Abstract
Objective: To analyze the short-term efficacy and safety over menopausal symptoms of three low-dose continuous sequential 17β-estradiol (E)/progesterone (P) parental monthly formulations using novel non-polymeric microspheres.
Methods: This was a multicenter, randomized, single blinded study in which peri- and postmenopausal women were assigned to receive a monthly intramuscular injection of 0.5 mg E + 15 mg P (Group A, n = 34), 1 mg E + 20 mg P (Group B, n = 24) or 1 mg E + 30 mg P (Group C, n = 26) for 6 months. Primary efficacy endpoints included mean change in the frequency and severity of hot flushes and the effect over urogenital atrophy symptoms at 3 and 6 months. Safety variables included changes in the rate of amenorrhea, endometrial thickness and histopathology, and local and systemic adverse events.
Results: Compared to baseline at month 6, the three treatment schemes significantly decreased the rate of urogenital atrophy symptoms and the frequency (mean number per day) and severity (mean number graded as moderate and severe per month) of hot flushes. No differences in studied efficacy parameters were observed between studied groups at baseline or at the end of the study. For all groups the most frequent adverse event was pain at the injection site; however they were all rated as mild. At the end of the study peri- and postmenopausal women displayed no significant changes in endometrial thickness or histopathology in all treated groups. The rate of amenorrhea at the end of the study decreased for all studied groups yet was less evident among postmenopausal women as compared to perimenopausal ones.
Conclusions: The three low-dose continuous sequential intramuscular monthly treatments of E/P using novel microsphere technology were effective at reducing menopausal symptoms at short-term with a low rate of adverse events. More long-term and comparative research is warranted to support our positive findings.
Introduction
More than a decade has passed since the first publication of the results of the Women's Health Initiative (WHI) study that reported a negative cost–benefit ratio and several clinical adverse events with a specific oral hormone therapy (HT) regimen (0.625 mg of conjugated equine estrogens (CEEs) + 2.5 mg of medroxyprogesterone acetate (MPA)) [Citation1]. Despite this, to-date, HT is still the most effective option for the relief of vasomotor and other symptoms related to the menopause [Citation2]. Moreover, current consensus highly recommends the use of lower dosages and the non-oral route for the control of these symptoms [Citation3].
An important goal for HT is to provide clinical efficacy with the lowest possible risk for women [Citation4]; aim that has been evidenced in multiple clinical studies, meta-analyses [Citation5] and a wide variety of hormone presentations offered on the market [Citation6]. A low dose of transdermal estrogen releases 25 μg of 17β-estradiol (E) daily, whereas an ultra-low dose releases 14 μg per day. Low-dose treatments reduce hot flushes between 60% and 70%, whereas with standard dosages this efficacy increases to 80–90% [Citation7]. Despite this, in general, low doses will confer more benefits than risks when compared to standard HT doses [Citation8]; hence research regarding HT at low dosages is still an ongoing challenge.
Recently, a new technology called “Stable Shaped Particles of Crystalline Organic Compounds” was developed for the controlled release of parental products (TechSphere®, Patent Nº US 6,287,693). This technology consists of the creation of non-polymeric crystalline structures in the form of microspheres that use cholesterol as a carrier instead of polymers. Cholesterol has the advantage of being an FDA approved ingredient for the manufacturing of drugs and is of endogenous origin, therefore providing better biocompatibility than polymers. This non-polymeric microsphere technology was used to develop a first of its kind parental HT product for the management of menopausal symptoms. This novel product would contain E microspheres (using cholesterol as carrier) and natural progesterone (P) microspheres (without cholesterol) all in an aqueous suspension, which would allow: (a) an extended E release for 30 days and P for 10–14 days (continuous sequential scheme) while maintaining plasmatic therapeutical concentrations. Estradiol dosages would be up to 30 times lower than those provided by the oral and transdermal route (b) fulfilling current recommendations regarding the use of a low-dose E (0.5–1 mg per month) and the non-oral route (intramuscular [IM]) and (c) incorporating natural P to the parental formulation, hence provide endometrial protection while avoiding the adverse effects of synthetic progestins [Citation9].
