Abstract
Natural estrogens such as estradiol (E2) or its valerate ester (E2V) offer an alternative to ethinyl estradiol (EE). E2-containing combined oral contraceptives (COCs) have demonstrated sufficient ovulation inhibition and acceptable contraceptive efficacy. However, earlier formulations were generally associated with unacceptable bleeding profiles. Two E2V-containing preparations have been approved to date for contraceptive use: E2V/cyproterone acetate (CPA) (Femilar®; only approved in Finland and only in women >40 years or women aged 35–40 years in whom a COC containing EE is not appropriate) and E2V/dienogest (DNG; Qlaira®/Natazia®). The objective of the current review is to provide an overview of the development of COCs containing natural estrogen, highlighting past issues and challenges faced by earlier formulations, as well as the current status and future directions. The majority of information to date pertains to the development of E2V/DNG.
Introduction
Combined oral contraceptives (COCs) consist of estrogen and progestogen components. Although contraceptive effects can largely be achieved with progestogen alone, as is the case for progestogen-only pills, the estrogen component in COCs avoids symptoms of hypoestrogenism [Citation1], enhances contraceptive efficacy and helps regulate bleeding. Although a number of progestogens have been introduced into clinical practice over the last five decades since the introduction of COCs [Citation2–5], the estrogen component has remained predominantly ethinyl estradiol (EE). This reliance on EE to date is due mainly to its good oral bioavailability (38–48%) [Citation6] relative to other estrogens. Recently, a new COC containing estradiol valerate (E2V) combined with dienogest (DNG) has been approved for contraceptive use worldwide.
The objective of the current review is to provide an overview of the development of COCs containing natural estrogen, highlighting past issues and challenges faced by earlier formulations, as well as the present status and future directions.
Past
Reducing the estrogen dose
In 1970, Inman and colleagues demonstrated that COC use was associated with an increased risk of thromboembolic disease [Citation7]. Thrombotic risk was attributed largely to the estrogen component, and increased with increasing doses of estrogen (at that time mestranol or EE) [Citation7].
Efforts were therefore made to reduce the EE dose in COCs. These dose reductions have been highly successful in reducing the risk of venous thromboembolism (VTE); current estimates put the incidence of VTE at between 8 and 10 per 10,000 women-years in users of COCs containing <50 µg of EE, compared with 4.7 per 10,000 women-years in non-pregnant, non-COC users and around 20 per 10,000 women-years during pregnancy and the post-partum period [Citation8].
Recent years have seen further reductions in the EE dose, to 20 µg and even 15 µg. However, these low doses have been associated with higher rates of discontinuation from clinical trials (mainly due to adverse events including bleeding) and bleeding disturbances (amenorrhea/infrequent bleeding, irregular, prolonged or frequent bleeding or spotting) compared with higher doses of EE [Citation9]. In particular, preparations containing EE 15 μg have a somewhat higher incidence of breakthrough bleeding and/or spotting than COCs containing EE 20 μg [Citation10], and may be associated with premature discontinuation because of bleeding irregularities [Citation11]. These observations, together with the finding that even EE doses as low as 10 µg have been associated with negative effects on hemostatic surrogate parameters [Citation12], mean that it is unlikely that a COC containing lower EE dosages will be well accepted.
Estradiol versus EE: pharmacological effects
Exogenously administered estradiol is chemically identical to endogenous 17β-estradiol (E2), the most potent of the natural estrogens [Citation13]. In the past, a major obstacle to using E2 in hormonal contraceptives was its relative inactivity when administered orally [Citation13]. As outlined previously, EE was first used in COCs because of its good oral bioavailability (38–48%) [Citation6] compared with E2 (5%) [Citation14]. Different approaches have been undertaken to overcome the low bioavailability of E2, including micronization and esterification [Citation15]. E2V is the valerate ester of natural E2. The estrogenic effects and pharmacokinetic profile of E2V and E2 are comparable as E2V is rapidly converted to E2 in the gut and liver [Citation16]. Following oral administration of E2V (in combination with DNG), serum concentrations of E2 remain fairly stable during a 24-h period () [Citation17]. In contrast, peak serum EE levels following administration of EE (in combination with levonorgestrel [LNG]) are reached after 1.5 h and reduce thereafter () [Citation18].
Figure 1. Mean serum estradiol (E2) concentration over 24 h following oral administration of estradiol valerate (E2V)/dienogest (DNG) [Citation17]. Zeun S, et al., Eur J Contracept Reprod Health Care, 2009;14(3):221–32, copyright© 2009, Informa Healthcare. Reproduced with permission of Informa Healthcare.
![Figure 1. Mean serum estradiol (E2) concentration over 24 h following oral administration of estradiol valerate (E2V)/dienogest (DNG) [Citation17]. Zeun S, et al., Eur J Contracept Reprod Health Care, 2009;14(3):221–32, copyright© 2009, Informa Healthcare. Reproduced with permission of Informa Healthcare.](/cms/asset/31ae1906-4d6e-403a-8948-c392f0b6fc24/igye_a_662547_f0001_b.gif)
Figure 2. Mean ethinyl estradiol (EE) concentration over 24 hours following oral administration of EE 20 µg/levonorgestrel (LNG) 100 µg [Citation18]. Endrikat J, et al., Eur J Contracept Reprod Health Care, 2002;7(2):79-90, copyright© 2002, Informa Healthcare. Reproduced with permission of Informa Healthcare.
