heart rate, blood pressure, respiratory rate, temperature, and body weight), or systemic clinical findings. Response to gevokizumab, as defined above, and treatment efficacy were secondary endpoints measured in the response phase. The efficacy of gevokizumab in uveitis was evaluated by changes in ophthalmological examination findings in the index eye, including BCVA using the standard ETDRS chart, vitreous haze, and retinal infiltrates and retinal vasculitis by indirect ophthalmoscopy.Citation10,Citation11 Data were also collected for the contralateral eye, and reported where appropriate. Retinal findings were scored using the uveitis scoring system of BenEzra et al.Citation12 Non-ocular manifestations of Behçet’s disease were also recorded at each study visit and only those considered as clinically relevant by the investigator were to be reported as an adverse event.
Predose serum samples were collected at every visit from the arm opposite to the injection arm (in case of IV infusions) and tested for anti-drug antibodies (ADAs) using an electrochemiluminescence (ECL)-based anti-gevokizumab-specific antibody immunoassay and for serum gevokizumab concentrations by an ECL-based bridging immunoassay in samples collected prior to infusion, at the end of infusion, and 30 minutes after infusion, and also prior to dosing at visits on days 1, 4, and 7 for acute patients. In the event of an ocular exacerbation during the study in acute or at-risk patients, a serum sample was collected for evaluation of ADAs and pharmacokinetics.
Statistical Methods
There was no formal sample size calculation for this exploratory study. Considering the amount of information already on file for the pharmacokinetics of gevokizumab, inclusion of 21 subjects was considered sufficient to document pharmacokinetic profiles for the three dosage regimens. The results of the study are presented as descriptive statistics with numbers for categorical variables and means and standard deviations (SD) for continuous variables. In view of the small sample size, statistical testing between different dose and administration modes was not performed. The statistical analysis was performed by the Biostatistics Division of the Institut de Recherches Internationales Servier using SAS®/PC Software.
RESULTS
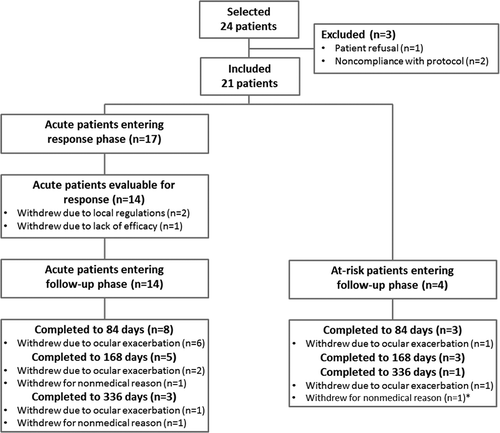
A total of 21 patients were included (17 in the acute group and 4 in the at-risk group); 8 patients were recruited in Turkey, 11 in South Korea, and 2 in Tunisia. The flow of participants through the study is shown in . Of the 17 acute patients, evaluation was not possible in 3 patients due to premature withdrawal, but all of the remaining 14 patients responded within 21 days and entered the follow-up period.
The baseline characteristics of the population were typical of patients with Behçet’s disease uveitis (). All included patients fulfilled the International Study Group criteria for Behçet’s disease diagnosis,Citation9 except for one patient who fulfilled the Japanese diagnostic criteria for Behçet’s Disease with recurrent oral ulcers and typical uveitis.Citation14 This patient was excluded from the study because of Quantiferon test positivity according to local Ethics Committee requirements. The median duration of Behçet’s disease uveitis was 25.0 months (range 5 to 114 months) and most patients had panuveitis (18 patients). In acute patients at baseline, mean BCVA in the index eye was 35.4 ± 22.7 letters (median 37.0, range 0 to 87); 13 (77%) patients had vitreous haze grade ≥2+; 12 patients (71%) had retinal infiltrates, 11 patients (65%) had acute retinal vasculitis and mean BenEzra score was 5.13±3.96. In at-risk patients at baseline, mean BCVA was 61.5.4 ± 21.3 letters (median 63, range 37 to 83); three out of the four patients had vitreous haze grade of 0 and one patient had 2+ vitreous haze in the index eye (this patient could not be considered as “acute” for the study because the last ocular exacerbation occurred 9 months before selection).