The aim of the present research was to analyze the short-term efficacy and safety over menopausal symptoms of three low-dose continuous sequential E/P parental monthly formulations using novel non-polymeric microspheres.
Methods
Study design
This was a multicenter randomized, single-blinded clinical trial with an 8-month follow-up period carried out at five primary care clinics in Mexico City affiliated to the Institute of Social Security for Government Employees (ISSSTE) and in one clinical research unit in Pachuca, near Mexico City. The Institutional Review Board of ISSSTE and the Federal Regulatory Office of the Ministry of Health reviewed and approved the research protocol. The study was conducted according to the provisions of the Declaration of Helsinki and its amendments; hence all women received a thorough explanation about the study before providing signed consent.
Study population
The present study included peri- and postmenopausal women aged 40–65 years with at least three hot flushes per day or 21 per week at baseline. Participants were allocated to randomly receive for six months (every 30 ± 3 days) an IM application of one of the following three continuous sequential schemes for the management of menopausal symptoms: 0.5 mg E + 15 mg P (Group A), 1 mg E + 20 mg P (Group B) and 1 mg E + 30 mg P (Group C). This document reports the outcomes (efficacy and safety) of these three treatments. Perimenopausal women were defined as those having irregular menses or less than 12 menstruations in the last 12 months; and postmenopausal women defined as those having no menses in the last 12 months in addition to a FSH of >40 mIU/mL [Citation10].
All recruited women were otherwise healthy. This was based on background clinical history, general clinical evaluation (including gynecological examination), clinical laboratory parameters, a normal abdominal pelvic ultrasound and mammography and a body mass index (BMI) ± 20% of the ideal range. Participants should not have been administered any type of HT in the previous 90 days. Exclusion criteria were having a chronic condition (diabetes or hypertension), refusal to participate, a history of endometrial hyperplasia or endometrial cancer, hypersensitivity to sex steroids and personal or family history of breast cancer. The appearance of adverse events and/or if participant took a drug that interacted with the treatments being tested were considered as criteria for treatment discontinuation.
The sample size was estimated using a formula for clinical trials with binary outcomes [Citation11]. The assumptions of the hypothesis were that the number of hot flushes would decrease by ≥40% after 4 weeks of exposure to any of the three treatments under study (Pendline = 0.6, Pbaseline = 1.0; α = 0.05, β = 0.2). Treatment safety was expected to be similar among them. The required sample size was 27 participants in each treatment.
Recruitment process
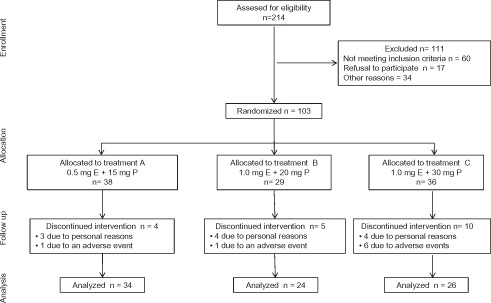
presents the CONSORT Diagram [Citation12] displaying the process of participant recruitment of this study. A total of 214 women were assessed for eligibility of which 111 did not meet the inclusion criteria. Hence, 103 subjects were included and finally randomized to one of the previously mentioned groups: A: n = 38; B: n = 29 and C: n = 36. Four participants of group A discontinued treatment (personal reasons, n = 3; adverse event, n = 1); five in group B (personal reasons, n = 4; adverse event, n = 1) and 10 in group C (personal reasons, n = 4; adverse events, n = 6). This left 84 subjects who completed the 6 months of treatment administration and follow-up (A = 34, B = 24, C = 26).
The research-coordinating center randomly allocated sets of the three treatments to each participating clinic. Treatment sequence was concealed at the study sites according to the list generated at the coordinating center.