![Figure 2. Mean ethinyl estradiol (EE) concentration over 24 hours following oral administration of EE 20 µg/levonorgestrel (LNG) 100 µg [Citation18]. Endrikat J, et al., Eur J Contracept Reprod Health Care, 2002;7(2):79-90, copyright© 2002, Informa Healthcare. Reproduced with permission of Informa Healthcare.](/cms/asset/fc4b6550-7724-40f2-bb76-46fbb1564ad4/igye_a_662547_f0002_b.gif)
At equivalent dosages (i.e. E2 2 mg has biologic effects that are equivalent to EE 4–20 µg, depending on the target organ) E2 has been shown to have a lesser impact on metabolic and hepatic parameters than EE in several studies. This is manifested in a more favorable effect of E2 versus EE on lipids [Citation19] and a reduced effect of E2 versus EE on the synthesis of hepatic proteins, including sex hormone-binding globulin (SHBG) and angiotensinogen [Citation6,Citation20,Citation21]. In addition, E2 appears to have a reduced impact on markers of hemostasis than EE [Citation12,Citation22,Citation23].
Clinical experience with estradiol-containing COCs
The design and results of clinical studies investigating E2-containing COCs are shown in [Citation24–43]. These studies have shown that, in terms of efficacy, these preparations demonstrate sufficient ovulation inhibition and an acceptable level of contraceptive efficacy. Seven studies examined preparations that no longer appear to be in development [Citation25,Citation29,Citation36,Citation38–42]. Three of these studies investigated the combination of E2 with norethisterone, with or without the addition of E3 [Citation25,Citation39,Citation40,Citation42]. In all studies, the E2 dose was 4 mg, the norethisterone dose was 3 mg, and, where applicable, the E3 dose was 2 mg. Effective inhibition of ovulation was noted with all regimens, but unacceptable bleeding profiles limited the use of this combination [Citation25,Citation39,Citation40,Citation42]. Four of the studies examined the combination of E2 with desogestrel (DSG) [Citation29,Citation36,Citation38,Citation41]. The dose of E2 varied from 1 to 3 mg, and the dose of DSG was 150 µg in all studies. The studies were very small (20–31 subjects). As described previously, ovulation inhibition was noted in all studies, but this benefit was outweighed by an unacceptable bleeding profile [Citation29,Citation36,Citation38,Citation41]. Similar trends were observed when the combination of E2 cyclo-octyl acetate/DSG was examined [Citation38].
The underlying reasons for the unacceptable bleeding profiles observed in these studies may include an inappropriate estrogen/progestogen ratio [Citation36,Citation38,Citation41,Citation42] or suboptimal E2 doses [Citation40,Citation42].
Table I. Summary of clinical studies in contraception involving combined oral contraceptives (COC) that incorporated estradiol (E2) rather than ethinyl estradiol (EE).
Present
Combinations of estradiol plus different progestogens
Estradiol/NOMAC
Nomegestrol acetate (NOMAC) is a 17-hydroxy-progesterone derivative [Citation30]. A recent study compared E2 1.5 mg/NOMAC 2.5 mg with EE 30 μg/drospirenone 3 mg in healthy women (n = 32) () [Citation30]. In this randomized, six-cycle study, ovulation inhibition was noted in all women in both treatment groups. Ovarian suppression was similar between treatments; progesterone was fully suppressed to levels <2 nmol/L in both groups () [Citation30]. A more recent publication noted that NOMAC 2.5 mg inhibited both ovulation and follicular maturation, and the antigonadotropic effects of NOMAC 2.5 mg were reinforced when it was combined with E2 1.5 mg [Citation27]. This dose-finding study underlines the role of the estrogen component in inhibiting ovulation. A third study assessed ovarian activity with two different E2 (1.5 mg)/NOMAC (2.5 mg) regimens (21/7 [n = 37] and 24/4 [n = 40]) [Citation28]. The 24/4 regimen was associated with greater inhibition of follicular activity. A shorter duration of total and withdrawal bleeding with the 24/4 regimen compared with the 21/7 regimen was described as secondary outcome (p < 0.05) [Citation28]. By cycle 3, the incidence of breakthrough bleeding was similar between regimens, but the duration of breakthrough bleeding was slightly longer with the 21/7 regimen than with the 24/4 regimen [Citation28]. The bleeding profile was also assessed as a secondary outcome in a study comparing the hemostatic effects of E2/NOMAC in a 24/4 regimen with those of EE/LNG in a 21/7 regimen. In this short-term study, also based on a limited number of participants and thus not powered to investigate the bleeding profile (n = 45 in each group), the duration of total bleeding, withdrawal bleeding and breakthrough bleeding appeared to be shorter with E2/NOMAC than with EE/LNG () [Citation43]. Meaningful investigations of the bleeding profile of E2/NOMAC in a 24/4 regimen and comparisons with other combined oral formulations in a larger more diverse group of women are currently lacking. Contraceptive efficacy was not assessed in these studies.
