Antiplatelet therapy plays a central role in the management of ischaemic heart disease [Citation1]. While poor inhibition of P2Y12 receptor has been linked to higher incidence of ischaemic events, enhanced inhibition has been associated with increased risk of bleeding events [Citation2]. Platelet function testing has been suggested as a way to individualise therapy in order to minimize the risk of both bleeding and ischaemic events [Citation3]. The VerifyNow analyser (VN) (Accumetrics, San Diego, CA) is a point-of-care device that provides rapid assessment of P2Y12 inhibition and results obtained with VN are reported as P2Y12 reaction units (PRU) [Citation4]. Traditionally, trisodium citrate (“citrate”) has been used with the VN and measurements with citrate require an incubation period of at least 10 minutes and can be performed up to 4 hours post sampling, as per manufacturer’s instruction. Recently, we have demonstrated the possibility for the test to be performed immediately post sampling with hirudin anticoagulation and showed that measurements with hirudin were stable over 20 minutes whereas PRU results obtained with citrate immediately were significantly lower than those obtained after 20 minutes incubation [Citation5]. In this study, we assessed the stability of PRU results obtained with citrate between the recommended minimum incubation time of 10 minutes compared to 20 minutes post sampling as well as compared to measurements with hirudin performed immediately or 20 minutes post sampling.
Ten patients with stable coronary artery disease undergoing elective percutaneous angioplasty provided informed consent according to a protocol approved by the local ethics committee. All patients had been pretreated with aspirin and either 600 mg clopidogrel at least 2 hours pre-sampling (n = 6) or 75 mg daily for at least 5 days (n = 4).
All blood samples for VN testing were obtained by venipuncture using a syringe and a needle. The first 2 mls were discarded and blood was added gently, avoiding turbulence, to uncapped anticoagulant tubes without the use of vacuum. Blood tubes were then capped and inverted gently to mix the blood with the anticoagulant. We used two tubes containing 3.2% citrate (Vacuette, Greiner Bio-One, Kremsmünster, Austria) and two tubes containing >15 µg/ml hirudin (Double-wall hirudin blood tube, Verum Diagnostica, Munich, Germany) for every patient. The VN P2Y12 test was performed at two time points for each anticoagulant: immediately and at 20 min post sampling with hirudin and at 10 minutes and 20 minutes post sampling with citrate.
All statistical analyses were performed using PASW statistic version 19 (IBM SPSS Inc., New York, NY). Data are presented as mean ± SD and analysis was carried out using paired samples t-test for continuous variables.
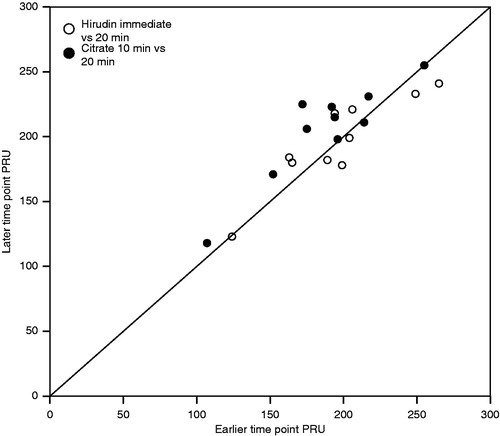
Measurements with hirudin provided more consistent results over the studied period as compared to measurements with citrate where PRU values tended to be significantly lower at 10 minutes (187 ± 40) versus at 20 minutes (205 ± 38; p = 0.009) (, ).
Table I. P2Y12 reactivity units (mean ± SD) as measured by both anticoagulation methods.
Measurements with hirudin as compared to measurements with citrate at 20 min further confirmed our previous study where optimum correlation between both measurements was estimated by the equation PRU_H_Imm = 0.95 X PRU_C_20.
Base PRU (BASE) results representing platelet aggregation in response to thrombin receptor activating peptide (TRAP) were stable over the studied time with each anticoagulant, however measurements with hirudin were significantly different to measurements with citrate. These results are consistent with the results shown in our previous study [Citation5].
