A 55-year-old female with a history of chronic idiopathic thrombocytopenic purpura (ITP) presented in the spring of 2012 to the emergency department (ED) with a 1-day history of severe generalized headache, nausea, and vomiting. A diagnosis of ITP was made in 1994, and the patient was treated intermittently with prednisone for many years with good response. On two occasions in 2010, her platelet count dropped to <5 K/µl while on prednisone for which she received intravenous immunoglobulin. The response with immunoglobulin did not last long and she was started in November 2010 on romiplostim injections 1 mcg/kg weekly, gradually increased to 4 mcg/kg weekly in order to keep the platelet count above 50 K/µl. Thirteen days prior to presentation, her outpatient hematologist switched her from romiplostim 250 mcg weekly to oral eltrombopag 25 mg daily for the ease of administration. Although the recommended starting dose of eltrombopag is 50 mg daily, it was decided to start the drug at a lower dose to avoid any potential complications in a clinically stable patient. There was no overlap between eltrombopag and romiplostim. Six days before presentation, she was found to be severely thrombocytopenic (platelet count 3 K/µl) and started on 80 mg of prednisone daily. Three days before admission, the dose of eltrombopag was increased to 50 mg daily and prednisone was decreased to 60 mg daily.
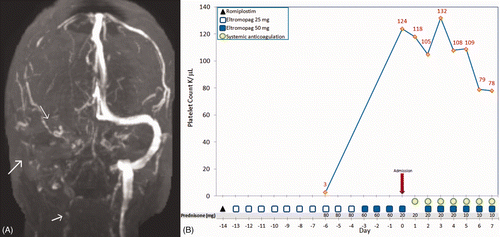
Routine laboratory tests in the ED were remarkable only for a mild thrombocytopenia (platelet count 124 K/µl). A computed tomography scan of the head showed an abnormal density in the deep white matter of the right temporal and parietal lobes suggestive of a hemorrhagic infarct. Magnetic resonance venography demonstrated extensive cerebral venous sinus thrombosis (CVST) with occluded right transverse sinus, sigmoid sinus, and high cervical internal jugular vein due to thrombosis with a large right intracranial hematoma (). A thrombophilia work-up including factor V Leiden, prothrombin gene mutation, and the antiphospholipid antibody syndrome, including lupus anticoagulant, anti-β2-glycoprotein I antibodies, and anticardiolipin antibodies was negative, and she had no other obvious risk factor for CVST. Systemic anticoagulation with heparin was initiated with a diagnosis of CVST and she was discharged home a few days later on warfarin, prednisone 10 mg daily, and eltrombopag 50 mg daily. Her symptoms resolved and she had a platelet count of 78 K/µl at the time of discharge. shows the timeline of events in our patient.
Figure 1. Imaging findings and time course of events in a patient with CVST. A magnetic resonance venogram shows extensive CVST with no flow in the high right jugular vein as well as transverse and sigmoid sinuses (A) and time course of events (B).
What made our patient hypercoagulable is a matter of speculation. Chronic ITP appears to be associated with an increased risk of thrombosis; the presence of larger and more adhesive platelets has been proposed to be a contributing factor Citation[1–3]. It is more likely, however, that since CVST in our patient happened shortly after significant changes were made to her medications (i.e., switch to eltrombopag and steroid dose increase), one of such changes was responsible for the thrombotic event. The dose of eltrombopag was increased 3 days after the prednisone dose was increased, making eltrombopag a more likely culprit in thrombosis in this case although steroids may have played a role as well. Platelet count per se does not seem to be associated with increased risk of thrombosis in patients with ITP treated with thrombopoietin receptor agonists (TPO-RAs) Citation[4], Citation[5]. Half of the eltrombopag-treated ITP patients who developed thrombosis in a recent study were thrombocytopenic at the time of diagnosis Citation[6]. Interestingly, the thrombotic event in 3 of the 22 patients who experienced thromboembolic complications in this study was a cerebrovascular accident (other than CVST). In another recent study, on the other hand, there was an association between high platelet counts (>200 K/µl) and the risk of thrombosis, and eltrombopag increased the risk of portal vein thrombosis in cirrhotic patients Citation[7]. It is plausible that the dramatic increase in platelet count (from 3 K/µl to 124 K/µl) that occurred in a short period of time may have been a significant contributor to thrombosis in our case. Eltrombopag did not affect platelet activation in vivo in two recent studies Citation[8], Citation[9], although it is known to increase P-selectin (a cell adhesion molecule that is expressed on the surface of activated platelets and endothelial cells) Citation[8], Citation[10], and in another study platelet activation was higher in the early period during treatment Citation[10]. Although there is no general agreement on the role of platelets in venous thromboses, the presence of deep venous thrombosis (DVT) was significantly associated in a recent study with increased platelet activation as measured by three platelet indices (mean platelet volume, mean platelet mass, and mean platelet component) Citation[11].
