Background
Hypertensive disorders of pregnancy (HDP) are associated with severe obstetric complications representing a leading cause of maternal mortality [Citation1]. Furthermore, HDP can be related to pregnancy complications like iatrogenic preterm delivery, intrauterine growth restriction (IUGR) and perinatal death [Citation2].
Traditional classification of HDP includes preeclampsia (PE), chronic hypertension with superimposed PE, gestational hypertension (GH) and chronic hypertension [Citation3,Citation4]. Its incidence greatly varies according to local population characteristics as a consequence of ethnicity, obesity, parity and other maternal risk factors. The incidence of PE alone is reported to be between 2% and 8% [Citation5,Citation6].
Scientific societies and committees attempted to improve the applicability of the traditional classification criteria of HDP to clinical practice by means of a detailed description of maternal functions and organs associated with hypertension in pregnancy [Citation7,Citation8]. The list of complications that classify GH as a preeclamptic phenotype had been recently enriched by IUGR, even in the absence of proteinuria [Citation8]. In fact, beyond different maternal complications and despite intensive research in the past years, the diagnosis of PE is still based on clinical manifestations, as it has been defined many decades ago as a disease characterized by hypertension and proteinuria with or without organ failure [Citation9].
An attempt to fit clinical phenotypes into this generalist criterion is the partition of HDP based on the temporal classification according to the gestational age at diagnosis (and in some cases gestational age at delivery) into early- and late-onset, with 34 weeks of gestation as the most commonly used cut-off [Citation10].
Although early-onset PE, which is more frequently associated with IUGR [Citation10], is related with a higher rate of neonatal mortality and morbidity, the late-onset PE is almost three times more frequent and accounts worldwide for the substantial proportion of late preterm birth [Citation11] and severe adverse outcome for the mother. Thus, it is not surprising that recently the members of the Global Pregnancy CoLaboratory [Citation12] suggested a revision of the main phenotypes of PE based not only on gestational age at onset but also on the co-occurrence of IUGR, deficient angiogenic factors, maternal risk conditions such as obesity, diabetes and others.
It is very likely, as it has been supported by histopathological and clinical studies since the late 1990s, that the common pathway underlying the clinical signs and symptoms of PE is represented by endothelial damage [Citation13]. A recent contribution has been added by Baschat [Citation14] and co-workers who revised in a seminal work the main risk profiles of pregnant women that might sum up to placental disease and trigger the development of cardiovascular and endothelial dysfunction. Maternal body mass index (BMI) and uterine arteries Doppler velocimetry resulted to be the two major determinants among the risk factors for PE. These findings on the role of BMI underline, on one side, the role of maternal predisposing conditions for metabolic syndrome, low-grade inflammation, the endothelial dysfunction that might be associated to late placental damage and hypertension not associated with IUGR. On the other side, shallow trophoblastic placentation, reflected by abnormal uterine arteries Doppler velocimetry, is at the basis of the trophoblastic oxidative stress and disbalance of angiogenic and anti-angiogenic factors [Citation15,Citation16]. The latter, ultimately, might damage maternal endothelium at the basis of systemic hypertension and/or cause other function or organ damage. In this case, the clinical manifestation will be associated with IUGR caused by placental insufficiency.
These findings are in agreement with epidemiological studies [Citation11] and with predictive studies as we will see in the next paragraph [Citation15–19]. Indeed, the multi-facets clinical manifestations of HDP [Citation9,Citation10] could fit well into two different pathophysiological models and, consequently, two completely different patterns of placental damage and clinical phenotypes: on one hand, the placenta with incomplete spiral artery remodeling resulting in HDP associated with IUGR (HDP-IUGR), and on the other hand, the placenta with deregulated perfusion due to an over-expansion of terminal villi [Citation20,Citation21] resulting in HDP associated with fetuses with appropriate for gestational age growth (HDP-AGAf).
The Placenta
The presence of the placenta is the main determinant of HDP, whatever are the underlying lesions and downstream effects. Once the placenta is removed the clinical symptoms of HDP ebb away, although, sometimes, there might persist residual permanent organ damage. The placental pathology is of a great help in the classification of HDP. In fact, recently, a different histology has been observed in pregnant women affected by HDP-IUGR and in those associated with HDP-AGAf [Citation12,Citation22].