Estradiol/drospirenone
Two randomized, double-blind, parallel-group Phase II studies have been completed with a COC containing E2/drospirenone administered in either a monophasic or triphasic regimen (dosages not defined). Both studies were conducted in healthy women aged 18–35 years. The first study (n = 116) assessed ovulation inhibition (ClinicalTrials.gov identifier: NCT00631124), while the second (n = 575) evaluated cycle control and safety (ClinicalTrials.gov identifier: NCT00653614). Results are anticipated.
Estradiol valerate/CPA
The combination of E2V and CPA has been marketed as a COC (Femilar®) in Finland since 1993, however it is only indicated in women >40 years or women aged 35–40 years in whom a COC containing EE is not appropriate. This formulation comprises E2V 1 mg/CPA 1 mg on days 1–10, E2V 2 mg/CPA 2 mg on days 11–21 and a pill-free interval on days 22–28. During 12 cycles of treatment with the E2V/CPA combination (n = 288), ovulation inhibition was observed in 95% of women. The cumulative pregnancy rate was 0.4% () [Citation32]. Intermenstrual bleeding/spotting was observed in 35.5% of women in cycle 3 and 24.5% of women in cycle 12 [Citation32]. Bleeding became less frequent over time in the majority of women, and dysmenorrhea subsided [Citation32]. Similar findings were observed in a second study (n = 50) comparing E2V/CPA with E2V/norethisterone in a biphasic regimen (E2V/CPA: as described above; E2V/norethisterone: E2V 1 mg/norethisterone 1 mg on days 1–10, E2V 2 mg/norethisterone 2 mg on days 11–21 and a pill-free interval on days 22–28) () [Citation33]. Ovulation was inhibited in all women (except for one woman who ovulated during the first treatment cycle) in the E2V/CPA group. In the E2V/norethisterone group, ovulation occurred in 8 women. One additional woman in this group ovulated during all treatment cycles; treatment was discontinued in this subject. Contraceptive efficacy was not assessed in this study. Menstrual blood loss was reduced in all women in the E2V/CPA group. However, in the E2V/norethisterone group, menstrual blood loss reduced in 40% and increased in 10% of women. The total number of bleeding days reduced with E2V/CPA and increased with E2V/norethisterone () [Citation33].
Estradiol valerate/DNG
The combination of E2V/DNG was approved as a COC in the European Union (EU) in 2008, where it is marketed as Qlaira®/Klaira®. FDA approval for Natazia® was obtained in May 2010. Qlaira® received regulatory approval in the EU for the treatment of heavy menstrual bleeding (HMB) in October 2010 and in the USA (Natazia®) in March 2012. The E2V/DNG combination provides early estrogenic dominance to ensure initial endometrial proliferation and endometrial stroma stability during the progestogen-dominated mid-to-late part of the cycle [Citation24]. DNG has potent endometrial activity [Citation44–46] and a bioavailability of >90% after oral intake [Citation47].
Early investigations with E2V/DNG employed biphasic or triphasic regimens, which provided effective ovulation inhibition but unacceptable bleeding profiles (). The unacceptable bleeding profile with E2V/DNG in a biphasic or triphasic regimen prompted the introduction of an E2V/DNG combination in an estrogen step-down/progestogen step-up approach. In a pilot study in healthy women (n = 100), a dynamic dosing regimen (E2V 3 mg for 3 days, E2V 2 mg/DNG 1 mg for 4 days, E2V 2 mg/DNG 2 mg for 16 days, E2V 1 mg for 2 days and finally placebo for 3 days) was associated with a far more favorable profile than the biphasic or triphasic regimens () [Citation34,Citation35].
Four variations of E2V/DNG in dynamic phasic regimens were investigated in two sequential Phase II studies designed to determine the optimal daily application and the required dose of DNG for effective inhibition of ovulation () [Citation31]. In the first study it was shown that a dosing regimen that incorporated 26 rather than 25 days of active treatment was associated with greater ovulation inhibition. In the second study, which examined two 26-day regimens with doses of DNG that were distinctly increased versus those used in the first study, it was shown that a dose of DNG of 2 mg on days 3–7 and 3 mg on days 8–24 (both in combination with E2V 2 mg) was the lowest effective dose of DNG for efficient ovulation inhibition. This regimen comprised E2V 3 mg alone for 2 days, E2V 2 mg/DNG 2 mg for 5 days, E2V 2 mg/DNG 3 mg for 17 days, E2V 1 mg alone for 2 days then placebo for 2 days ( and ). There are few data on compliance with COCs, but one would expect that reducing the hormone-free interval to only 2 days (i.e. 26 days of active treatment, 2 days of placebo [26/2 regimen]) would improve tolerability and, in turn, improve compliance.
Figure 3. Dosing regimen of an estradiol valerate (E2V)/dienogest (DNG)-containing oral contraceptive administered using an estrogen step-down and progestogen step-up approach over a 28-day treatment cycle (with 26 days of active tablets). Numbers along the bottom of the figure correspond to days of the 28-day cycle.
