Abstract
Platelet P2Y12 inhibitors have become a central component of the treatment strategy for patients with atherothrombosis due to the importance of platelet P2Y12 receptors in arterial thrombosis. P2Y12 inhibitors effectively reduce the risk of adverse cardiovascular events in patients with acute coronary syndromes (ACS) and patients undergoing percutaneous coronary intervention (PCI). However, despite this, patients with ACS continue to suffer from recurrent atherothrombosis and an increased risk of mortality. In addition, P2Y12 inhibitors increase the risk of bleeding, thereby limiting their clinical benefit. It is therefore clear that further optimizations are needed in the pharmacology and treatment strategies of P2Y12 inhibitors. The objective of these optimizations is to maximize cardiovascular benefit whilst minimizing adverse effects on haemostasis. This review article summarizes the most successful recent strategies in P2Y12 inhibition in order to identify the optimizations and developments that are most likely to be successful in the future.
Introduction
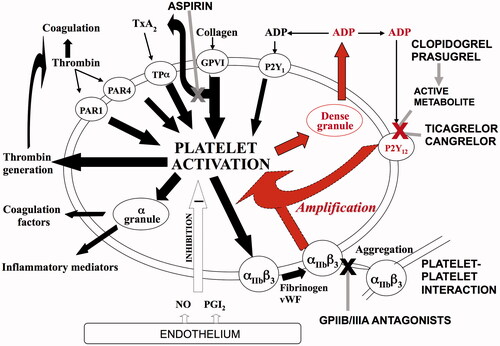
Platelet P2Y12 inhibitors are some of the most commonly used medications worldwide, due to their established benefit in the treatment and prevention of arterial thrombosis, as reviewed by Heptinstall and colleagues [Citation1]. Following atherosclerotic plaque rupture, platelets are exposed to potent agonists that trigger platelet activation and aggregation. Subsequent platelet release of ADP and corresponding activation of platelet P2Y12 ADP receptors has a central role in amplifying the response of platelets to the initial stimulus () [Citation2]. Therefore, platelet P2Y12 receptors are an attractive target for pharmacotherapy.
Figure 1. The role of P2Y12 receptors in platelet activation. Platelet activation is induced by collagen, thromboxane A2, thrombin and ADP, as well as other agonists. This triggers the release of dense granules, which contain ADP. The released ADP then acts on platelet ADP receptors, of which, the P2Y12 receptor has a major role in amplifying the response of the platelet to the initial agonist (highlighted in red).
The first-generation thienopyridine ticlopidine was the first P2Y12 inhibitor to be used in clinical practice, although its use was limited by adverse effects including neutropaenia [Citation3]. The second-generation thienopyridine clopidogrel had a superior safety profile and therefore replaced ticlopidine. Clopidogrel is effective at reducing the risk of adverse cardiovascular events in patients with acute coronary syndromes (ACS) and following percutaneous coronary intervention (PCI) [Citation4–6]. However, it has become increasingly clear that clopidogrel does not satisfactorily inhibit the platelets of approximately one-third of patients [Citation7]. This is in part due to its reliance on multiple cytochrome P450 (CYP) enzymes for conversion into its active metabolite. The third-generation thienopyridine prasugrel is less dependent on CYP enzymes and therefore causes a more potent and consistent decrease in platelet reactivity [Citation8]. In keeping with this, prasugrel decreases the risk of adverse cardiovascular events compared to clopidogrel in invasively-managed ACS patients, albeit at the expense of an increase in spontaneous and surgery-related bleeding [Citation9]. Ticagrelor is another recently introduced potent P2Y12 inhibitor, although it is a nucleoside analogue, representing a novel class of non-thienopyridine P2Y12 inhibitor that is used in ACS, as reviewed by Heptinstall and colleagues [Citation10]. Ticagrelor is also more effective than clopidogrel at reducing the risk of adverse cardiovascular events in patients with ACS, but also increases the risk of spontaneous bleeding [Citation11].
By identifying the most successful advancements in P2Y12 inhibitors to date, this review article aims to predict the optimizations and developments that are likely to be most successful in the future.