The consistency in PRU results obtained with hirudin over the studied time might be explained by the mechanism of action of hirudin, which directly inhibits thrombin without affecting divalent cation levels [Citation6] and this might help provide a stable medium for homeostasis over a 20-min period. Other studies assessing the use of hirudin with Multiple Electrode Aggregometry (MEA) demonstrated stability in results obtained with hirudin up to 12 hours post sampling [Citation7, Citation8].
Citrate, on the other hand, appears to transiently blunt platelet reactivity, possibly due to the sudden shift in extracellular ionized cations affecting receptor activity and/or intracellular signalling.
The limitations of this study are small sample size (although the results are consistent with our previous larger study) and assessment of only a 20-minute window since our primary focus related to rapid testing of P2Y12 inhibition in the clinical setting. We did not assess other assays, such as the VN aspirin assay since this is not currently recommended in clinical practice [Citation9], and further work is needed to assess the impact of anticoagulant on these other assays.
In the coronary angiography suite, it is likely that VN P2Y12 test will be performed within the first 20 minutes post sampling. In these situations, we suggest the use of hirudin as the anticoagulant of choice in order to ensure reliability of the results and, when appropriate, guide therapeutic decision-making in line with current expert consensus [Citation9]. In other situations where the test will be performed beyond 20 minutes post sampling, then citrate would be appropriate for use as anticoagulant in order to accurately guide therapy.
Declaration of interest
R.F.S. has received consultancy fees and/or honoraria from AstraZeneca, Eli Lilly, Daiichi Sankyo, Accumetrics, Merck, Novartis, Roche, Sanofi Aventis, Regeneron, Bristol Myers Squibb, Iroko, and Medscape and institutional grants/research support from AstraZeneca, Eli Lilly/Daiichi Sankyo, Merck and Accumetrics. The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollendberg SM, et al. ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol 2011;58:e44–e122
- Grove EL, Hossain R, Storey RF. Platelet function testing and prediction of procedural bleeding risk. Thromb Haemost 2013;109:817–824
- Siller-Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle-Karth G, Gouya G, Ruzicka K, Podczeck-Schweighofer A, Christ G. Personalized antiplatelet treatment after percutaneous coronary intervention: The MADONNA study. Int J Cardiol 2013;167:2018–2023
- Malinin A, Pokov A, Swaim L, Kotob M, Serebruany V. Validation of a VerifyNow-P2Y12 cartridge for monitoring platelet inhibition with clopidogrel. Methods Find Exp Clin Pharmacol 2006;28:315–322
- Sumaya W, Daly RL, Mehra S, Dhutia AJ, Howgego KE, Ecob R, Judge HM, Morton AC, Storey RF. Hirudin anticoagulation allows more rapid determination of P2Y(1)(2) inhibition by the VerifyNow P2Y12 assay. Thromb Haemost 2013;109:550–555
- Pittens CA, Bouman HJ, van Werkum JW, ten Berg JM, Hackeng CM. Comparison between hirudin and citrate in monitoring the inhibitory effects of P2Y12 receptor antagonists with different platelet function tests. J Thromb Haemost 2009;7:1929–1232
- Kaiser AF, Neubauer H, Franken CC, Kruger JC, Mugge A, Meves SH. Which is the best anticoagulant for whole blood aggregometry platelet function testing? Comparison of six anticoagulants and diverse storage conditions. Platelets 2012;23:359–367
- Zhang HZ, Yu LH, Kim MH. Effect of different anticoagulants on multiple electrode platelet aggregometry after clopidogrel and aspirin administration in patients undergoing coronary stent implantation: A comparison between citrate and hirudin. Platelets 2013;24:339–347
- Aradi D, Storey RF, Komocsi A, Trenk D, Gulba D, Kiss RG, Husted S, Bonello L, Sibbing D, Collet JP, et al. Expert position paper on the role of platelet function testing in patients undergoing percutaneous coronary intervention. Eur Heart J 2013. Available from: http://eurheartj.oxfordjournals.org/content/early/2013/09/24/eurheartj.eht375.full.pdf+html [last accessed 14 Nov 2013]