To the editor
GpIIb/IIIa receptor inhibitors (abciximab, eptifibatide, and tirofiban) which are used to treat the patients with acute coronary syndrome (ACS) cause thrombocytopenia with or without bleeding. The incidence of thrombocytopenia during the clinical use of tirofiban which is given to the patient with ACS ranges from 1% to 2% Citation[1]. On the other hand, the incidence of severe thrombocytopenia ranges from 1% to 5% Citation[2].
We have presented a profound-severe thrombocytopenia case which developed 24 hours after the tirofiban infusion and was treated with intravenous immunoglobulin (IVIG).
In an 87-year-old female patient, coronary angiography was performed after the patient was diagnosed with ACS who was admitted to the emergency service with the complaint of chest pain. Percutaneous coronary angioplasty and stent placement to the right coronary artery were performed. The patient was treated with aspirin and beta blocker; 5000 IU, IV bolus of unfractionated heparin (UFH) followed by an UFH infusion of 1000 IU/hour and tirofiban 0.4 µg/kg/min for 30 min followed by 0.1 µg/kg/min IV were started. When the patient was first admitted, her hemoglobin level was 10.5 g/dl, white blood cell level was 13 × 103 /uL, platelet level was 242 × 103 /uL, PT-aPTT, and fibrinogen levels were normal. When it was found out that her aPTT level was 115 at the 12th hour, heparin infusion, was stopped. Because the patient had active melena, the patient's blood count showed that her hemoglobin level was 8.5 g/dl, and platelet level was 3 × 103 /uL. Tirofiban infusion which she had been taking for 24 hours was stopped because of the gastrointestinal system (GIS) bleeding and thrombocytopenia. The patient was examined by the hematology department because of the thrombocytopenia. The physical examination results of the patient showed the presence of active hematemesis in nasogastric catheter and ecchymosis in injection area. Platelet count was confirmed by peripheral blood smear. Peripheral blood smear showed the presence of one platelet in each area, platelet clumping, and fragmentation in erythrocyte were not observed, pathologic cell was not found out. PT-aPTT, fibrinogen, and D-dimmer tests performed for disseminate intravascular coagulopathy were normal. Because the patient had an active GIS bleeding problem, she received erythrocyte and platelet suspension transfusion, and was started with 400 mg/kg/day IVIG. One day after the tirofiban was stopped and at the 12th hour of IVIG treatment her platelet count was found as 26 × 103 /uL. On the third day of the IVIG treatment, thrombocyte levels increased up to 152 × 103 /uL. Platelet counts variance after the tirofiban was stopped and during the IVIG treatment was given in . After the signs of GIS bleeding disappeared, the patient was discharged taking antiaggregant, and antianginal. Clinical observations did not show any problem concerning thrombocytopenia or bleeding. Thrombocytopenia mechanism, caused by GpIIb/IIIa receptor inhibitors, could not be understood completely. This may result from the antiplatelet antibodies caused by the use of medicine Citation[3]. Another hypothesis is the antibodies, produced naturally against the platelet neoepitopes during the use of GpIIb/IIIa receptor inhibitors Citation[4].
Figure 1. Thrombocyte counts variance after the tirofiban was stopped and during the IVIG treatment.
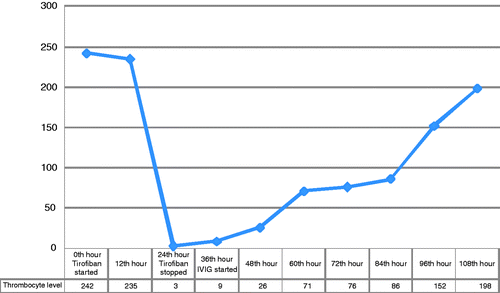
When the thrombocytopenia develops in the patients treated with GpII/bIIIa receptor inhibitors and heparin, differential diagnosis should be applied to search for other reasons. Peripheral blood smear should be examined in terms of pseudothrombocytopenia and clumping platelets should be verified to confirm EDTA phenomenon. In our case, clumping platelets were not observed in peripheral blood smear, one platelet was observed in each area when it was examined by microscope. The platelet count was presented to be lower in blood samples anticoagulated with citrate. Lower platelet count was confirmed by these tests results. Since, our patient used heparin, heparin induced thrombocytopenia (HIT) was among the differential diagnosis. To confirm the diagnosis, antibodies to platelet factor 4/heparin should be examined by enzyme-linked immunoabsorbant assay. Our patient was not examined for the antibodies resulting from HIT. Clinically, our patient is not likely to have HIT, which is typically characterized by thrombocytopenia occurring 5 days after the start of heparin Citation[5]. Since our patient did not have a prior exposure to heparin, and took heparin only for 12 hours, and since the thrombocytopenia developed 24 hours after the use of heparin, the patient was thought not to have HIT. In our case, profound thrombocytopenia, being 3 × 103 /uL, was most probably caused by “tirofiban” which developed 24 hours after it was used.