Optimizing the pharmacology of P2Y12 inhibition
Pharmacokinetics
Thienopyridines, such as clopidogrel and prasugrel, are prodrugs that require conversion into their active metabolites by hepatic cytochrome P450 (CYP) enzymes in vivo to reduce platelet reactivity. Clopidogrel is converted into its active metabolite in two metabolic steps by CYP enzymes, in particular CYP2C19 [Citation12]. Generation of the active metabolite of clopidogrel is therefore influenced by drugs that affect CYP2C19 [Citation13] and by loss-of-function polymorphisms of the CYP2C19 gene [Citation14]. In contrast, prasugrel is converted into its intermediate form by plasma esterases, requiring just one CYP-mediated step to generate its active metabolite, and has little dependence on CYP2C19 [Citation15, Citation16]. Whilst the active metabolites of clopidogrel and prasugrel are structurally very similar (but not identical), the more efficient and extensive metabolism of prasugrel compared to clopidogrel results in higher and more consistent generation of the active metabolite of prasugrel [Citation17]. Consequently, the pharmacokinetics of prasugrel are not significantly affected by drugs that affect CYP2C19 [Citation13] or by genetic polymorphisms of CYP2C19 [Citation14]. In contrast to the thienopyridines, ticagrelor is direct acting and therefore does not require conversion into an active metabolite to reduce platelet reactivity, thereby resulting in a predictable pharmacokinetic profile [Citation18]. However, ticagrelor is metabolized by CYP3A into at least 10 different active metabolites (some of which are equipotent with ticagrelor) and therefore has drug–drug interactions with CYP3A inhibitors [Citation19].
Since they provide a more predictable pharmacokinetic profile, direct acting P2Y12 inhibitors or P2Y12 inhibitors that require minimal biotransformation are likely to be the most successful in the future. Avoidance of CYP metabolism is also preferable to minimize drug–drug interactions.
Pharmacodynamics
A major limitation of the use of clopidogrel is its variability of response, since approximately one-third of clopidogrel-treated patients do not achieve satisfactory platelet inhibition [Citation7]. A poor response to clopidogrel can be detected using platelet function testing, including a P-selectin-based test developed by Heptinstall and colleagues, and is associated with an increased risk of cardiovascular events [Citation20, Citation21]. Conversely, a high level of response to clopidogrel is associated with an increased risk of bleeding [Citation21–23]. This has led to the concept of a therapeutic window of P2Y12 inhibition, which aims to achieve an optimal balance between maximizing cardiovascular benefit whilst minimizing bleeding [Citation21–23]. Tailoring antiplatelet therapy on the basis of measurements of platelet reactivity is an attractive concept for achieving an optimal level of P2Y12 inhibition. Various attempts have been made to personalize antiplatelet therapy in clopidogrel-treated patients, but the strategies tested so far have not provided additional cardiovascular benefit compared to traditional antiplatelet therapy [Citation24–26]. The more favourable pharmacokinetics of prasugrel and ticagrelor compared to clopidogrel result in more rapid, consistent and potent platelet inhibition than clopidogrel [Citation8, Citation27]. Prasugrel reduces the risk of adverse cardiovascular events compared to clopidogrel in invasively-managed ACS patients [Citation9]. Similarly, ticagrelor reduces the risk of adverse cardiovascular events compared to clopidogrel in patients with ACS managed both invasively and non-invasively, including a reduced risk of cardiovascular death [Citation11, Citation28]. However, the cardiovascular benefit of both drugs is counter-balanced by increased rates of spontaneous bleeding [Citation9, Citation11]. Ideal strategies for P2Y12 inhibition would achieve an optimal level of P2Y12 inhibition that maximizes reductions in risk of adverse cardiovascular events without an excessive increase in risk of bleeding. Ticagrelor maintenance therapy achieves a very high level of P2Y12 inhibition [Citation27, Citation29], so aiming for a higher level than this is not necessary or desirable since this may only result in increases in bleeding that outweigh potential cardiovascular benefits. It is therefore unlikely that future strategies of P2Y12 inhibition will involve greater levels of P2Y12 inhibition than current strategies, in the majority of patients at least.