TABLE 1. Baseline characteristics of patients.
There were no drug-related adverse events during the trial. Twenty-six adverse events were reported in 14 of 17 acute patients, and 15 adverse events were reported in the 4 at-risk patients. Adverse events were mostly associated with Behçet’s disease or its usual complications. Six serious adverse events, all unrelated to the study drug, were reported in four patients. One patient in the acute group, who had no improvement in vitreous haze, underwent a pars plana vitrectomy, which revealed an intravitreal hemorrhage as the underlying cause explaining the lack of response to any previous treatment; this patient withdrew from the study for “lack of efficacy” before evaluation of response () and was not included in subsequent response analyses. There was also one patient with pheochromocytoma, one patient with uveitic glaucoma and optic nerve atrophy treated with trabeculectomy (elevated intraocular pressure was already present at selection), and one patient with cataract surgery. This study did not aim to evaluate the efficacy of gevokizumab for other manifestations of Behçet’s disease; however, considering known medical history, there was no significant worsening of non-ocular Behçet’s disease-related manifestations during the study, which may suggest inadequate disease control. Adverse events related to infections included pyelonephritis (one patient), upper respiratory tract infection (one patient), and dental abscess (one patient); none of these were serious or were considered to be related to treatment with gevokizumab. There were no allergic reactions. A summary of the most frequently reported emergent adverse events per system organ class is provided in . No clear conclusion could be drawn regarding the comparison of adverse event frequencies for the various dose regimens of gevokizumab.
TABLE 2. Emergent adverse events most frequently reported during the treatment period in acute and at-risk patients according to system organ classes.
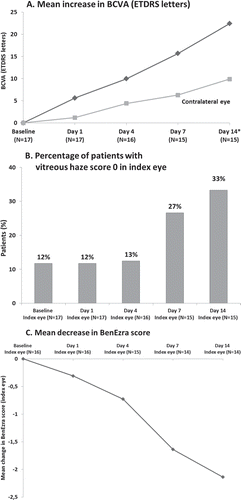
Response to gevokizumab treatment, as defined in the protocol, could not be evaluated for the three patients who withdrew prematurely (see below). All remaining acute patients (N = 14) responded within 21 days, with response observed as early as day 1 in one patient. The response per protocol definition was observed in 7 patients (50%) by day 4, 10 patients (71%) by day 7, and all except one patient by day 14 (). Main ophthalmologic parameters were markedly improved in all acute patients (BCVA, vitreous haze, and BenEzra score) during the response evaluation phase () with a mean improvement of BCVA from baseline of 22.40 ± 15.37 letters in the index eye, an increase in the number of acute patients reaching vitreous haze score of zero, and a reduction in BenEzra scoreCitation12 by 2.14 ± 1.83 in the index eye. No difference in ophthalmologic parameters could be observed between the three dose regimens, possibly due to the small sample size in each group. An improvement in the BCVA was also observed in the contralateral eye ().
FIGURE 3. Rapid increase in cumulative percentage of responders in 14 acute patients evaluable for treatment response during the response phase.
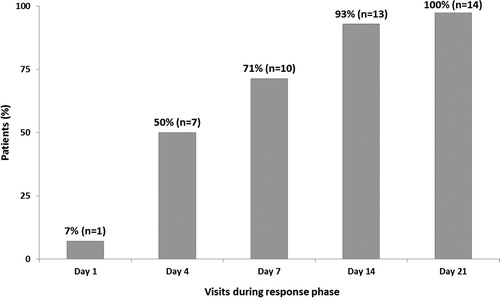
FIGURE 4. Marked improvement of ophthalmologic parameters in acute patients during response phase (including acute patients withdrawn before evaluation of response): (a) BCVA; (b) vitreous haze; and (c) BenEzra score (one eye not evaluated due to high vitreous haze). Data during response phase are provided up to the day 14 visit (all but one patient responded by day 14 although one patient had a supplementary visit to confirm response at day 21).