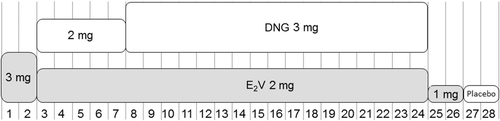
The efficacy, bleeding profile and safety of E2V/DNG in a dynamic dosing regimen has been examined in three Phase III trials (). The first of these trials enrolled 1377 women aged 18–50 years and was conducted in Europe over twenty 28-day cycles [Citation37]. All women received the regimen outlined above. The E2V/DNG combination was associated with an adjusted Pearl Index of 0.34 (upper limit of 95% CI = 0.73), together with good tolerability and a high degree of user satisfaction. Only 2.5% of 1377 women treated for up to 20 cycles prematurely discontinued treatment because of menstrual bleeding irregularities [Citation37]. The second study, conducted in the USA and Canada, was an open-label, non-comparative study designed to assess the contraceptive efficacy, cycle control, safety and tolerability of E2V/DNG. A total of 490 women aged 18–35 years received E2V/DNG for up to 28 cycles [Citation48]. The third study, conducted in Europe, compared the E2V/DNG regimen with a monophasic COC (EE 20 µg/LNG 100 µg) over seven cycles (n = 798) [Citation24]. Scheduled withdrawal bleeding occurred in 77.7–83.2% of E2V/DNG recipients and 89.5–93.8% of EE/LNG recipients. The maximum intensity of withdrawal bleeding was significantly different in women treated with E2V/DNG and EE/LNG; a greater proportion of women who received E2V/DNG versus EE/LNG experienced spotting or light bleeding and a smaller portion of women who received E2V/DNG versus EE/LNG experienced normal or heavy bleeding. In addition, the mean duration of withdrawal bleeding was reduced with E2V/DNG compared with EE/LNG (4.1–4.7 vs. 5.0–5.2 days; p < 0.05 per cycle). Intracyclic bleeding was similar between the two COCs (10.5–18.6% vs. 9.9–17.1% per cycle; p > 0.05) () [Citation24].
Based on data from the three Phase III trials performed in Europe, the USA and Canada, E2V/DNG was associated with a typical-use Pearl Index of 0.79 (upper limit of 95% CI = 1.23) and a perfect-use Pearl Index of 0.42 (upper limit 95% CI = 0.77) in women aged 18–50 years [Citation49]. In women aged 18–35 years, the corresponding Pearl Indices were 1.01 (upper limit 95% CI = 1.59) and 0.51 (upper limit 95% CI = 0.97) [Citation49].
Pharmacokinetics of estradiol valerate/DNG
The pharmacokinetics of E2V/DNG were analyzed in 15 healthy women aged 18–50 years who participated in a Phase I, open-label, single-cycle study [Citation17]. E2V/DNG was associated with stable serum levels of E2 throughout the 28-day period of treatment () [Citation17,Citation50]. Minimum mean serum E2 levels during E2V administration (days 1–26) were 33.6–64.7 pg/mL, similar to those seen in the mid-follicular phase of normal ovulatory cycles, while minimum mean serum DNG levels were 6.8–15.1 ng/mL during DNG administration (days 3–24). Minimum concentrations of DNG showed only minor accumulation within each phase of the regimen during which DNG was administered. On day 24, the geometric mean maximum concentration of DNG was 82.9 ng/mL, while the average concentration and terminal half-life were 33.7 ng/mL and 12.2 h. The median time to maximum observed drug concentration for DNG was 1.5 h. Serum SHBG concentrations increased by 40%, but remained within the normal range. Cortisol-binding-globulin levels remained essentially unchanged.
Figure 4. Minimum mean (standard deviation [SD]) serum concentrations of estradiol during daily administration of a 28-day oral contraceptive containing estradiol valerate (E2V)/dienogest (DNG) [Citation50].
![Figure 4. Minimum mean (standard deviation [SD]) serum concentrations of estradiol during daily administration of a 28-day oral contraceptive containing estradiol valerate (E2V)/dienogest (DNG) [Citation50].](/cms/asset/366b9b26-4094-467e-9f22-92e416905ce0/igye_a_662547_f0004_b.gif)
Metabolic and vascular effects of estradiol valerate/DNG
Data have shown that E2V/DNG has an impact on various metabolic and hemostatic parameters that is comparable to or less than that of EE/LNG-containing COCs [Citation51,Citation52]. The effect on prothrombin and D-dimer levels is marginal [Citation52], and the effects on high- and low-density lipoprotein cholesterol [Citation51], insulin [Citation51], and carbohydrate metabolism [Citation51] are, in general, more favorable with E2V/DNG than with EE/LNG.