The active metabolites of clopidogrel and prasugrel covalently bind to P2Y12 receptors, causing irreversible inhibition that lasts for the lifespan of the platelet, which is approximately 10 days. Since new, uninhibited platelets are constantly generated, platelet function recovers approximately 5–7 days after clopidogrel and prasugrel discontinuation [Citation30]. In contrast, ticagrelor is a reversibly-binding P2Y12 inhibitor, which results in a more rapid offset of platelet inhibition, within approximately 72 hours [Citation31]. This may have contributed to the particular benefit of ticagrelor over clopidogrel in patients undergoing CABG, with a reduction in all-cause mortality of approximately 50% and bleeding contributing to more deaths in the clopidogrel group following CABG surgery [Citation32]. Whilst the rapid offset of ticagrelor potentially allows for a shorter interruption of P2Y12 inhibition than clopidogrel, it would theoretically be appealing to continue P2Y12 inhibition until immediately prior to surgery. In the BRIDGE study, treatment with cangrelor after discontinuation of thienopyridine was able to reduce platelet reactivity in the interval before surgery without increasing bleeding [Citation33]. An antidote to ticagrelor has recently been developed and its efficacy is currently being tested [Citation34]. In the future, it may be possible to continue ticagrelor up until the time of surgery and reverse its effect immediately prior to surgery. This strategy could only be possible with P2Y12 inhibitors that do not cause irreversible inhibition of the P2Y12 receptor.
Route of administration
In healthy volunteers and stable patients, both prasugrel and ticagrelor achieve a high level of P2Y12 inhibition within approximately 30 minutes [Citation31, Citation35]. However, in patients with ST-elevation myocardial infarction (MI), the inhibitory effect of both drugs is delayed by as much as 2–6 hours [Citation36]. This may be due to delayed absorption and the effect of morphine on delaying gastric emptying has been implicated as a likely cause for this [Citation37, Citation38]. Strategies that can help to overcome this have therefore been investigated. In the Mashed Or Just Integral Tablets of ticagrelOr (MOJITO) study, crushed tablets achieved a significantly greater reduction in platelet reactivity than ordinary tablets [Citation39]. Another intuitive pharmacological strategy to address this would be initiation of immediate parenteral P2Y12 inhibitors in patients with ACS, followed by oral maintenance therapy once sufficient platelet inhibition has been achieved. The intravenous P2Y12 inhibitor cangrelor can achieve almost immediate potent P2Y12 inhibition [Citation40]. Meta-analysis of studies investigating cangrelor shows that it reduces the risk of periprocedural thrombotic events in patients undergoing PCI, but increases the risk of bleeding [Citation41]. Elinogrel was developed as a direct acting P2Y12 inhibitor that could be administered intravenously or orally. In the phase II study INNOVATE PCI, elinogrel did not increase major bleeding compared to clopidogrel [Citation42] but was subsequently withdrawn from further development.
Logically, a strategy of intravenous administration of P2Y12 inhibitor followed by oral maintenance therapy is particularly appealing. Recently cangrelor has been approved by the European Commission and has received favourable opinion from the FDA advisory committee. The availability of cangrelor therefore offers the opportunity to circumvent the problem of delayed absorption of oral P2Y12 inhibitors in opiate-treated patients undergoing emergency coronary stenting. This will likely reduce the subsequent risk of acute stent thrombosis in these patients, as long as clinicians take care in transitioning to oral P2Y12 inhibitors. This is a concern as cangrelor blocks the binding of clopidogrel and prasugrel active metabolites to the receptor, as shown by Heptinstall and colleagues and other groups [Citation43–45].
Safety, tolerability and minimization of adverse effects
Although bleeding is clearly a core concern in the development of antiplatelet strategies, other adverse effects are also important considerations. The first thienopyridine P2Y12 inhibitor to be used in clinical practice, ticlopidine, caused neutropaenia [Citation3], leading to its replacement by clopidogrel. Whilst subsequent thienopyridines have been generally well-tolerated, it has become apparent that the reversibly-binding agents ticagrelor, cangrelor and elinogrel cause dyspnoea [Citation46]. In the case of ticagrelor, it has been hypothesized that this may be related to the finding that ticagrelor inhibits cellular uptake of adenosine [Citation47] and therefore increases plasma levels of adenosine [Citation48], which can cause dyspnoea [Citation49]. Alternatively, it has been suggested that reversible P2Y12 inhibitors may affect P2Y12 receptors on sensory neurons to a greater degree than thienopyridines, thereby causing dyspnoea [Citation46]. As dyspnoea can rarely lead to discontinuation of the P2Y12 inhibitor, it would be preferable to avoid dyspnoea. However, in the case of ticagrelor, it is unknown whether the mechanism that causes dyspnoea contributes to the clinical benefit of the medication. For example, a significant reduction in sudden cardiac death was noted in PLATO with ticagrelor compared to clopidogrel [Citation50], raising the hypothesis that ischaemic preconditioning by increased extracellular adenosine might reduce the risk of arrhythmic death, although reduction in MI via platelet P2Y12 inhibition is an alternative explanation. The Trial of Caffeine to Alleviate Dyspnoea Related to Ticagrelor (TROCADERO) study (clinicaltrials.gov reference NCT02311088) will clarify the cause of ticagrelor-associated dyspnoea by investigating whether it can be relieved by caffeine, which is an adenosine antagonist.