The study protocol required patient withdrawal with the exacerbation of ocular manifestations, regardless of severity, and did not aim to evaluate the efficacy with respect to the recurrence rate. There were 17 withdrawals during the study, including one patient who did not continue in the optional period beyond 168 days (). The most frequent reason for withdrawal was ocular exacerbation (11 patients). These exacerbations were mostly mild and due to emergence of new retinal infiltrates or vasculitis (6 patients) without worsening of BCVA or vitreous haze; 7 out of these 11 patients had no clinically relevant decrease in BCVA (i.e., ≥15 letters). In two cases, ocular exacerbations occurred after unplanned tapering of corticosteroids. Three acute patients withdrew prematurely before evaluation of response: two because a positive QuantiFERON® TB test result became available after enrollment in South Korean centers, and one patient whose findings did not respond to gevokizumab, or later to high-dose corticosteroids, but improved after a pars plana vitrectomy that revealed intravitreal hemorrhage but no active disease as the cause of the vitreous haze. In addition, three patients withdrew during the follow-up phase due to non-medical reasons (withdrawal of consent). Out of the four patients who completed 1 year of treatment, two were on repeated-dose regimen of 30 mg IV (one acute and one at-risk patient) and two acute patients were on 60 mg SC (regimens 2 and 3, respectively).
Vital signs remained stable throughout the infusion or injection of gevokizumab and for the duration of the study. There were no reported significant ECG abnormalities. No neutralizing ADA for gevokizumab was detected in the acute or at-risk patients at any time for any dose. Observed serum concentrations of gevokizumab were consistent with the values expected for the assessed dosing regimens. The pharmacokinetics were linear in the administered dose range and exhibited a biexponential decline with initial and terminal half-lives, clearance, and volume of distribution typical for a human antibody subject to a non-specific clearance mechanism (data not shown).
DISCUSSION
The rationale for developing gevokizumab in Behçet’s disease uveitis is mainly based on the role of IL-1β as a key proinflammatory cytokine in the pathogenesis of Behçet disease.Citation15 Results of this phase 2 exploratory study indicate that gevokizumab is safe and well tolerated in patients with Behçet’s disease uveitis. In patients with acute exacerbations, rapid improvements in ophthalmologic inflammatory parameters were observed without any increase in systemic corticosteroid dosages, similar to those observed with anti-TNF treatments.Citation16
There were no safety concerns with the use of gevokizumab on top of standard-of-care corticosteroid plus immunosuppressive treatment in Behçet’s disease uveitis. The reported adverse events were associated with underlying disease or its usual complications, and there were no events such as allergic reactions, or serious or opportunistic infections.
All 14 evaluable acute patients responded to gevokizumab, thereby avoiding the need for high-dose corticosteroids. Response appeared to be rapid: the majority (71%) responded within 1 week, and a half responded by the fourth day of treatment. Our results confirm and extend those of a single-center, open-label pilot study with a single body-weighted dose of gevokizumab in patients with Behçet’s disease uveitis.Citation8 Indeed, our findings may be considered to be more robust, since they were obtained within the context of a multicenter randomized trial in countries where Behçet’s disease is prevalent, and with a study design in which patients received a monthly dose of gevokizumab on top of background immunosuppressive drugs and limited doses of corticosteroids. Together, these findings imply that gevokizumab may be a promising treatment in the management of Behçet’s disease uveitis.
As a safety procedure, our study protocol required withdrawal of patients in case of signs of new ocular exacerbations regardless of severity, and this occurred in 11 patients. The stringent definition of ocular exacerbation employed in our study may not be entirely representative of routine clinical practice. Indeed, most of the ocular exacerbations leading to withdrawal were mild and were due to emergence of retinal infiltrates or vasculitis without clinically significant worsening of visual acuity or vitreous haze.
No neutralizing ADAs for gevokizumab was detected and gevokizumab serum concentrations were consistent with expected values based on data from previous clinical trials with gevokizumab.Citation8,Citation17 Pharmacokinetic data were in the range of expected values for the tested dose regimens.
There are a number of limitations of this exploratory study, notably the small sample size. The absence of a control group precluded evaluation of efficacy on frequency of recurrent exacerbations. This is an important limitation since Behçet’s disease is a relapsing disease and there is a great variability in the recurrence rate of individual exacerbations.Citation18
CONCLUSIONS
Gevokizumab was well tolerated and rapidly controlled acute exacerbations of Behçet’s disease uveitis without the need for high-dose corticosteroid on top of immunosuppressive drugs, confirming the proof-of-concept study results.Citation8 However, because of the small sample size and the lack of a control group, our results regarding recurrent exacerbations should be interpreted with caution.