Tirofiban induced thrombocytopenia usually develops after 24 hours from the start of the treatment, however, it may also develop 10 days after the treatment Citation[6]. In our case, thrombocytopenia was observed 24 hours after the tirofiban infusion. In this situation, if the platelet count was not severely low, medicine treatment may be stopped, and the patient may be observed. In the literature, platelet counts may turn out to be normal within 3 or 6 days, even just after the stop of tirofiban, in tirofiban induced thrombocytopenia cases Citation[4], Citation[7]. If a severe thrombocytopenia and/or clinical bleeding are observed at the same time, the patient can be treated with steroid, and platelet transfusions. Moreover, because the reason of thrombocytopenia is drug dependent antibodies (DDAbs) in cases of tirofiban induced thrombocytopenia, IVIG treatment which inhibited DDAb and include FcχRIIA blockade of reticuloendothelial cells, caused a good and long-lasting recovery of the platelet count may be applied Citation[6]. Since our patient has active GIS bleeding, platelet, and erythrocyte transfusion were given to the patient. Steroid could not be given because of the active bleeding. Since DDAbs induced thrombocytopenia may be present, the patient was treated with IVIG for 5 days (400 mg/kg/day). According to the research, in the patients’ plasma, who were treated with abciximab, eptifibatide, and tirofiban, the presence of DDAbs was demonstrated only in the patients who took tirofiban. Therefore, especially in tirofiban induced thrombocytopenia cases, the clinically response to IVIG was quite good because of the presence of DDAbs Citation[6]. However, there are some cases in which thrombocytopenia continues despite the stop of tirofiban or active bleeding is observed which requires IVIG treatment Citation[4], Citation[6], Citation[8]. A quick recovery was observed in the platelet count just after the 20 hours of the IVIG treatment Citation[8]. In our case, it was observed that the platelet counts increased at the 12th hour of the IVIG treatment and became normal after the second day. This has shown that IVIG should be preferred to get quick response in case of tirofiban induced severe thrombocytopenia and clinical bleeding.
In conclusion, GpIIb/IIIa receptor inhibitor induced thrombocytopenia mechanism could not been understood exactly, it is thought to be immune-mediated. Therefore in the case of severe thrombocytopenia resulting from tirofiban and active clinical bleeding, IVIG treatment should be used along with the discontinuation of tirofiban and transfusion of erythrocyte-platelets. If tirofiban is stopped during the early periods in tirofiban induced thrombocytopenia, platelets increase more quickly. Therefore, the blood counts of patients who use GpIIb/IIIa receptor inhibitors should be examined at 2–6 hours intervals and as soon as thrombocytopenia is diagnosed, tirofiban should be discontinued.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. The RESTORE Investigators. Randomized Efficacy Study of Tirofiban for Outcomes and Restenosis. Circulation 1997;96:1445–53.
- Weitz J. Antithrombotic drugs. Hematology basic principles and practice5th, R Hoffman, E Benz, S Shattil. Elsevier, Philadelphia 2009; 2070
- Pedersen BU, Andersen M, Hansen PB. Drug induced thrombocytopenia: Clinical data on 309 cases and the effect of corticosteroid therapy. Eur J Clin Pharmacol 1997; 52: 183–189
- Bougie DW, Wilker PR, Wuitschick ED, Curtis BR, Malik M, Levine S, Lind RN, Pereira J, Aster RH. Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GpIIb/IIIa. Blood 2002; 100: 2071–2076
- Warkentin TE, Kelton JG. Temporal aspects of heparin-induced thrombocytopenia. N Engl J Med 2001; 344: 1286–1292
- Sanchez GC, Harizi H, Nurden A, Coste P, Jais C, Nurden P. A case of profound and prolonged tirofiban-induced thrombocytopenia and its correction by intravenous immunglobulin G. International society on thrombosis and haemostasis. J Thromb Haemost 2007; 5: 1068–1070
- Panduranga P, Sulaiman K. Severe thrombocytopenia following tirofiban infusion. Indian J Pharmacol 2011; 43: 726–728
- Sakellariou D, Pastromas S, Koulouris S, Manolis A. First report of tirofiban-induced anemia. Tex Heart Inst J 2009; 36: 55–57