The most widely known placental lesions derive from an abnormal early shallow trophoblastic invasion, anomalous changes of the spiral arteries, a small placenta with fewer and poorly branched terminal villi [Citation23–25] and trophoblastic oxidative stress. In this case, the oxidative stress leads to the release of anti-angiogenic factors like soluble FMS-like tyrosine kinase-1 (sflt-1) and to the reduction of proangiogenic factors like placental growth factor (PlGF).
A second placental pathological description proved an increased terminal villi branching with a relative reduction and an under-perfusion of the intervillous space resulting in a similar trophoblastic oxidative stress.
The former placental lesion is associated with HDP-IUGR, the latter with HDP-AGAf. In cases of HDP-AGAf, the early placental development is normal, and the trophoblastic oxidative stress and its related lesions occur only late in pregnancy, worsen the anti-angiogenic condition that normally develops in late placental growth and overlap to the proinflammatory status typical of the third trimester ().
Figure 1. Markers of trophoblast stress in normal pregnancy (adapted from [Citation21] and [Citation43]).
![Figure 1. Markers of trophoblast stress in normal pregnancy (adapted from [Citation21] and [Citation43]).](/cms/asset/623b9e72-efe5-42ff-8ee1-4b5695519258/igye_a_1115833_f0001_c.jpg)
In agreement with this data, our preliminary results confirmed different placental histology in the two different clinical phenotypes of HDP. In 15 cases of HDP-IUGR, we observed a highly significant association with lesions on the maternal vascular side, while in 10 cases of HDP-AGAf there was an association with fetal vascular side lesions, characterized by villi immaturity and excessive branching with consequent restricted inter-villous perfusion [Citation23].
The Mother
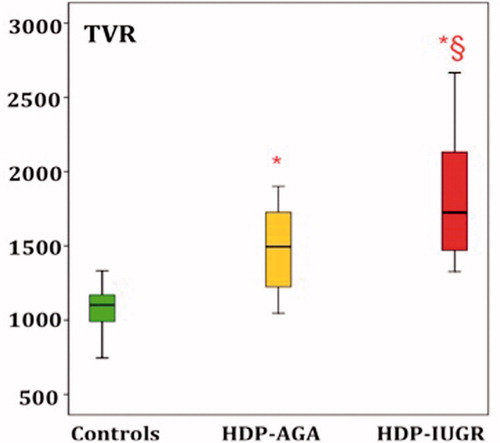
More than 10 years ago, the group of Valensise started to study the maternal hemodynamic changes in pregnancies complicated by PE. The aim of those studies was to evaluate the changes in cardiac output and total vascular resistance (TVR) in different subtypes of PE. Indeed, the main findings were that the early-onset PE was characterized by high TVR and low cardiac output and the late-onset PE by low TVR and higher cardiac output [Citation26]. Other research groups confirmed these findings supporting the role of maternal hemodynamic as an additional tool to study PE, even though some results, especially for late-PE, are still contradictory [Citation27].
In the preliminary data from our group, we analyzed the same maternal hemodynamic parameters using the clinical classification of HDP phenotypes. We found that TVR was the highest in HDP-IUGR group than in HDP-AGAf and uneventful controls ().
Predictivity
A proof of principle that maternal risk factors bear a significant impact on the applicability of predictive models based on first-trimester assessment of placental and maternal risk factors is represented by the work of Oliveira et al. [Citation28]. When applied to a population affected by obesity epidemics such as the large metropolitan area of Baltimore, MD, USA the predictive algorithms developed in Europe underperformed compared to the original predictive studies. Most likely, this is due to the fact that the risk factors for metabolic syndrome, both cardiovascular and diabetogenic, were not comparable with those of populations on which the original models were developed. Under these epidemiological conditions, the higher prevalence of HDP-AGAf, caused by high maternal BMI, alters the expected relative prevalence of HDP-IUGR and HDP-AGAf both in early and late-onset hypertension. Consequently, the predictive algorithm in the first trimester, that is strictly associated with shallow trophoblastic invasion, loses its predictive value.
The main criticism to many existing predictive algorithms for PE is that they are modeled to predict, starting from the first trimester, a “single disease” [Citation18] and not a syndrome characterized by the presence of different phenotypes. If, indeed, we try to separate the two major clinical phenotypes we have on one hand a valid predictive algorithm for HDP-IUGR based on uterine arteries Doppler velocimetry, anamnestic characteristics, angiogenic and anti-angiogenic factors. However, on the other hand, the predictive model for HDP-AGAf, based on these first trimester parameters, will have a low sensibility and specificity, as shown by our data and as we will discuss below [Citation29].
