Abstract
Prothrombin mutation G20210A, anti-phospholipid syndrome as well as iron overload has previously been shown to cause thrombotic events. The main reason for this is the involvement of these anomalies in causing hypercoagulability of the coagulation system, which frequently leads to venous and arterial thrombotic events. We report the case of a 37-year-old white female with prothrombin mutation G20210A, anti-phospholipid syndrome, as well as an increased serum ferritin level, who experienced two transient ischemic attacks and suffers from regular amaurosis fugax. We present an ultrastructural depiction of erythrocytes, platelets, and the fibrin network, to explain the clinical manifestations of the thrombotic state seen in this patient.
A polymorphism in the 3′-UTR (untranslated region) of the prothrombin gene, due to a G to A transition at nucleotide 20210 was first described in 1996 [Citation1]. This abnormality is associated with elevated plasma prothrombin (factor II) levels, resulting in hypercoagulability and a 2.8-fold increased possibility of carriers to develop venous thrombosis [Citation2]. Anti-phospholipid syndrome was recently described as an intriguing clinical entity due to the wide range of clinical manifestations involving every organ system [Citation3]. Reddy, in 2013, mentioned that there are overlapping but distinct autoantibodies, and a positive result in one assay is conclusive despite a negative result in another [Citation3]. Importantly, the pathogenesis of anti-phospholipid syndrome includes venous, as well as arterial thrombotic risks [Citation4].
We report a case of a 37-year-old female, diagnosed with a pro-thrombin mutation (G20210A – heterozygous) as well as anti-phospholipid syndrome, presenting with an increased serum ferritin level of 177 ng/ml (healthy female upper limit: 150 ng/ml), and a platelet count of 382 × 109 g/l (normal ranges: 150–480 × 109 g/L). She previously experienced two transient ischemic attacks (TIAs) and suffers from regular amaurosis fugax (AF) episodes.
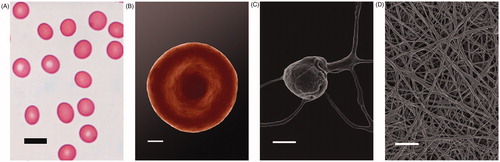
Here we compare blood smears (light microscopy), as well as scanning electron micrographs from erythrocytes (RBCs), platelets and fibrin networks from the patient, with our database of thousands of micrographs of healthy individuals. Healthy RBCs are typically discoid in shape (), while platelets are bulbous, with a few pseudopodia () – this morphology is typically seen when a smear is made on a glass slide. Fibrin networks in healthy individuals, created by adding thrombin to platelet-rich plasma, typically show fibrin nets, without platelets ().
Figure 1. Light microscopy of a healthy individual’s whole blood smear (scale = 10) (A); SEM micrograph of a typical discoid RBC (scale = 1 μm) (B); Typical bulbous platelet with some pseudopodia (scale = 1 μm) (C); Fibrin network (scale = 1 μm) (D).
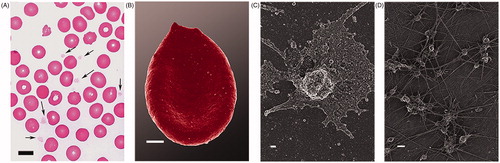
A blood smear from the patient showed platelets between non-discoid erythrocytes (). Scanning electron microscopy (SEM) confirmed numerous platelets in whole blood smears, showing spreading, activation, and major membrane changes (). In fibrin micrographs, numerous platelets were associated with fibrin fibers (). Although the patient’s iron levels are only slightly elevated, similar RBC shape changes were previously noted in iron overload [Citation5, Citation6]. Here we suggest that the pro-thrombin mutation is responsible for the atypical platelets and fibrin, and that the increased iron levels may be responsible for the changed RBCs. Increased iron, the pro-thrombin mutation as well as the presence of anti-phospholipid syndrome are all associated with hypercoagulability and may be responsible for the recurring episodes of AF and previous TIAs.
Figure 2. Light microscopy of a whole blood smear with arrows showing platelets between erythrocytes (scale = 10 μm) (A); Erythrocyte with changed ultrastructure (scale = 1 μm) (B); SEM micrograph of activated platelet with spreading and membrane changes (scale = 2 μm) (C); Numerous platelets within dense fibrin networks (scale = 2 μm) (D).
Ethical approval disclosure
Ethical approval was granted by the University of Pretoria (Human Ethics Committee: Faculty of Health Sciences) under the name of E. Pretorius. Human blood sample was obtained and analysed at the University of Pretoria and the participant filled in an informed consent form.
Declaration of interest
E. Pretorius analysed SEM samples and prepared the manuscript. N. Vermeulen and J. Bester prepared all samples. There is no conflict of interest.
References
- Poort SR, Rosendaal FR, Reitsma PH, Bertina RM. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood 1996;88:3698–3703
- Gonzalez Ordonez AJ, Medina Rodriguez JM, Fernandez Alvarez CR, Macias Robles MD, Coto Garcia E. A patient homozygous for mutation 20210A in the prothrombin gene with venous thrombosis and transient ischemic attacks of thrombotic origin. Haematologica 1998;83:1050–1051
- Reddy P. Laboratory diagnosis of antiphospholipid syndrome. South Med J 2013;106:439–446
- Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013;368:1033–1044
- Pretorius E, Lipinski B. Iron alters red blood cell morphology. Blood 2013;121:9
- Pretorius E. The adaptability of red blood cells. Cardiovasc Diabetol 2013;12:63--69