DECLARATION OF INTEREST
Ahmet Gül has received lecture fees, honoraria and travel grants from Servier and Novartis. Sibel Kadayifcilar has acted as an investigator for Servier, Santen, Novartis, and Bayer, and has received travel grants from Novartis, Allergan, and Bayer, and honoraria for advisory board meetings from Bayer and Allergan. Moncef Khairallah has acted as a consultant for Servier. Sung Chul Lee, none. Pinar Ozdal, none. Yilmaz Özyazgan is Consultant for the Advisory Board of Servier. Ji Hun Song has received lecture fees from Allergan and a travel grant from Servier. Ilknur Tugal-Tutkun has received lecture fees, honoraria and travel grants from Servier. Valerie Lehner, Agnès de Cordoue and Oana Bernard are employees of Servier. Hyeong Gon Yu, none.
Additional information
Notes on contributors
Ilknur Tugal-Tutkun
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Sibel Kadayifcilar
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Moncef Khairallah
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Sung Chul Lee
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Pinar Ozdal
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Yilmaz Özyazgan
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Ji Hun Song
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Hyeong Gon Yu
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Valerie Lehner
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Agnès de Cordoue
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Oana Bernard
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Ahmet Gül
All authors participated in the study design, interpretation of the results, development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
REFERENCES
- Sakane T, Takeno M, Suzuki N, et al. Behcet’s disease. N Engl J Med. 1999;341:1284–1291.
- Hatemi G, Yazici Y, Yazici H. Behcet’s syndrome. Rheum Dis Clin North Am. 2013;39:245–261.
- Tugal-Tutkun I, Onal S, Altan-Yaycioglu R, et al. Uveitis in Behcet disease: an analysis of 880 patients. Am J Ophthalmol. 2004;138:373–380.
- Hatemi G, Silman A, Bang D, et al. EULAR recommendations for the management of Behcet disease. Ann Rheum Dis. 2008; 67:1656–1662.
- Owyang AM, Issafras H, Corbin J, et al. XOMA 052, a potent, high-affinity monoclonal antibody for the treatment of IL-1beta-mediated diseases. MAbs. 2011;3:49–60.
- Roell MK, Issafras H, Bauer RJ, et al. Kinetic approach to pathway attenuation using XOMA 052, a regulatory therapeutic antibody that modulates interleukin-1beta activity. J Biol Chem. 2010;285:20607–20614.
- Issafras H, Corbin JA, Goldfine ID, et al. Detailed mechanistic analysis of gevokizumab, an allosteric anti-IL-1beta antibody with differential receptor-modulating properties. J Pharmacol Exp Ther. 2014;348:202–215.
- Gul A, Tugal-Tutkun I, Dinarello CA, et al. Interleukin-1beta-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet’s disease: an open-label pilot study. Ann Rheum Dis. 2012;71:563–566.
- International Study Group for Behcet’s Disease. Criteria for diagnosis of Behcet’s disease. Lancet. 1990;335:1078–1080.
- Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–516.
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806.
- BenEzra D, Forrester JV, Nussenblatt RB, et al. Uveitis Scoring System. Berlin, Germany: Springer Verlag; 1991.
- Tugal-Tutkun I, Herbort CP, Khairallah M. Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol. 2010;30:539–552.
- Mizushima Y, Inaba G, Mimura Y. Guide for Diagnosis of Behcet’s Disease, Behcet’s Diesase Research Committee, Japan. Tokyo, Japan: Ministry of Health and Welfare; 1987.
- Lachmann HJ, Quartier P, So A, et al. The emerging role of interleukin-1beta in autoinflammatory diseases. Arthritis Rheum. 2011;63:314–324.
- Sfikakis PP, Theodossiadis PG, Katsiari CG, et al. Effect of infliximab on sight-threatening panuveitis in Behcet’s disease. Lancet. 2001;358:295–296.
- Cavelti-Weder C, Babians-Brunner A, Keller C, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012; 35:1654–1662.
- Gul A. Standard and novel therapeutic approaches to Behcet’s disease. Drugs. 2007; 67:2013–2022.