The hemostatic effects of E2V/DNG and E2/NOMAC have not been compared in a head-to-head trial; however, a comparison of data from different trials suggests that the overall magnitude of changes in hemostatic parameters during treatment with E2V/DNG is comparable to that reported with the E2/NOMAC combination [Citation43,Citation51,Citation52]. For example, E2V/DNG and E2/NOMAC were associated with similar effects on SHBG, prothrombin fragment 1+2, fibrinogen and thrombin generation (either determined by the activated protein C (APC) sensitivity ratio or by APC resistance) as measured by the endogenous thrombin generation method [Citation43,Citation51–53]. This suggests that the hemostatic effects of COCs comprising either E2 or E2V in equimolar dosages are comparable, as one would expect.
Although these results are reassuring, one should bear in mind that the lipid, metabolic and hemostatic parameters are only surrogate markers, and have yet to be fully validated as good predictors of the occurrence of clinical events such as VTE. Furthermore, even oral intake of E2 is associated with a hepatic first pass effect [Citation6,Citation16] that may impair the biosynthesis and clearance of proteins involved in hemostasis or blood pressure regulation. Further clinical and long-term epidemiological studies of E2V/DNG in large populations are needed before any safety conclusions can be made. A large international prospective, controlled, non-interventional cohort active surveillance study (INAS-SCORE) to investigate the occurrence of cardiovascular events over a 3- to 5-year period in COC users (including E2V/DNG) is currently underway (ClinicalTrials.gov identifier: NCT01009684).
Additional non-contraceptive benefits of estradiol valerate/DNG
E2V/DNG has been shown to be effective in women with HMB (defined as menstrual blood loss >80 mL). The registration procedure has been successfully concluded for an indication of HMB without organic pathology in the EU, Switzerland and USA and for heavy and/or prolonged menstrual bleeding in Australia and several Latin American and Asian countries. License applications for the HMB indication have also been submitted to health authorities in other countries. Overall, E2V/DNG was associated with an 88% reduction in median menstrual blood loss (from 142 mL to 17 mL/cycle) after 6 months of treatment, compared with a 24% reduction with placebo (from 154 mL to 117 mL/cycle) [Citation54]. Reductions in MBL volume were rapid and sustained and were deemed clinically meaningful [Citation54].
The efficacy of this combination in HMB is thought to be due to its unique dosing regimen, which enables estrogen dominance during the early part of the cycle and progestogen dominance in the late part of the cycle [Citation24]. Women receive 26 days of E2V, which supports endometrial stability [Citation17], and 22 days of DNG, a progestogen with high endometrial potency [Citation44–46].
In summary, the development of dynamic regimens has yielded acceptable bleeding patterns whilst maintaining a reliable level of contraceptive efficacy. Data have shown this regimen to provide effective ovulation inhibition with an acceptable level of cycle control in healthy women.
Future directions
In modern contraception, one focus lies in additional health benefits. There is, therefore, a great deal of interest in whether E2-containing COCs have known or even new benefits. In order to establish these benefits, extensive efforts have been put into the development program for E2V/DNG, with one of the most comprehensive Phase IIIb clinical programs for a COC.
The HARMONY I and II studies are multicenter, randomized, double-blind, active control group studies comparing the efficacy of the E2V/DNG combination with that of EE/norgestimate (ClinicalTrials.gov identifier: NCT00754065) or EE/LNG (ClinicalTrials.gov identifier: NCT00778609) for the treatment of hormone withdrawal-associated symptoms (HWAS), including headache, pelvic pain and bloating. The studies aim to demonstrate the superiority of E2V/DNG over the comparators with regards to HWAS improvement after six cycles of treatment. Both studies are underway and results are anticipated in 2011. It is expected that E2V/DNG will have a beneficial effect on cycle-related hormone withdrawal symptoms owing to less hormonal fluctuations. Specifically, E2V/DNG is associated with levels of E2 that are stable and are comparable to those during the first week of the follicular phase of a spontaneous menstrual cycle [Citation17]. In addition, the hormone-free interval with E2V/DNG (of 2 days) is shorter than that of conventional COCs (7 days). It has been shown previously that hormone-related symptoms are worse during the 7-day hormone-free interval than during active treatment, [Citation55] and shortening the hormone-free interval from 7 to 4 days may decrease the number of days of symptoms typically associated with hormone withdrawal [Citation55–57].
The STABLE study (ClinicalTrials.gov identifier: NCT00764881) aims to establish the non-inferiority of E2V/DNG over EE/LNG for improving libido in women with OC-associated female sexual dysfunction (FSD). Women presenting with OC-associated FSD are often switched to a COC containing EE/LNG, in the belief that the partial androgenic effects of LNG will alleviate FSD symptoms. STABLE is, to our knowledge, one of the first, if not the first, comprehensive, randomized controlled trial with a comparator arm, using validated questionnaires to investigate the effects of different COCs on OC-associated FSD. The benefit of E2V/DNG on libido in women with OC-induced FSD is thought to be due to the low impact of E2V/DNG on SHBG and its beneficial effect on vaginal cell maturation, demonstrating the complex and multifactorial nature of OC-associated FSD. Therefore, effects other than just the androgenic nature of the progestogen should also be taken into account in women with OC-associated FSD.