Summary of predictions for developments in the pharmacology of P2Y12 inhibitors
Following on from the most successful developments to date described above, drugs with the following pharmacological features are likely to be most successful in the future: (1) direct acting or requiring minimal biotransformation; (2) minimal interaction with CYP enzymes; (3) level of P2Y12 inhibition no greater than currently achieved by standard doses of ticagrelor; (4) reversible; (5) readily available antidote; (6) safe and well-tolerated. Intravenous P2Y12 inhibitors offer an attractive adjunct to oral inhibitors in particular clinical settings such as opiate-treated patients undergoing emergency PCI.
Optimizing the application of P2Y12 inhibitors
Patient populations
Clopidogrel, in addition to aspirin, reduces the risk of adverse cardiovascular events in patients with ST-elevation MI, non-ST-elevation MI and in patients undergoing PCI compared to placebo [Citation4–6]. In the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel – Thrombolysis in Myocardial Infarction (TRITON – TIMI) 38 study, prasugrel reduced the incidence of adverse cardiovascular events compared to clopidogrel in ACS patients with a planned invasive strategy [9.9% vs. 12.1%; hazard ratio (HR), 0.81; 95% confidence interval (CI), 0.73–0.90; p < 0.001] [Citation9]. However, prasugrel did not significantly reduce the incidence of adverse cardiovascular events compared to clopidogrel in medically managed ACS patients in the TRILOGY study (13.9% vs. 16.0%; HR, 0.91; 95% CI, 0.79–1.05; p = 0.21) [Citation51]. In contrast, ticagrelor reduces the risk of adverse cardiovascular events compared to clopidogrel in both medically-managed and invasively-managed ACS patients [Citation11, Citation52]. It has not yet been shown whether potent P2Y12 inhibitors offer additional benefit compared to clopidogrel in patients with stable coronary artery disease undergoing PCI. The STEEL PCI study (clinicaltrials.gov reference NCT02327624) will investigate two doses of ticagrelor compared to clopidogrel in these patients. In the future, it is clear that potent P2Y12 inhibitors will be used in ACS patients with a planned invasive strategy. Further investigation is needed to determine the reason why ticagrelor is of benefit to medically-managed ACS patients, whilst it would appear that prasugrel is not [Citation28, Citation51].
Duration of P2Y12 inhibition
The treatment options for patients with ACS are complex and have evolved substantially over the last decade. There is an increasing focus on treatment with PCI and drug-eluting stents in particular. Whilst drug-eluting stents are effective at preventing restenosis compared to bare metal stents, there has been concern that a prolonged duration of P2Y12 inhibition may be required to prevent stent thrombosis. Guidelines have previously recommended 12 months of dual antiplatelet therapy following PCI for ACS [Citation53, Citation54]. However, the recent Dual Antiplatelet Therapy (DAPT) study has shown that 30 months of dual antiplatelet therapy reduces the risk of stent thrombosis and adverse cardiovascular events compared to 12 months at the expense of an increased risk of bleeding [Citation55]. On the other hand, recent meta-analyses have suggested that prolonged dual therapy with aspirin and either clopidogrel or prasugrel might increase all-cause mortality [Citation56]. The recent Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin – Thrombolysis in Myocardial Infarction 54 (PEGASUS-TIMI 54) study showed that ticagrelor reduces the risk of adverse cardiovascular events when started 1–3 years after MI at the expense of an increased risk of bleeding [Citation57]. Interestingly, in this setting, a lower dose of ticagrelor (60 mg twice daily) provided as much cardiovascular benefit as the dose normally used after ACS (90 mg twice daily) but caused slightly less bleeding. Although longer durations of dual antiplatelet therapy provide a reduction in adverse cardiovascular events, this is to some extent balanced by an increase in bleeding. Therefore, in the future, it is likely that there will be an increased focus on determining which patients are likely to benefit the most from long-term dual antiplatelet therapy. In the future, higher doses of P2Y12 inhibitors may be used for a set period of time following ACS, followed by a lower long-term dose, particularly in patients who may not tolerate higher doses.