Study endpoints
The primary efficacy endpoint was the effect over hot flushes in terms of frequency (decrease of the daily number) and intensity (changes in the total number of moderate and severe hot flashes per month). The secondary efficacy endpoint was the decrease of urogenital atrophy symptoms, such as dysuria, dyspareunia, vaginal atrophy, vaginal dryness and post-coital vaginal bleeding.
Safety variables
The safety variables included changes in the rate of baseline amenorrhea (bleeding and spotting profiles), local and systemic tolerability to the drugs and changes in endometrial thickness and histopathology. Changes in the rate of amenorrhea were evaluated according to the menopausal stage of the participant (peri- and postmenopausal). Local tolerability refers to the appearance of local symptoms or signs, such as pain, edema, skin lesions or color changes at the injection site. Systemic tolerability aimed at identifying whether the adverse events were or were not related to the drug under study.
Follow-up of participants
All participants received treatment for six months and were followed-up for eight months since baseline. Laboratory exams and gynecological examinations were performed only at baseline and at the end of the study (visit six). During each monthly visit, a pregnancy test was performed among perimenopausal women. To ensure compliance each month (for six months) a nurse administered the corresponding treatment. All laboratory tests were processed and interpreted in a certified laboratory. Likewise a certified blinded pathologist examined endometrial biopsies.
Assessment of menopausal symptoms
Hot flushes
Women consenting participation and fulfilling inclusion criteria were provided with a diary in order to register for one month the number and intensity of hot flushes per day. This was considered the baseline determination after which women received the first assigned treatment. Subsequently at each monthly visit she was provided with a new diary which was analyzed by the researcher in the next visit. Hot flush frequency was assessed each month and registered as the mean daily number. Hot flush severity was assessed at baseline and at three and six months and registered as the total number of moderate or severe hot flushes registered during each assessment period.
Symptoms of urogenital atrophy
The following symptoms were assessed at baseline and at three and six months: vaginal dryness, vaginal atrophy, dysuria, dyspareunia and post-coital vaginal bleeding.
Endometrial evaluation
Endometrial changes were evaluated through endometrial biopsies and vaginal ultrasound (endometrial thickness) performed at baseline and at the sixth month. Endometrial hyperplasia was classified according to the International Society of Gynecologic Pathologists [Citation13].
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences version 20.0 (IBM SPSS®, Armonk, NY). Data are presented as mean ± standard deviations, medians (intervals), frequencies and percentages. The Kolmogorov–Smirnov test was used to determine the normality of data distribution. According to this, comparison of continuous data within groups was performed with paired Student's t-test and between groups with the Mann–Whitney test. Comparison of percentages was performed with the chi-square test. A p value <0.05 was considered as statistically significant.
Results
Baseline demographic characteristics and reproductive history were similar between studied groups (p > 0.05) (). Median age of all participants ranged from 47 to 49 years (interval 38–62). Peri- and postmenopausal women were evenly distributed among the three studied groups (p > 0.05).
Table 1. Demographic and baseline characteristics of participants.
Efficacy
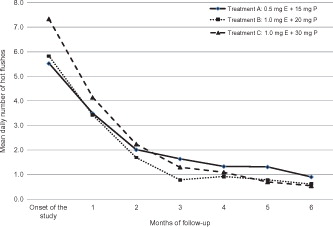
Changes in the mean daily number of hot flushes are presented in . Compared to baseline, all treatment groups displayed a significant decrease (p < 0.01) in the mean daily number of hot flushes at the third and sixth month of follow-up. For all treatment groups, the daily number of hot flushes ranged from 5 to 7 at baseline, from 0.7 to 1.6 at month three and from 0.5 (one every other day) to one per day at month six. No statistically significant differences were observed at each time interval between groups (p > 0.05).