The CALM study (ClinicalTrials.gov identifier: NCT00909857) is investigating the effect of E2V/DNG on primary dysmenorrhea. In this multicenter, randomized, double-dummy, parallel-group study, women will receive E2V/DNG or EE/LNG for three cycles. Results for both STABLE and CALM are expected in 2011.
Conclusions
The quest to identify an effective, well tolerated COC using natural estrogens, such as E2, has encompassed a number of clinical trials over several decades. Earlier attempts to use E2 in COCs explored various doses, progestogens and regimens, but yielded unfavorable results in terms of bleeding and cycle control. Recent data suggest that the combination of E2/NOMAC may yield promising results. Further studies are, however, needed.
Two E2V-containing COCs are currently available; E2V/CPA has been marketed as Femilar® in Finland since 1993 (only indicated in women >40 years or women aged 35–40 years for whom a COC containing EE is not appropriate), and E2V/DNG has recently been launched in the EU as Qlaira®/Klaira® and in the USA as Natazia®. There are extensive data available regarding the E2V/DNG combination, which is administered in a dynamic dosing regimen. This combination has been shown to provide women with reliable contraceptive efficacy whilst maintaining acceptable cycle control. It is also effective for the treatment of HMB, and may offer additional non-contraceptive benefits, such as improvements in OC-associated FSD, dysmenorrhea and hormone withdrawal-associated adverse events.
Acknowledgements
Funding for this study was provided by Bayer HealthCare Pharmaceuticals. Medical writing assistance was provided by Claire Byrne and Danielle Turner of inScience Communications. The funding for this assistance was provided by Bayer HealthCare Pharmaceuticals.
Declaration of interest: F. F. has served on advisory boards for Bayer HealthCare Pharmaceuticals. F. T. has received research grants from Amgen, Nycomed and Pierre Fabre Médicament and has served on advisory boards and/or received honoraria for lectures from Amgen, Bayer HealthCare Pharmaceuticals, Codépharma, Daiichi Sankyo France, GlaxoSmithKline, Merck Sharp & Dohme (MSD), and Pierre Fabre Médicament. J. B. has participated in advisory boards for Bayer HealthCare Pharmaceuticals, Merck Sharp & Dohme (MSD), Lilly, Solvay Pharma and Boehringer Ingelheim and has lectured at meetings supported by Bayer HealthCare Pharmaceuticals, MSD, Lilly, Solvay Pharma and Boehringer Ingelheim.
References
- Lobo RA, Stanczyk FZ. New knowledge in the physiology of hormonal contraceptives. Am J Obstet Gynecol 1994;170:1499–1507.
- Düsterberg B, Ellman H, Müller U, Rowe E, Mühe B. A three-year clinical investigation into efficacy, cycle control and tolerability of a new low-dose monophasic oral contraceptive containing gestodene. Gynecol Endocrinol 1996;10:33–39.
- Huber J, Foidart JM, Wuttke W, Merki-Feld GS, The HS, Gerlinger C, Schellschmidt I, Heithecker R. Efficacy and tolerability of a monophasic oral contraceptive containing ethinylestradiol and drospirenone. Eur J Contracept Reprod Health Care 2000;5:25–34.
- Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update 2006;12:169–178.
- Spona J, Feichtinger W, Kindermann C, Moore C, Mellinger U, Walter F, Gräser T. Modulation of ovarian function by an oral contraceptive containing 30 micrograms ethinyl estradiol in combination with 2.00 mg dienogest. Contraception 1997;56:185–191.
- Kuhl H. Pharmacology of estrogens and progestogens: influence of different routes of administration. Climacteric 2005;8 Suppl 1:3–63.
- Inman WH, Vessey MP, Westerholm B, Engelund A. Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. Br Med J 1970;2:203–209.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception 2007;75:344–354.
- Gallo MF, Nanda K, Grimes DA, Schulz KF. 20 mcg versus >20 mcg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev 2005:CD003989.
- Gestodene Study Group 324. Cycle control, safety and efficacy of a 24-day regimen of gestodene 60 microg/ethinylestradiol 15 microg and a 21-day regimen of desogestrel 150 microg/ethinylestradiol 20 microg. Eur J Contracept Reprod Health Care 1999;4(Suppl 2):17–25.
- Gestodene Study Group 322. The safety and contraceptive efficacy of a 24-day low-dose oral contraceptive regimen containing gestodene 60 microg and ethinylestradiol 15 microg. Eur J Contracept Reprod Health Care 1999;4(Suppl 2):9–15.
- Lindberg UB, Crona N, Stigendal L, Teger-Nilsson AC, Silfverstolpe G. A comparison between effects of estradiol valerate and low dose ethinyl estradiol on haemostasis parameters. Thromb Haemost 1989;61:65–69.
- Speroff L, Fritz MA. Clinical Gynecologic Endocrinology and Infertility., 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005.
- Kuhnz W, Gansau C, Mahler M. Pharmacokinetics of estradiol, free and total estrone, in young women following single intravenous and oral administration of 17 beta-estradiol. Arzneimittelforschung 1993;43:966–973.