The anatomical distribution of thrombotic events in patients treated with TPO-RAs such as eltrombopag and romiplostim will be delineated as more cases are reported. The incidence of thrombotic events in chronic ITP patients during two phase 3, randomized, placebo-controlled, 24-week studies of romiplostim and during subsequent treatment in an open-label extension study was not different from the placebo group Citation[12]. There are more data available on thrombotic complications of eltrombopag. The three thrombotic events reported among 135 ITP patients treated with eltrombopag in a recent study were two cases of pulmonary thromboembolism (PTE) and one case of DVT Citation[4]. All three patients had significant risk factors for thromboembolism. A review of 446 ITP patients treated with eltrombopag in 3 placebo-controlled and 2 open-label studies estimated the incidence of thrombotic complications to be 3.8%. Twenty-two thromboembolic events were reported in 17 patients. The majority of these events were DVT (n = 8) and PTE (n = 6) Citation[6]. Our case demonstrates that headache and nausea, which are among the most common side effects of eltrombopag Citation[4], have to be taken seriously if the severity of symptoms is concerning.
Declaration of interest: The authors report no conflicts of interest.
References
- Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J. ITP Study Group. Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol 2004; 76(3)205–213
- Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM, Sanz MA, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: A double-blind randomised controlled trial. Lancet 2008; 371(9610)395–403
- Sarpatwari A, Bennett D, Logie JW, Shukla A, Beach KJ, Newland AC, Sanderson S, Provan D. Thromboembolic events among adult patients with primary immune thrombocytopenia in the United Kingdom general practice research database. Haematologica 2010; 95(7)1167–1175
- Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. Lancet 2011; 377(9763)393–402
- Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer B, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: A randomised, double-blind, placebo-controlled trial. Lancet 2009; 373(9664)641–648
- Bussel JB, Cheng G, Saleh MN, Vasey S, Aivado M, Brainsky A. Thromboembolic events observed in eltrombopag clinical trials in chronic immune thrombocytopenic purpura. ASH Annual Meeting Abstracts 2009; 114: 2423
- Afdhal NH, Giannini EG, Tayyab G, Mohsin A, Lee JW, Andriulli A, Jeffers L, McHutchison J, Chen PJ, Han KH, et al. ELEVATE study group. Eltrombopag before procedures in patients with cirrhosis and thrombocytopenia. N Engl J Med 2012; 367(8)716–724
- Psaila B, Bussel JB, Linden MD, Babula B, Li Y, Barnard MR, Tate C, Mathur K, Frelinger AL, Michelson AD. In vivo effects of eltrombopag on platelet function in immune thrombocytopenia: No evidence of platelet activation. Blood 2012; 119(17)4066–4072
- Erhardt JA, Erickson-Miller CL, Aivado M, Abboud M, Pillarisetti K, Toomey JR. Comparative analyses of the small molecule thrombopoietin receptor agonist eltrombopag and thrombopoietin on in vitro platelet function. Exp Hematol 2009; 37: 1030–1037
- Haselboeck J, Pabinger I, Ay C, Koder S, Panzer S. Platelet activation and function during eltrombopag treatment in immune thrombocytopenia. Ann Hematol 2012; 91(1)109–113
- Cay N, Ipek A, Gumus M, Birkan Z, Ozmen E. Platelet activity indices in patients with deep vein thrombosis. Clin Appl Thromb Hemost 2012; 18(2)206–210
- Gernsheimer TB, George JN, Aledort LM, Tarantino MD, Sunkara U, Matthew Guo D, Nichol JL. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenia (ITP). J Thromb Haemost 2010; 8(6)1372–1382