Assessment of the severity of hot flushes, symptoms of urogenital atrophy and endometrial thickness per treatment group at proposed timelines are presented in . At baseline, the mean number of monthly moderate and severe hot flushes did not differ among studied groups and ranged from 150.6 to 157.5 and 23.3 to 57.3, respectively. All studied groups displayed a significant decrease at the sixth month in the mean number of monthly registered moderate and severe hot flushes with no differences determined between groups. Moderate severe hot flushes were reduced on average 87% for all groups; whereas for severe hot flushes this reduction was 97.3% average for all studied groups.
Table 2. Assessment of the severity of hot flashes, symptoms of urogenital atrophy and endometrial thickness per treatment group at proposed timelines.
The percentage of urogenital atrophy symptoms was similar at baseline among studied groups. Vaginal dryness was the most frequent symptom. In general, all studied groups displayed a trend toward a reduction in the percentage of symptoms at the sixth month of evaluation. As with hot flushes there were no differences between groups at month six.
Endometrial thickness among studied groups was analyzed separately for peri- and postmenopausal women. Endometrial thicknesses were similar among studied groups at baseline (for peri- and postmenopausal women); although baseline postmenopausal values were lower than perimenopausal ones. No significant differences were found in endometrial thickness at month six among studied groups (for peri- and also postmenopausal women). Endometrial thickness for postmenopausal women of all studied groups was <5 mm at both baseline and at final evaluation ().
Safety
Frequency of adverse events among studied groups is displayed in . Pain at the injection site was most commonly reported for all studied groups (A = 18.4%, B = 24.1%, C = 16.6%, p > 0.05); however this pain was considered for all women as mild. Participants receiving treatment A reported mastalgia and myalgias in 2.6%. Those receiving treatment C reported myalgias, nervousness and induration of the injection site in 2.8%.
Table 3. Frequency of adverse events per type of treatment.
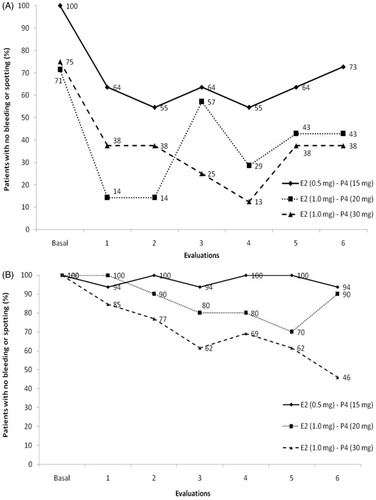
The percentage of amenorrhea among studied groups throughout the 6-month period is presented in (A for peri- and B for postmenopausal women). At the end of the study period, perimenopausal women of all the groups displayed a lower rate of amenorrhea as compared to baseline (mean 38.7% decrease for all studied groups). All postmenopausal women were in amenorrhea at baseline and at the end of the study a decrease was observed for all studied groups. However, this decrease was less evident as compared to the decrease observed for perimenopausal women. Among postmenopausal women, rate of amenorrhea was lower in group C (46%) as compared to groups A (94%) and B (90%). Participants of all studied groups presented normal endometrial biopsies at baseline with no changes (cancer or hyperplasia) found at the end of study. Equally all mammographic evaluations were normal at baseline and after treatment.
Discussion
The present study found that the three proposed continuous sequential treatment schemes using E and P non-polymeric microspheres were able to effectively reduce menopausal symptoms: hot flushes and symptoms of urogenital atrophy. At week 4 of treatment there was an overall 40% reduction of symptoms; rate that continued to decline at months 3 and 6. Regarding adverse events, all treatment schemes had acceptable local and systemic tolerability.
The aforementioned positive results, although short-term, seem to support the use of E and P non-polymeric microspheres for IM administration. Although this novel form of administration seems interesting, more long-term data are required. Nevertheless, our data may provide the basis to support a safe and innovative way of delivering drugs for the long-term treatment of menopausal symptoms. In this sense, the multiplicity of clinical high risk conditions requiring treatment added to current risk-benefit concerns for HT use have created a complex scenario that urge the need to explore new drug delivery presentations and administration routes. Bearing this in mind transdermal E was created [Citation14]. Our novel microsphere E/P presentation seems to follow the same principle.