- Kuhnz W, Blode H, Zimmermann H. Pharmacokinetics of exogenous natural and synthetic estrogens and antiestrogens. In: Oettel, M, Schillinger, E. editors. Handbook of Experimental Pharmacology, Estrogens and Antiestrogens II. Berlin: Springer Verlag; 1999. p. 261–322.
- Düsterberg B, Nishino Y. Pharmacokinetic and pharmacological features of oestradiol valerate. Maturitas 1982;4:315–324.
- Zeun S, Lu M, Uddin A, Zeiler B, Morrison D, Blode H. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care 2009;14:221–232.
- Endrikat J, Blode H, Gerlinger C, Rosenbaum P, Kuhnz W. A pharmacokinetic study with a low-dose oral contraceptive containing 20 microg ethinylestradiol plus 100 microg levonorgestrel. Eur J Contracept Reprod Health Care 2002;7:79–90.
- Bostofte E, Hemmingsen L, Møller KJ, Serup J, Weber T. Serum lipids and lipoproteins during treatment with oral contraceptives containing natural and synthetic oestrogens. A controlled double-blind investigation. Acta Endocrinol 1978;87:855–864.
- Helgason S. Estrogen replacement therapy after the menopause. Estrogenicity and metabolic effects. Acta Obstet Gynecol Scand Suppl 1982;107:1–29.
- Mashchak CA, Lobo RA, Dozono-Takano R, Eggena P, Nakamura RM, Brenner PF, Mishell DR Jr. Comparison of pharmacodynamic properties of various estrogen formulations. Am J Obstet Gynecol 1982;144:511–518.
- Toy JL, Davies JA, Hancock KW, McNicol GP. The comparative effects of a synthetic and a ‘natural’ oestrogen on the haemostatic mechanism in patients with primary amenorrhoea. Br J Obstet Gynaecol 1978;85:359–362.
- Wiegratz I, Lee JH, Kutschera E, Winkler UH, Kuhl H. Effect of four oral contraceptives on hemostatic parameters. Contraception 2004;70:97–106.
- Ahrendt HJ, Makalová D, Parke S, Mellinger U, Mansour D. Bleeding pattern and cycle control with an estradiol-based oral contraceptive: a seven-cycle, randomized comparative trial of estradiol valerate/dienogest and ethinyl estradiol/levonorgestrel. Contraception 2009;80:436–444.
- Astedt B, Jeppsson S, Liedholm P, Rannevik G, Svanberg L. Clinical trial of a new oral contraceptive pill containing the natural oestrogen 17 beta-oestradiol. Br J Obstet Gynaecol 1979;86:732–736.
- Bayer Healthcare. Natazia Prescribing Information, 2010. Available from: http://berlex.bayerhealthcare.com/html/products/pi/natazia_pi.pdf [January 7th 2011].
- Chabbert-Buffet N, Chassard D, Ochsenbein E, Thomas JL, Christin-Maitre S. Inhibition of ovulation by NOMAC/E2, a novel monophasic oral contraceptive combining nomegestrol acetate and 17ß-oestradiol: a double-blind, randomised, dose-finding pilot study. Eur J Contracept Reprod Health Care 2011;16:76–84.
- Christin-Maitre S, Serfaty D, Chabbert-Buffet N, Ochsenbein E, Chassard D, Thomas JL. Comparison of a 24-day and a 21-day pill regimen for the novel combined oral contraceptive, nomegestrol acetate and 17ß-estradiol (NOMAC/E2): a double-blind, randomized study. Hum Reprod 2011;26:1338–1347.
- Csemiczky G, Dieben T, Coeling Bennink HJ, Landgren BM. The pharmacodynamic effects of an oral contraceptive containing 3 mg micronized 17 beta-estradiol and 0.150 mg desogestrel for 21 days, followed by 0.030 mg desogestrel only for 7 days. Contraception 1996;54:333–338.
- Duijkers IJ, Klipping C, Grob P, Korver T. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17 beta-oestradiol on ovarian function in comparison to a monophasic combined oral contraceptive containing drospirenone and ethinylestradiol. Eur J Contracept Reprod Health Care 2010;15:314–325.
- Endrikat J, Parke S, Trummer D, Schmidt W, Duijkers I, Klipping C. Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies. Contraception 2008;78:218–225.
- Hirvonen E, Allonen H, Anttila M, Kulmala Y, Ranta T, Rautiainen H, Sipilä P, Ylöstalo P. Oral contraceptive containing natural estradiol for premenopausal women. Maturitas 1995;21:27–32.
- Hirvonen E, Stenman UH, Mälkönen M, Rasi V, Vartiainen E, Ylöstalo P. New natural oestradiol/cyproterone acetate oral contraceptive for pre-menopausal women. Maturitas 1988;10:201–213.
- Hoffmann H, Moore C, Kovacs L, et al. Alternatives of the replacement of ethinylestradiol by natural 17beta-estradiol in dienogest-containing oral contraceptives. Drugs Today 1999;35:105–113.
- Hoffmann H, Moore C, Zimmermann H, Elger W, Schwarz S, Gräser T, Oettel M. Approaches to the replacement of ethinylestradiol by natural 17beta-estradiol in combined oral contraceptives. Exp Toxicol Pathol 1998;50:458–464.