Initiation of P2Y12 inhibition
Guidelines recommend that antiplatelet therapy should be initiated as early as possible after the diagnosis of ACS is made [Citation53, Citation54]. In patients with non-ST-elevation ACS, the ACCOAST trial recently showed that pretreatment with prasugrel before coronary angiography offers no additional benefit compared to later administration once coronary anatomy has been defined [Citation58]. However, studies that have shown a benefit of clopidogrel and ticagrelor in non-ST-elevation ACS have used pretreatment strategies and so the findings from ACCOAST cannot be extrapolated to these drugs. In patients with ST-elevation ACS, the Administration of Ticagrelor in the Cath Lab or in the Ambulance for New ST Elevation Myocardial Infarction to Open the Coronary Artery (ATLANTIC) study showed a similar effect of ticagrelor on parameters of reperfusion regardless of whether it was administered before or after arrival at the hospital, although a benefit of early ticagrelor treatment might have been masked by morphine treatment [Citation59]. Further studies are required to determine the optimal time for starting ticagrelor in different clinical settings and to assess how intravenous P2Y12 inhibitors may be used as adjunctive therapy to optimize risk.
Summary of predictions for future optimizations in use of P2Y12 inhibitors
It is clear that potent P2Y12 inhibitors will continue to play an important role in invasively-managed ACS patients in the future. There may be an increasing focus on identifying patients who are also likely to receive a cardiovascular benefit from long-term P2Y12 inhibition without an excessive risk of bleeding. Pretreatment with P2Y12 inhibitors prior to coronary angiography is likely to continue in ticagrelor- and clopidogrel-treated patients, unless future studies indicate a more favourable alternative, such as initial use of an intravenous P2Y12 inhibitor.
New frontiers of P2Y12 inhibition
New indications for P2Y12 inhibitors
In the PLATO study, ticagrelor was unexpectedly associated with fewer deaths following pulmonary infections and sepsis than clopidogrel [Citation32, Citation50, Citation60]. It is unclear whether this was due to ticagrelor causing greater P2Y12 inhibition than clopidogrel or whether it was due to inhibition of adenosine uptake by ticagrelor. Alternatively, it could have been due to an adverse off-target effect of clopidogrel or could simply have been due to chance. The Examining the Effect of Ticagrelor on Platelet Activation, Platelet-Leukocyte Aggregates, and Acute Lung Injury in Pneumonia (XANTHIPPE) study (clinicaltrials.gov reference NCT01883869) and the Randomized Trial of Ticagrelor for Severe Community Acquired Pneumonia (TCAP) trial (clinicaltrials.gov reference NCT01998399) will investigate this by determining whether ticagrelor is of benefit compared to placebo in patients with pneumonia.
Clopidogrel is currently used in the management of stroke and peripheral arterial disease. AstraZeneca's PARTHENON programme of clinical trials includes EUCLID, SOCRATES and THEMIS, which will determine the benefit of ticagrelor in peripheral arterial disease, stroke and patients with type 2 diabetes, respectively. It is therefore possible that P2Y12 inhibitors, particularly ticagrelor, may take more important roles in the management of stroke and peripheral arterial disease in the future and may develop new indications for the treatment of stable patients with diabetes and patients with pneumonia.
