To the editor
Some papers have reviewed immune thrombocytopenic purpura (ITP) associated with lymphoma Citation[1], Citation[2]. The prevalence of ITP was more common in chronic lymphocytic leukemia/small cell lymphocytic lymphoma (CLL/SLL) and hodgkin's disease (HD) than in non-hodgkin's lymphoma (NHL) without CLL Citation[1]. Although diffuse large B cell lymphoma (DLBL) is the most frequent subtype of NHL, ITP associated with DLBL was rarely reported. We report a case of 11 months ITP preceding manifestation of DLBL.
A 21-year-old man was referred to our hospital with the complaint of ecchymoses and gingival bleeding for 5 days. He denied fever, weight lose, or other systemic symptom. No history of infection preceded the onset of bleeding. Initial physical examination showed scatter petechias on his extremities, tongue, and soft palate. There was no hepatosplenomegaly or palpable lymphadenopathy. Laboratory test revealed his platelet count was 12 × 109/L, and his hemoglobin level, white blood cell counts, differentials, and mean platelet volume were normal. Serum lactate dehydrogenase (LDH) and beta 2-microglobulin were within normal range. An antinuclear antibody was also negative. Bone marrow smear showed normal erythropoiesis and granulopoiesis, an increase in the number of megakaryocytes with dysmaturity. He was treated with prednisone in a dosage of 1 mg/kg/day, which led to an increase in his platelet count to 61 × 109/L after 5 days. Platelet count reached 126 × 109/L after 2 weeks of treatment. When the prednisone was tapered down to 30 mg daily for 1 week, his platelet count had decreased to 15 × 109/L. He again suffered from skin and mucosal bleeding. High dose dexamethasone (40 mg) was given for 4 consecutive days, which led to a transient increase of his platelet count to 48 × 109/L. Two more cycles of dexamethasone were given. Thereafter, his platelet count remained in the normal range. During the follow-up period, his platelet count was above 90 × 109/L.
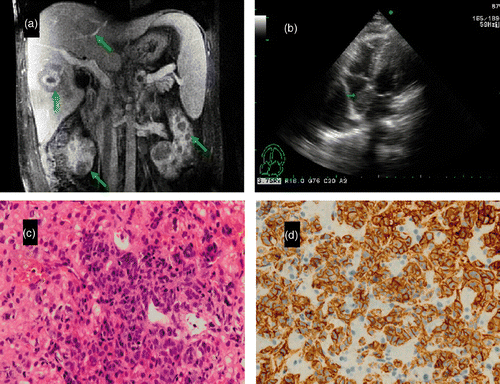
Eight months later, the patient was admitted to our hospital with yellow complexion, fatigue, and abdominal distension. Physical examination revealed jaundice, hepatosplenomegaly, and extremity edema. Enlarged lymph node was not palpated. Laboratory tests revealed liver dysfunction, with alanine transaminease (ALT) 215.6 U/L, albumin 30.7 g/L, total bilirubin (TB) 52.4 µmol/L, alkaline phosphatase (ALP) 535.3 U/L, and LDH 1390 U/L. Complete blood count was normal. Serology was negative for hepatitis B, hepatitis C virus and cytomegalovirus. Antimitochondrial antibody, anti-liver/kidney microsomal antibodies, and antibodies to soluble liver antigen were negative. Computed tomography (CT) and MRI scans of the abdomen and chest showed multiple nodules scattered in the liver, spleen, pancreas, and bilateral kidneys (). They also revealed hepatosplenomegaly, porta hepatic and peri-pancreatic lymphadenopathy, ascites, and bilateral hydrothorax. An echocardiography showed a mass measuring 2.6 × 2.4 cm in the right atrium, suggesting a right atrial tumor or a thrombus (), but a negative D-dimer test ruled out thromboembolism. A percutaneous CT guided liver biopsy was performed. The histopathology showed diffuse lymphoid proliferation with clear cytoplasmic cells and large, round nuclei (). Cells in the lesion were positive for CD20 (), CD30, BCL-6 (week) and CD45. CD10, MPO, ALK and Mum-1 were negative; Ki-67 labeling index was 50–60%. Based on the morphology and immunophenotype of liver biopsy, the diagnosis of DLBL was made. Pleural effusion revealed a predominant large lymphocyte and high LDH level. Flow cytometry test showed the cells were positive for CD20 and CD45. A biopsy of the bone marrow showed it was not involved by lymphoma. Based on these findings, the patient was diagnosed with DLBL with liver, kidney, pancreas, cardiac, and serous cavity involvement. The patient was immediately started on R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) and after the first cycle, distension, dyspnea and jaundice were relieved. Transient ALT and LDH increase was observed following chemotherapy, which represented lysis of lymphoma cells. ALT, LDH and TB decreased to the normal range before the second cycle of chemotherapy. Echocardiography revealed a mass in the right atrium that decreased to 1.6 × 1.0 cm. The treatment response confirmed cardiac involvement of lymphoma. The patient received consecutive R-CHOP. MRI revealed a striking decrease in the size of masses in the liver, spleen, kidney and pancreas, and no abdominal lymphadenopathy after three cycles of R-CHOP. During chemotherapy, the patient had mild leucocytopenia and thrombocytopenia, which recovered at the intermission of chemotherapy. This patient is receiving further cycles of R-CHOP chemotherapy.
Figure 1. (A) MRI scan of the abdomen revealed multiple nodules scattered in the liver, kidney (arrows). (B) Echocardiography showed a mass in the right atrium (arrow). (C) Liver histopathology showed diffuse lymphoid proliferation, with clear cytoplasmic cells and large, round nuclei. HE staining; original magnification ×400. (D) Immunohistochemisty revealed cells were CD20 positive; original magnification ×400.