- Kivinen S, Saure A. Efficacy and tolerability of a combined oral contraceptive containing 17 beta-estradiol and desogestrel. Eur J Contracept Reprod Health Care 1996;1:183.
- Palacios S, Wildt L, Parke S, Machlitt A, Römer T, Bitzer J. Efficacy and safety of a novel oral contraceptive based on oestradiol (oestradiol valerate/dienogest): a Phase III trial. Eur J Obstet Gynecol Reprod Biol 2010;149:57–62.
- Schubert W, Cullberg G. Ovulation inhibition with 17 beta-estradiol cyclo-octyl acetate and desogestrel. Acta Obstet Gynecol Scand 1987;66:543–547.
- Serup J, Bostofte E, Larsen S, Westergaard J. Effectivity and acceptability of oral contraceptives containing natural and artificial estrogens in combination with a gestagen. A controlled double-blind investigation. Acta Obstet Gynecol Scand 1981;60:203–206.
- Serup J, Bostofte E, Larsen S, Westergaard J, Lebech PE. Natural oestrogens for oral contraception. Lancet 1979;2:471–472.
- Wenzl R, Bennink HC, van Beek A, Spona J, Huber J. Ovulation inhibition with a combined oral contraceptive containing 1 mg micronized 17 beta-estradiol. Fertil Steril 1993;60:616–619.
- World Health Organization Task Force on Oral Contraception. A randomized, double-blind study of two combined oral contraceptives containing the same progestogen, but different estrogens. Contraception 1980;21:445–459.
- Gaussem P, Alhenc-Gelas M, Thomas JL, Bachelot-Loza C, Remones V, Ali FD, Aiach M, Scarabin PY. Haemostatic effects of a new combined oral contraceptive, nomegestrol acetate/17ß-estradiol, compared with those of levonorgestrel/ethinyl estradiol. A double-blind, randomised study. Thromb Haemost 2011;105:560–567.
- Oettel M, Breitbarth H, Elger W, et al. The pharmacological profile of dienogest. Eur J Contracept Reprod Health Care 1999;4(Suppl 1):2–13.
- Oettel M, Graeser T, Hoffmann H, Moore C, Zimmermann H, Zimmermann T. The preclinical and clinical profile of dienogest. A short overview. Drugs Today 1999;35(Suppl C):3–12.
- Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, Kato S, Mizuguchi K. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids 2008;73:222–231.
- Oettel M, Carol W, Elger W, et al. A 19-norprogestin without 17alfa-ethinyl group II: dienogest from a pharmacodynamic point of view. Drugs Today 1995;31:517–536.
- Nelson A, Sampson-Landers C, Parke S, Jensen J. Efficacy of estradiol valerate/dienogest OC: Results of 3 large studies in North America and Europe. Presented at the American Congress of Obstetricians and Gynecologists 57th Annual Clinical Meeting, Chicago, May 2–9 2009.
- Electronic Medicines Compendium. Qlaira Summary of Product Characteristics, 2011. Available from: http://www.medicines.org.uk/emc/medicine/21700/SPC/Qlaira/ [12 Apr 2011].
- Lu M, Uddin A, Foegh M. Pharmacokinetics and pharmacodynamics of a new four-phasic estradiol valerate and dienogest oral contraceptive. Obstet Gynecol 2007;109(4 Suppl):61S (abstract plus poster presentation at the 55th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists: 2007 May 5–9; San Diego, CA, USA).
- Junge W, Mellinger U, Parke S, Serrani M. Metabolic and haemostatic effects of estradiol valerate/dienogest, a novel oral contraceptive: a randomized, open-label, single-centre study. Clin Drug Investig 2011;31:573–584.
- Klipping C, Duijkers I, Parke S, Mellinger U, Serrani M, Junge W. Hemostatic effects of a novel estradiol based oral contraceptive: An open-label, randomized, crossover study of estradiol valerate/dienogest versus ethinylestradiol/levonorgestrel. Drugs R D 2011;11:159–170.
- Tans G, van Hylckama Vlieg A, Thomassen MC, Curvers J, Bertina RM, Rosing J, Rosendaal FR. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol 2003;122:465–470.
- Fraser IS, Parke S, Mellinger U, Machlitt A, Serrani M, Jensen J.. Effective treatment of heavy and/or prolonged menstrual bleeding without organic cause: pooled analysis of two multinational, randomised, double-blind, placebo-controlled trials of oestradiol valerate and dienogest. Eur J Contracept Reprod Health Care 2011;16:258–269.
- Sulak PJ, Scow RD, Preece C, Riggs MW, Kuehl TJ. Hormone withdrawal symptoms in oral contraceptive users. Obstet Gynecol 2000;95:261–266.
- Klipping C, Duijkers I, Trummer D, Marr J. Suppression of ovarian activity with a drospirenone-containing oral contraceptive in a 24/4 regimen. Contraception 2008;78:16–25.
- Spona J, Elstein M, Feichtinger W, Sullivan H, Lüdicke F, Müller U, Düsterberg B. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception 1996;54:71–77.