Current recommendations are to continue carrying out clinical trials to test the efficacy and safety of low and ultra-low dose HT schemes [Citation15]. The observed efficacy for our microsphere proposed schemes (84–93% reduction in the number of daily hot flushes at month six) is comparable to the efficacy of the standard dose of oral 0.625 mg of CEE that reaches 80–90% reduction [Citation16,Citation17]. Previous studies have reported that low-dose treatments reduce the risk of endometrial hyperplasia [Citation18] and the rate of side effects, such as bleeding [Citation19]. Our study found that the mean endometrial thickness of postmenopausal women at baseline and final evaluation was <5 mm. Endometrial biopsies did not report endometrial hyperplasia and there were no cases of spotting. This finding suggests that the use of microspheres may be safe as it does not induce hyperplasia; however, this result should be interpreted with caution because this study was a short-term safety evaluation.
The inclusion of natural P was taken into account to provide endometrial protection and better effects over vessels and the brain than the use of synthetic progestins. As an added value, the monthly dose of P used in our study is lower than the one provided by oral micronized P. Indeed, P has poor oral bioavailability and presentations currently available on the market require high doses to achieve adequate plasma concentrations. Using a lower monthly dose could help to reduce the appearance of progesterone-related side effects.
Finally, our study has strengths and weaknesses. The use of microspheres that provides a monthly continuous delivery of a low E dose and natural P is indeed a potential strength. Our preliminary data contribute at exploring suitable and innovative low-dose alternatives that can minimize HT-related risks [Citation20]. Prevalence of overweight/obesity is increasing in the world and is considered a problem in aging women [Citation21]. Moreover, an important proportion of women still present hot flushes 5 years after menopause onset and related to impaired quality of life. These women definitely need to be treated [Citation22]. Symptomatic high risk women could be a potential target for treatment using our novel low dose presentation. In this sense, it has been reported that low-dose HT may in fact help to reduce many of the parameters of the metabolic syndrome [Citation23].
Not comparing with another hormonal route and the short-term follow-up period are potential weaknesses of our study. It is recommended that studies evaluating safety should complete follow-up at least 12 months and endometrial hyperplasia should not exceed a 1% [Citation24]. Our initiative aimed at testing the efficacy and adverse events of E/P microspheres at different doses in order to gather evidence that would help to define optimal doses for the monthly administration of the microspheres. The efficacy of E and P is already well known, but the optimal and innovative form of administration is not. Thus, our results are useful for the designing of a randomized clinical trial where the microspheres would be compared with a product having similar pharmacokinetic characteristics, such as transdermal patches.
To the best of our knowledge reports using this form of novel hormonal delivery is lacking in the literature; however a longer period of follow-up is warranted. Despite this, our preliminary data have an interesting potential and require more investigation.
In conclusion, the three low-dose continuous sequential intramuscular monthly E/P treatments using novel microsphere technology were effective at reducing menopausal symptoms at short-term with a low rate of adverse events. More long-term and comparative research is warranted to support our positive findings.
Acknowledgements
The authors would like to thank the support of the authorities and health personnel at the five primary care clinics affiliated to the Institute of Social Security for Government Employees (ISSSTE) of West Zone of Mexico City.
Declaration of interest
The authors declare no conflicts of interest.
This research was funded by Productos Científicos, S.A. de C.V. (Carnot Laboratories, Mexico DF, Mexico).