Combination of P2Y12 inhibitors with other platelet inhibitors
Platelet P2Y12 inhibitors are routinely used in combination with low dose aspirin at an optimal dose of 75–100 mg [Citation61]. However, it is not known whether aspirin is still needed in all situations when a potent P2Y12 inhibitor such as prasugrel or ticagrelor is used. The GLOBAL LEADERS study (clinicaltrials.gov reference NCT01813435) will investigate this by randomizing PCI patients to receive 1 month of aspirin in combination with ticagrelor, followed by 23 months of ticagrelor alone compared to a normal treatment strategy (12 months of dual antiplatelet therapy, followed by aspirin alone). There is also interest in whether a third antiplatelet medication can be added in on top of aspirin and a P2Y12 inhibitor. The thrombin receptor inhibitor vorapaxar initially showed potential in this role, but unfortunately did not significantly reduce the risk of adverse cardiovascular events when commenced urgently in ACS patients and increased the risk of bleeding, including intracranial haemorrhage [Citation62]. However, vorapaxar has been approved by the FDA for use in combination with other antiplatelet drugs as secondary prevention therapy. The prostaglandin E receptor 3 (EP3) antagonist DG-041 has been identified as a promising platelet inhibitor for use in conjunction with aspirin and P2Y12 inhibitors by Heptinstall and colleagues [Citation63–66]. In vitro, ex vivo and animal studies have suggested that DG-041 inhibits platelets without significantly impairing haemostasis [Citation67, Citation68], which holds promise for future clinical investigation. Strategies involving dual antiplatelet therapy with aspirin and a potent P2Y12 inhibitor currently have the most momentum, although it is possible that aspirin may play a lesser role in this relationship in the future.
Conclusion
There is still much scope for optimizing the pharmacology and treatment strategies of P2Y12 inhibitors (). P2Y12 inhibitors used in the future are likely to be direct acting and reversible and are unlikely to be significantly more potent than current treatments. Theoretically, compounds that are available in both oral and intravenous formulations would be ideal although different oral and intravenous agents that interact favourably should suffice. Invasively-managed ACS patients are likely to continue to derive the most benefit from P2Y12 inhibitors in the future. There may also be an increasing focus on risk stratification to determine whether patients may benefit from long-term P2Y12 inhibition and which patients may be optimally treated by P2Y12 inhibitors alone without aspirin. In addition, P2Y12 inhibitors may find a more prominent role in stroke, peripheral arterial disease and stable patients with diabetes.
Table 1. Summary of our predictions for the most successful strategies for P2Y12 inhibition in the future.
Acknowledgements
The views expressed are those of the authors and not necessarily those of the MRC.
Declaration of interest
Dr. Mark Thomas is funded by a Medical Research Council (MRC) Clinical Research Training Fellowship. Dr Mark Thomas has no conflicts of interest. Prof Robert Storey reports institutional grants from AstraZeneca and Merck; research support from Accumetrics; honoraria from AstraZeneca, Accumetrics, and Medscape; consultancy fees from AstraZeneca, Correvio, Merck, Accumetrics, Sanofi-Aventis, Regeneron, Roche, The Medicines Company, Aspen, PlaqueTec and Thermo Fisher Scientific.
References
- Wijeyeratne YD, Heptinstall S. Anti-platelet therapy: ADP receptor antagonists. Br J Clin Pharmacol 2011;72:647–657
- Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol 2000;110:925–934
- Gent M, Blakely JA, Easton DJ, Ellis DJ, Hachinkski VC, Harbison JW, Panak E, Roberts RS, Sicurella J, Turpie AG. The Canadian American ticlopidine study (CATS) in thromboembolic stroke. Lancet 1989;333:1215–1220
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502
- Sabatine MS, Cannon CP, Gibson CM, López-Sendón JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179–1189
- Steinhubl SR, Berger PB, Mann JT, Fry ETA, DeLago A, Wilmer C, Topol EJ, Observation CREDO Investigators Clopidogrel for the Reduction of Events During. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002;288:2411–2420
- Breet NJ, Van Werkum JW, Bouman HJ, Kelder JC, Ruven HJT, Bal ET, Deneer VH, Harmsze AM, van der Heyden JAS, Rensing BJWM, et al. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA 2010;303:754–762
- Michelson AD, Frelinger AL, Braunwald E, Downey WE, Angiolillo DJ, Xenopoulos NP, Jakubowski JA, Li Y, Murphy SA, Qin J, et al., for the TRITON-TIMI 38 Investigators. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753–1763