The poor angiogenic performance of the placenta since the first trimester in HDP-IUGR and its worsening status can be predicted by assessing the uterine arteries Doppler velocimetry, vascular growth factors and eventually placental volume [Citation30]. These markers persist throughout gestation and are eventually met by reduced fetal growth observed by ultrasound fetal biometry and, at birth, by the restricted and abnormal histology of the placental tissue.
In our recent prospective study [Citation29], in an unselected cohort of more than 4000 women examined in the first trimester, the model based on the uterine arteries Doppler velocimetry performed far more better in predicting HDP-IUGR than HDP-AGAf in the gestational period from 25 to 40 weeks. The better prediction of women that will develop HDP-IUGR, was observed in spite of the fact that the most “severe” cases occurred, as expected, prior to 34 weeks of gestation. However, additional cases of HDP-IUGR occurred beyond 34 weeks of gestation. In fact, according to the temporal classification the “early” group included a mix of cases with HDP-IUGR and HDP-AGAf. The latter had normal uterine arteries Doppler velocimetry in the first trimester hence lowering the predictive values of the model based on the temporal classification. Again, these data confirmed that the time-domain approach to the HDP classification is unessential: in fact, although less frequently, we observed late PE with IUGR and early PE with normally grown fetuses and placenta not affected by shallow trophoblastic invasion.
In a subset of this cohort, additional items of maternal history and central aortic pressure were collected and included in the predictive model. The BMI, the family history of hypertension and the mean aortic and brachial pressure were significantly higher in women who later developed HDP-AGAf than in uncomplicated pregnancies. There were no significant differences of these first trimester parameters between HDP-IUGR and uncomplicated pregnancies.
Bedside diagnosis of two different phenotypes of HDP
On the basis of these considerations, we can hypothesize two models of HDP, one associated with shallow trophoblastic invasion, the abnormal vascular impedance of uterine arteries, the early imbalance between proangiogenic factors (PlGF) and anti-angiogenic factors (sflt-1; vascular endothelial growth factor (VEGF)-R) and IUGR. The other type is substantially associated with maternal risk factors blown up by placental oxidative stress due to placenta terminal villi overcrowding [Citation20] and decidual lesions [Citation21] associated with maternal chronic inflammation [Citation31] and AGA fetuses [Citation32].
To support this view, our group recently published a multicenter retrospective study [Citation33] proving the existence of two different clinical phenotypes of HDP through simple bedside diagnostic tools such as the measurement of the fetal abdominal circumference and the evaluation of the uterine arteries Doppler velocimetry (). As an additional proof of the role of maternal risk factors in HDP-AGAf, the odd ratio for metabolic syndrome was as high as 2.63. This study adds genuine new data that might help to differentiate HDP associated with IUGR from HDP associated with AGAf, based on prenatal simple examinations.
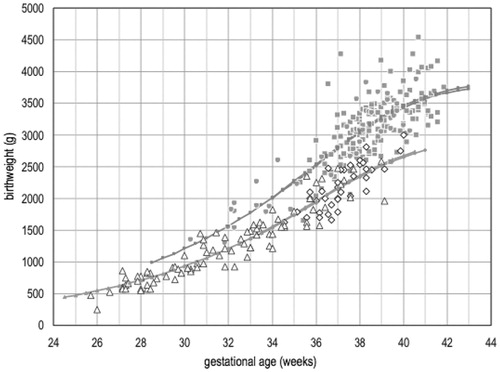
Figure 3. Birthweight as a function of gestational age at delivery in HDP-AGAf and HDP-IUGR groups with early (34 weeks of gestation) or late (≥34 weeks of gestation) gestational age at diagnosis. Grey circles: birthweight in HDP-AGAf group <34. Gray squares: birthweight in HDP-AGAf group ≥34. White triangles: birthweight in HDP-IUGR group <34. White rhombus: birthweight in HDP-IUGR group ≥34 (modified from reference 34).
Traditional and innovative therapies
A careful reconstruction of possible different physiopathological pathways at the basis of clinical phenotypes of HDP could allow to model tailored predicting strategies, better prevention interventions and to overcome the present deadlock by personalizing the therapy of HDP on the basis of the clinical subtype [Citation34].
