A standard treatment for acquired amegakaryocytic thrombocytopenic purpura (AATP) has not yet been established. Once AATP is diagnosed, a therapeutic trial of cyclosporine, with or without antithymocyte globulin (ATG), is indicated [Citation1]. Allogeneic bone marrow transplantation (BMT) is considered to be an appropriate treatment for patients with refractory or progressive AATP, who are relatively young and have matched siblings [Citation2]. Recently, successful treatment of AATP with a thrombopoietin (TPO) receptor agonist, eltrombopag, has been reported [Citation3]. We herein describe a patient with AATP refractory to other therapies, including eltrombopag, who was successfully managed with another TPO receptor agonist, romiplostim. This publication was approved by the ethics committee of Tokushima Prefectural Central Hospital.
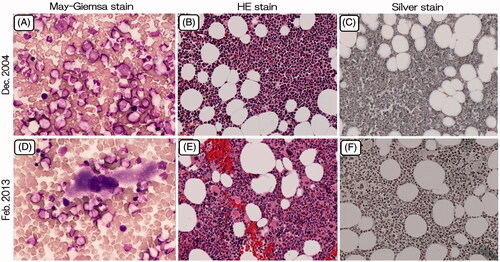
A 55-year-old male was referred to us for thrombocytopenia in December 2004. He had a past history of myocardial infarction at 51 years of age, and had therefore been taking numerous drugs, such as aspirin, nicorandil, quinapril hydrochloride, carvedilol, candesartan cilexetil, azosemide, spironolactone, and allopurinol. He was not a heavy drinker. A physical examination revealed many petechiae and ecchymoses on the extremities and the abdominal wall. The peripheral blood results were as follows: Hb, 13.2 g/dl; RBC, 4.19 × 106/μl; WBC, 7.6 × 103/μl (seg., 63.5%; eos., 7.0%; baso., 1.5%; mono., 7.5%; lym., 23.0%) and PLT, 4 × 103/μl. His blood chemistry values, including total bilirubin, AST, ALT, ALP, LDH, BUN and creatinine, were all within the normal limits. The prothrombin time, activated partial thromboplastin time, plasma fibrinogen level, and plasma FDP concentration were all normal. The platelet-associated IgG level was 57.6 ng/107 cells (reference range, 5–25 ng/107 cells). Tests for rheumatoid factor and antinuclear antibody were negative. Screening assays for the human immunodeficiency virus, human T-cell leukemia virus type-1, hepatitis B virus, hepatitis C virus and human parvovirus B19 (IgM) were negative. Bone marrow aspiration showed normal cellularity and the absence of megakaryocytes, with a normal appearance of erythroid and myeloid elements (). A bone marrow cytogenetic study showed normal karyotypes. The patient was therefore diagnosed with AATP. Unfortunately, a bone marrow biopsy was not carried out.
Figure 1. Bone marrow aspirate from the acquired amegakaryocytic thrombocytopenic purpura patient at the time of diagnosis (December 2004) and during treatment with romiplostim (February 2013). (A, D): Smear, May-Giemsa stain, ×400; (B, E): Clot section, HE stain, ×100; (C, F): Clot section, Silver stain, ×100.
Of the drugs that the patient had received, both aspirin and allopurinol had previously been given to patients with AATP [Citation1, Citation3, Citation4]. However, since it was impossible to show that none of the other drugs were associated with AATP, all medications were discontinued [Citation5]. Pulse therapy with methylprednisolone (1 g/day × 3), followed by prednisolone (1 mg/kg/day), was conducted at the same time, but his platelet count remained low for one month. Cyclosporine (5 mg/kg/day) was initiated in January 2005, but his thrombocytopenia had not improved after two months. Horse ATG (1 g/day × 5) was administered in March 2005. His platelet count oscillated between 15 × 103/μl and 470 × 103/μl in 35–39-day intervals, but became consistently low in December 2005. Bone marrow aspiration in March 2006 gave the same results as in December in 2004 (data not shown). Rabbit ATG (0.5 g/day × 5) was administered in March 2006. The change in his platelet count was similar to that after the administration of horse ATG. Danazol (300 mg/day) treatment was initiated in October 2006, but his thrombocytopenia had not improved after three months. Although he was referred to a hospital for allogeneic BMT in May 2007, he was refused a transplant because of his age, complications and the absence of related donors.
Cyclophosphamide hydrate (1 and 2 g) was intravenously administered in June and August 2007, respectively, with no improvement of his thrombocytopenia. Since successful treatment of AATP with rituximab (375 mg/m2 intravenously, weekly, for two consecutive weeks) in a patient with systemic lupus erythematosus was reported, he was given rituximab (375 mg/m2) twice in November 2008 [Citation6]. However, his platelet count did not increase at all. When a test dose of horse ATG was given in March 2009, he developed hypotension and a skin rash. We therefore abandoned a second course of both horse and rabbit ATG. Intravenous immunoglobulin was not tested due to its potential risk of thrombosis [Citation7]. Eltrombopag was initiated at 12.5 mg/day in September 2011, and was increased by 12.5 mg/day every two weeks. After one month of treatment with eltrombopag at 50 mg/day, his platelet count showed no increase.