ITP can be classified based on the absence or presence of other diseases (primary or secondary). In the reported case, ITP presented without any manifestation or evidence of any underlying disease at onset. Although ITP is believed to be caused by autoantibodies, experts disagree on the role of various tests for platelet antibodies in the diagnosis of ITP Citation[3]. The platelet antibody was not measured in the present patient. The patient was initially diagnosed with primary ITP based on clinical impression. Steroid treatment was effective, but he developed aggressive lymphoma 8 months after recovery from ITP. He maintained a normal platelet count during the course of DLBL. We considered if ITP could be pathogenetically related to DLBL. We retrieved published papers from 1960 to 2010, and in three reported cases ITP occurred 4, 18 and 46 months preceding DLBL Citation[4–6]. In five cases ITP and DLBL occurred concurrently Citation[7–11]. It was interesting that most of the sporadically reported cases had extranodal lymphoma including kidney Citation[1], mesentery Citation[8] and adrenal glands Citation[7], Citation[9], Citation[1]. Our case had multiple extranodal involvements including liver, kidney, pancreas, cardiac and serous cavity. It was uncertain whether ITP had any relationship with extranodal involvement. The present case achieved complete remission of ITP after steroid treatment alone. Only one case responded well to the prednisone and cyclophosphamide Citation[6], while the others had partial or no responses to the treatment for ITP. More than half of the ITP patients achieved complete remission after anti-lymphoma treatment Citation[2]. In some patients with ITP, rituximab has been associated with a reduction in specific platelet autoantibodies and an increase in platelet count Citation[1]. The sustained response of platelets might be attributed to the depletion of CD 20 B cells as precursors of antibody producing plasmablasts.
Lymphoma is commonly associated with autoimmune cytopenias. In most of the cases, ITP occurred at or after diagnosis of lymphoma, especially in cases of CLL and HD Citation[1], Citation[1]. Cases of ITP that occurred prior to diagnosis of lymphoma were also documented. A high rate of CLL phenotype lymphocytes have been discovered in seemingly primary ITP Citation[1]. It might be that ITP was a paraneoplastic phenomena in subclinical lymphoma. The finding of platelet antibody production by DLBL cells at the time of ITP supported the assumption that in some cases the platelet antibody was produced by lymphoma cells Citation[9].
In summary, ITP occurring prior to the diagnosis of aggressive DLBL is rare. We suppose that not only indolent lymphoma such as CLL, but also aggressive lymphoma like DLBL, might have a subclinical stage of autoimmune disorder. Limited data are available to draw the pathogenesis of ITP associated DLBL. The present case and published cases might still provide useful information in this matter.
Declaration of interest: The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- Visco C, Rodeghiero F. Immune thrombocytopenia in lymphoproliferative disorders. Hematol Oncol Clin North Am 2009; 23: 1261–1274
- Hauswirth AW, Skrabs C, Schutzinger C, Raderer M, Chott A, Valent P, Lechner K, Jager U. Autoimmune thrombocytopenia in non-Hodgkin's lymphomas. Haematologica 2008; 93: 447–450
- Chong BH, Ho SJ. Autoimmune thrombocytopenia. J Thromb Haemost 2005; 3: 1763–1772
- Fink K, Al-Mondhiry H. Idiopathic thrombocytopenic purpura in lymphoma. Cancer 1976; 37: 1999–2004
- Caminal Montero L, Susano RC, Marroquin AG, Taborcias D. [Immune thrombocytic purpura as the form of presentation of a non-Hodgkin's lymphoma]. Rev Clin Esp 1994; 194: 998–999
- Kaden BR, Rosse WF, Hauch TW. Immune thrombocytopenia in lymphoproliferative diseases. Blood 1979; 53: 545–551
- Baudard M, Pagnoux C, Audouin J, Buy JN, Bethoux JP, Delmer A, Zittoun R. Idiopathic thrombocytopenic purpura as the presenting feature of a primary bilateral adrenal non Hodgkin's lymphoma. Leuk Lymphoma 1997; 26: 609–613
- Moriwaki Y, Naka M, Yamamoto T, Takagi S, Hidaka T, Takahashi S, Hada T, Nishigami T, Higashino K. Malignant lymphoma in the mesentery with immune thrombocytopenia. Intern Med 1992; 31: 1185–1189
- Nobuoka A, Sakamaki S, Kogawa K, Fujikawa K, Takahashi M, Hirayama Y, Takayanagi N, Ikeda H, Sekiguchi S, Niitsu Y. A case of malignant lymphoma producing autoantibody against platelet glycoprotein Ib. Int J Hematol 1999; 70: 200–206
- Tadokoro J, Gunji H, Handa T, Aoyagi M, Nakamura Y, Saito K, Furusawa S. [Primary renal non-Hodgkin's lymphoma presenting as immune thrombocytopenia]. Rinsho Ketsueki 2001; 42: 41–46
- Yamamoto E, Ozaki N, Nakagawa M, Kimoto M. Primary bilateral adrenal lymphoma associated with idiopathic thrombocytopenic purpura. Leuk Lymphoma 1999; 35: 403–408
- Stasi R. Rituximab in autoimmune hematologic diseases: Not just a matter of B cells. Semin Hematol 2010; 47: 170–179
- Lechner K, Chen YA. Paraneoplastic autoimmune cytopenias in Hodgkin lymphoma. Leuk Lymphoma 2010; 51: 469–474
- Mittal S, Blaylock MG, Culligan DJ, Barker RN, Vickers MA. A high rate of CLL phenotype lymphocytes in autoimmune hemolytic anemia and immune thrombocytopenic purpura. Haematologica 2008; 93: 151–152