References
- Rossouw JE, Anderson GL, Prentice RL, et al., Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principles results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33
- De Villiers TJ, Pines A, Panay N, et al., on behalf of the International Menopause Society. Updated 2013 International Menopause Society recommendations on menopausal hormone therapy and preventive strategies for midlife health. Climacteric 2013;16:316–37
- Santen RJ, Allred DC, Ardoin SP, et al., Endocrine society: executive summary. Postmenopausal hormone therapy: an endocrine society scientific statement. J Clin Endocrinol Metab 2010;95:S1–66
- Pines A. Postmenopausal hormone therapy: the way ahead. Maturitas 2007;57:3–5
- Nelson H. Commonly used types of postmenopausal estrogen for treatment of hot flashes. JAMA 2004;291:1610–20
- Simon JA, Snabes MC. Menopausal hormone therapy for vasomotor symptoms: balancing the risks and benefits with ultra-low doses of estrogen. Exp Opin Investig Drugs 2007;16:2005–20
- Crandall C. Low-dose estrogen therapy for menopausal women: a review of efficacy and safety. J Women's Health (Larchmt) 2003;12:723–47
- Renoux C, Dell'aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case control study. BMJ 2010;340:c2519
- Utian WH, Archer DF, Bachmann GA, et al., North American Menopause Society. Position statement. Estrogen and progestogen use in postmenopausal women: July 2008 position statement of The North American Menopause Society. Menopause 2008;15:584–603
- Sierra B, Hidalgo LA, Chedraui PA. Measuring climacteric symptoms in an Ecuadorian population with the Greene Climacteric Scale. Maturitas 2005;51:236–45
- Meinert C. Clinical trials: design, conduct and analysis. New York: Oxford University Press; 1986
- Altman D, Schulz K, Moher D, et al., CONSORT GROUP (Consolidated Standards of Reporting Trials). The Revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663–94
- García AE, Cárdenas ML, Sandoval MD, Mayorga AH. Hiperplasia endometrial: análisis de serie de casos diagnosticados en biopsia endometrial. Rev Chil Obstet Ginecol 2010;75:146–52
- L'Hermite M, Simoncini T, Fuller S, Genazzani AR. Could transdermal estradiol + progesterone be a safer postmenopausal HRT? A review. Maturitas 2008;60:185–201
- Ettinger B. Rationale for use of lower estrogen doses for postmenopausal hormone therapy. Maturitas 2007;57:81–4
- Carranza LS. Dosis bajas de terapia hormonal durante el climaterio. Ginecol Obstet Mex 2008;76:267–74
- Velazco MV. Estrógenos a dosis bajas en el climaterio. Rev Med Inst Mex Seguro Soc 2007;45:381–7
- Trabal JF, Lenihan Jr JP, Melchione TE, et al. Low-dose unopposed estrogens: preliminary findings on the frequency and duration of vaginal bleeding in postmenopausal women receiving esterified estrogens over a two-year period. Menopause 1997;4:130–8
- Utian WH, Burry KA, Archer DF, et al. Efficacy and safety of low, standard, and high treatments of an estradiol transdermal system (Esclim) compared with placebo on vasomotor symptoms in highly symptomatic menopausal patients. The Esclim Study Group. Am J Obstet Gynecol 1999;181:71–9
- Paoletti R, Wenger N. Review of the International Position Paper on Women's Health and Menopause. A comprehensive approach. Circulation 2003;107:1336–9
- Lobo RA, Davis SR, De Villiers TJ, et al. Prevention of diseases after menopause. Climacteric 2014;17:540–56
- Blümel JE, Chedraui P, Baron G, et al., Collaborative Group for Research of the Climacteric in Latin America (REDLINC). Menopausal symptoms appear before the menopause and persist 5 years beyond: a detailed analysis of a multinational study. Climacteric 2012;15:542–51
- Lobo RA. Metabolic syndrome after menopause and the role of hormones. Maturitas 2008;60:10–18
- Committee for Proprietary Medicinal Products (CPMP). 1997. Points to consider on hormone replacement therapy. Available from: http://www.emea.eu.int/pdfs/human/ewp/002197en.pdf [last accessed Oct 2014]