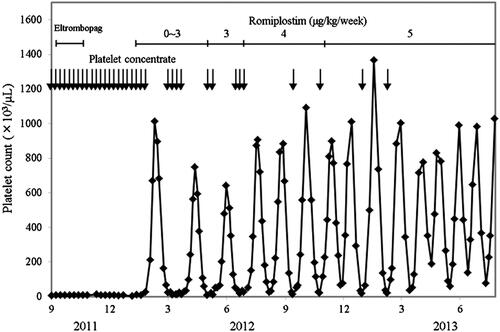
Romiplostim was initiated at 1 μg/kg once weekly in January 2012. The dose was adjusted on the basis of the patient’s platelet count according to the manufacturer’s instructions for use until April 2012. His platelet count oscillated between 9 × 103/μl and 1012 × 103/μl in 49–62-day intervals (). Although romiplostim has been administered at a fixed dose (3–5 μg/kg) once weekly since May 2012, his platelet count has oscillated between 18 × 103/μl and 1366 × 103/μl in 21–42-day intervals. His nadir platelet count has gradually increased since March 2013. Since romiplostim was initiated, his bleeding tendency has disappeared, and the frequency of platelet transfusion has been reduced. He has received 5895 units of platelet concentrate since December 2004, but received only 20 units of platelet concentrate in 2013. While his platelet count has exceeded 100 × 103/μl, he has taken clopidogrel sulfate (50 mg/day) to prevent the recurrence of myocardial infarction.
Figure 2. The platelet counts in the acquired amegakaryocytic thrombocytopenic purpura patient prior to and following treatment with romiplostim. Each arrow denotes a transfusion of 20 units of platelet concentrate.
Abundant megakaryocytes were observed in bone marrow aspirated from the sternum in February 2013, when his platelet count was 1002 × 103/μl (). No increase of reticulin was observed in the aspirate () [Citation8]. The cytogenetic findings were normal. Although his platelet count may be better stabilized by the combination of cyclosporine or danazol with romiplostim, he is currently doing well and has refused to receive additional treatment [Citation9].
This case suggests that romiplostim exerts potent effects on AATP, and that patients with refractory AATP who are unresponsive to eltrombopag may benefit from treatment with romiplostim. We believe that not only eltrombopag, but also romiplostim, is indicated for patients with refractory AATP [Citation10]. Romiplostin did not increase the incidence of neoplasms or hematological malignancies in patients with immune thrombocytopenia [Citation11]. However, since clonal evolution or progression to acute myeloid leukemia remains a concern when using TPO mimetics in bone marrow failure, this case should be carefully followed [Citation12]. Long-term follow-up studies are required to assess the efficacy and safety of romiplostim for the treatment of AATP.
Acknowledgements
The authors would like to thank Yoshiko Sato, Kazumi Kurahashi, and Kiyoshi Nishimura for their expert technical assistance.
Declaration of interest
The authors declare that they have no conflicts of interest associated with this study.
References
- Agarwal N, Spahr JE, Werner TL, Newton DL, Rodgers GM. Acquired amegakaryocytic thrombocytopenic purpura. Am J Hematol 2006;81:132–135
- Lonial S, Bilodeau PA, Langston AA, Lewis C, Mossavi-Sai S, Holden JT, Waller EK. Acquired amegakaryocytic thrombocytopenia treated with allogeneic BMT: A case report and review of the literature. Bone Marrow Transplant 1999;24:1337–1341
- Cela I, Miller IJ, Katz RS, Rizman A, Shammo JM. Successful treatment of amegakaryocytic thrombocytopenia with eltrombopag in a patient with systemic lupus erythematosus (SLE). Clin Adv Hematol Oncol 2010;8:806–809
- Kim HD, Boggs DR. A patient with apparent idiopathic a-megakaryocytic thrombocytopenia. Am J Hematol 1980;9:117–120
- Hoffman R. Acquired pure amegakaryocytic thrombocytopenic purpura. Semin Hematol 1991;28:303–312
- Fukushima T, Dong L, Sakai T, Sawaki T, Miki M, Tanaka M, Masaki, Y, Hirose Y, Kuwana M, Umehara H. Successful treatment of amegakaryocytic thrombocytopenia with anti-CD20 antibody (rituximab) in a patient with systemic lupus erythematosus. Lupus 2008;17:210–214
- Marie I, Maurey G, Hervé F, Hellot MF, Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br J Dermatol 2006;155:714–721
- Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood 2009;114:3748–3756
- Go RS. Idiopathic cyclic thrombocytopenia. Blood Rev 2005;19:53–59
- Lown R, Rhodes E, Bosworth J, Shannon MS, Stasi R. Acquired amegakaryocytic thrombocytopenia: Potential role of thrombopoietin receptor agonists. Clin Adv Hematol Oncol 2010;8:809–812
- Rodeghiero F, Stasi R, Giagounidis A, Viallard J, Godeau B, Pabinger I, Cines D, Liebman H, Wang X, Woodard P. Long-term safety and tolerability of romiplostim in patients with primary immune thrombocytopenia: A pooled analysis of 13 clinical trials. Eur J Haematol 2013;91:423–436
- Townsley DM, Desmond R, Dunbar CE, Young NS. Pathophysiology and management of thrombocytopenia in bone marrow failure: Possible clinical applications of TPO receptor agonists in aplastic anemia and myelodysplastic syndromes. Int J Hematol 2013;98:48–55