In this view, labetalol and hydralazine are not equipollent drugs for the treatment of HDP, whereas one has a predominantly peripheral vasodilatory action and the other has a combined blocking effect on alpha–beta-adrenergic receptors. If it is true that HDP-AGAf are associated with low peripheral TVR, the use of an alpha–beta-blocking drug might be the right choose to treat hypertensive women with a low cardiac output.
Moreover, it would be interesting to define which phenotypes of HDP might benefit from the prevention with low-molecular-weight-heparin (LMWH) and a low dose of acetylsalicylic acid (ASA, 100 mg).
As stressed before, dysfunctional placenta and endothelial damage, associated with IUGR, do not include all clinical phenotypes. A large majority of women with HDP give birth to normal weight babies and placentas. In these cases, endothelial dysfunction, hypertension and organ damage are mainly caused by maternal factors that result in the low-grade inflammation, and, most likely, the best preventive intervention could be the changing of the lifestyle, nutrition and physical exercise. On the contrary, the powerful immunomodulation of LMWH might play a role in the prevention of HDP-IUGR. Heparin is not primarily an antithrombotic peptide: it is parsimoniously released by mast cells in sites of tissue injury, with a substantial impact on inflammation and oxidative stress and displays a critical immunomodulation activity on trophoblast. This explains why LMWH worked better in trials focused on early severe HDP associated with IUGR. LMWH should be considered to prevent the recurrence of HDP-IUGR, or in other terms the HDP of placental origin [Citation35–39].
In the same way, ASA has been validated as an effective therapy in the prevention of the recurrence of PE and IUGR, even if without a clear distinction between the two types of diseases [Citation40,Citation41]. A physiopathological classification could better characterize the action and the efficacy of this drug.
In order to prevent not only the recurrence of the early placental cases but also the occurrence of new cases, there are ongoing studies that provide the use of a daily dose of ASA 100 mg in patients at high risk of PE screened in the first trimester.
Conclusion
New evidences suggest that there are different phenotypes of HDP, in a view that goes beyond the temporal classification. The distinction of HDP in a placental phenotype (associated to IUGR) and in a maternogenic phenotype (associated to AGAf) gives also a new light on the use of the ultrasound biophysical tools (evaluation of fetal growth, Doppler velocimetry of the uterine arteries), maternal hemodynamic evaluation or placental biomarkers. The application of these instruments, both early and late in pregnancy, could give us the tools to look at the HDP with new lenses [Citation33,Citation42].
Declaration of interest
The authors report no conflicts of interest.
References
- Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States 1998 to 2005. Obstet Gynecol 2010;116:1302–9
- Roberts JM, Pearson G, Cutler J, Lindheimer M. Summary of the NHLBI working group on research on hypertension in pregnancy. Hypertension 2003;41:437–45
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013;122
- Hypertension in pregnancy. The management of hypertensive disorders during pregnancy Issued: August 2010, last modified: January 2011. NICE clinical guideline 107
- Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol 2009;113:1299–306
- Hypertensive disorders in pregnancy, Guideline summary. New York State Department of Health, May 2013
- Lowe SA, Bowyer L, Lust K, et al. The SOMANZ guideline for the management of hypertensive disorders of pregnancy. 2014
- Magee LA, Pels A, Helewa M, et al. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 2014;29:416–38
- Ferrazzi E, Stampalija T, Aupont J. The evidence for late-onset pre-eclampsia as a maternogenic disease of pregnancy. Fetal Matern Med Rev 2013;24:18–31
- Steegers AE, von Dadelszen P, Duvekot JJ, Pijnenborg R. Seminar, preeclampsia. Lancet 2010;376:631–44
- Khan KS, Wojdyla D, Say L, et al. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367:1066–74
- Myatt L, Redman CW, Staff AC, et al. Strategy for standardization of pre-eclampsia research study design. Hypertension 2014;63:1293–301
- Roberts JM. Objective evidence of endothelial dysfunction in preeclampsia. Am J Kidney Dis 1999;33:992–7
- Baschat AA. First-trimester screening for pre-eclampsia: moving from personalized risk prediction to prevention. Ultrasound Obstet Gynecol 2015;45:119–29
- Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med 2009;22:1021–38
- Stampalija T, Chaiworapongsa T, Romero R, et al. Maternal plasma concentrations of sST2 and angiogenic/anti-angiogenic factors in pre-eclampsia. J Matern Fetal Neonatal Med 2013;26:1359–70
- Chappell LC, Duckworth S, Seed PT, et al. Diagnostic accuracy of placental growth factor in women with suspected preeclampsia: a prospective multicenter study. Circulation 2013;128:2121–31
- Akolekar R, Syngelaki A, Poon L, et al. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn Ther 2013;33:8–15
- Rana S, Karumanchi SA, Lindheimer MD. Angiogenic factors in diagnosis, management, and research in preeclampsia. Hypertension 2014;63:198–202
- Redman CW, Sargent IL, Staff AC. IFPA Senior Award Lecture: making sense of pre-eclampsia – two placental causes of preeclampsia? Placenta 2014;35:S20–5
- Staff AC, Redman CW. IFPA Award in Placentology Lecture: preeclampsia, the decidual battleground and future maternal cardiovascular disease. Placenta 2014;35:S26–31
- Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta 2009;30:32–7
- Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta 2008;29:S86–91
- Mayhew TM, Ohadike C, Baker PN, et al. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta 2003;24:219–26
- Egbor M, Ansari T, Morris N, et al. Maternal medicine: morphometric placental villous and vascular abnormalities in early- and late-onset pre-eclampsia with and without fetal growth restriction. BJOG 2006;113:580–9
- Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008;52:873–80
- Melchiorre K, Sutherland G, Sharma R, et al. Mid-gestational maternal cardiovascular profile in preterm and term pre-eclampsia: a prospective study. BJOG 2013;120:496–504
- Oliveira N, Magder LS, Blitzer MG, Baschat AA. First-trimester prediction of pre-eclampsia: external validity of algorithms in a prospectively enrolled cohort. Ultrasound Obstet Gynecol 2014;44:279–85
- Stampalija T, Quadrifoglio M, Di Martino D, et al. Differentiation of clinical phenotypes of hypertensive disorders in pregnancy by first trimester uterine artery Doppler velocimetry. Submitted for publication
- Schwartz N, Coletta J, Pessel C, et al. Novel 3-dimensional placental measurements in early pregnancy as predictors of adverse pregnancy outcomes. J Ultrasound Med 2010;29:1203–12
- Borzychowski AM, Sargent IL, Redman CWG. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med 2006;11:309–16
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2010;30:473–82
- Ferrazzi E, Zullino S, Stampalija T, et al. Bedside diagnosis of two major clinical phenotypes of hypertensive disorders of pregnancy. Ultrasound Obstet Gynecol 2015. [Epub ahead of print]. doi:10.1002/uog.15741
- Valensise H, Vasapollo B, Novelli GP, et al. Maternal and fetal hemodynamic effects induced by nitric oxide donors and plasma volume expansion in pregnancies with gestational hypertension complicated by intrauterine growth restriction with absent end-diastolic flow in the umbilical artery. Ultrasound Obstet Gynecol 2008;31:55–64
- Ferrazzi E, Muggiasca M, Gervasi MT. Low molecular weight heparin: does it represent a clinical opportunity for preventing preeclampsia associated with fetal growth restriction? J Matern Fetal Neonatal Med 2015;28:1525–9
- Dodd JM, McLeod A, Windrim RC, Kingdom J. Antithrombotic therapy for improving maternal or infant health outcomes in women considered at risk of placental dysfunction. Cochrane Database Syst Rev 2013;1:1–51
- Rodger MA, Carrier M, Le Gal G, et al. Meta-analysis of low-molecular-weight heparin to prevent recurrent placenta-mediated pregnancy complications. Blood 2014;123:822–8
- Martinelli I, Ruggenenti P, Cetin I, et al. Heparin in pregnant women with previous placenta-mediated pregnancy complications: a prospective, randomized, multicenter, controlled clinical trial. Blood 2012;119:3269–75
- Conserva V, Muggiasca M, Arrigoni L, et al. Recurrence and severity of abnormal pregnancy outcome in patients treated by low-molecular-weight heparin: a prospective pilot study. J Mater Fetal Neonatal Med 2012;25:1467–73
- Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol 2010;116:402–14
- Askie LM, Duley L, Henderson-Smart DJ, et al. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet 2007;369:1791–8
- Verlohren S, Melchiorre K, Khalil A, Thilaganathan B. Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: providing insights into the dual etiology of late-onset pre-eclampsia. Ultrasound Obstet Gynecol 2014;44:293–8
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672–83