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001–2015
- Wijeyeratne YD, Joshi R, Heptinstall S. Ticagrelor: A P2Y12 antagonist for use in acute coronary syndromes. Expert Rev Clin Pharmacol 2012;5:257–269
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057
- Kazui M, Nishiya Y, Ishizuka T, Hagihara K, Farid NA, Okazaki O, Ikeda T, Kurihara A. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 2009;38:92–99
- Thomas MR, Storey RF. Optimal management of antiplatelet therapy and proton pump inhibition following percutaneous coronary intervention. Curr Treat Options Cardiovasc Med 2011;14:24–38
- Thomas MR, Storey RF. Genetics of response to antiplatelet therapy. Prog Mol Biol Transl Sci 2014;124:123–153
- Farid NA, Kurihara A, Wrighton SA. Metabolism and disposition of the thienopyridine antiplatelet drugs ticlopidine, clopidogrel, and prasugrel in humans. J Clin Pharmacol 2013;50:126–142
- Rehmel JLF. Interactions of two major metabolites of prasugrel, a thienopyiridne antiplatelet agent, with the cytochromes P450. Drug Metab Dispos 2006;34:600–607
- Payne CD, Li YG, Small DS, Ernest CS, Farid NA, Jakubowski JA, Brandt JT, Salazar DE, Winters KJ. Increased active metabolite formation explains the greater platelet inhibition with prasugrel compared to high-dose clopidogrel. J Cardiovasc Pharmacol Ther 2007;50:555–562
- Husted SE, Storey RF, Bliden K, Tantry US, Hoimark L, Butler K, Wei C, Teng R, Gurbel PA. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease results from the ONSET–OFFSET and RESPOND studies. Clin Pharmacokinet 2012;51:397–409
- Teng R, Butler K. Effect of the CYP3A inhibitors, diltiazem and ketoconazole, on ticagrelor pharmacokinetics in healthy volunteers. J Drug Assess 2013;2:30–39
- Thomas MR, Wijeyeratne YD, May JA, Johnson A, Heptinstall S, Fox SC. A platelet P-selectin test predicts adverse cardiovascular events in patients with acute coronary syndromes treated with aspirin and clopidogrel. Platelets 2014;25:612–618
- Tantry US, Bonello L, Aradi D, Price MJ, Jeong Y-H, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Berg Ten J, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol 2013;62:2261–2273
- Aradi D, Kirtane A, Bonello L, Gurbel PA, Tantry US, Huber K, Freynhofer MK, Berg Ten J, Janssen P, Angiolillo DJ, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: Collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J 2015; ePub ahead of print
- Storey RF. More transparency for a therapeutic window in platelet P2Y12 inhibition? Eur Heart J, in press
- Price MJ. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention the GRAVITAS randomized trial. JAMA 2011;305:1097–1105
- Trenk D, Stone GW, Gawaz M, Kastrati A, Angiolillo DJ, Müller U, Richardt G, Jakubowski JA, Neumann F-J. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents. J Am Coll Cardiol 2012;59:2159–2164
- Collet J-P, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, Henry P, Motreff P, Carrié D, Boueri Z, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100–2109
- Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon CP, Becker RC, Wallentin L. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: The PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol 2010;56:1456–1462
- Lindholm D, Varenhorst C, Cannon CP, Harrington RA, Himmelmann A, Maya J, Husted S, Gabriel Steg P, Cornel JH, Storey RF, et al. Ticagrelor vs. clopidogrel in patients with non-ST-elevation acute coronary syndrome with or without revascularization: Results from the PLATO trial. Eur Heart J 2014;35:2083–2093
- Alexopoulos D, Xanthopoulou I, Storey RF, Bliden K, Tantry US, Angiolillo DJ, Gurbel PA. Platelet reactivity during ticagrelor maintenance therapy: A patient-level data meta-analysis. Am Heart J 2014;168:530–536
- Price MJ, Walder JS, Baker BA, Heiselman DE, Jakubowski JA, Logan DK, Winters KJ, Li W, Angiolillo DJ. Recovery of platelet function after discontinuation of prasugrel or clopidogrel maintenance dosing in aspirin-treated patients with stable coronary disease: The recovery trial. J Am Coll Cardiol 2012;59:2338–2343
- Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: The ONSET/OFFSET study. Circulation 2009;120:2577–2585
- Varenhorst C, Scirica BM, Hogue CW, Åsenblad N, Storey RF, Steg PG, Horrow J, Mahaffey KW, Becker RC, James S, et al. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol 2012;60:1623–1630
- Angiolillo DJ. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery a randomized controlled trial. JAMA 2012;307:265–274
- Nylander S, Pehrsson S, Inghardt T, Antonsson T, Svensson P, Sjögren T, Öster L, Janefeldt A, Sandinge A-S, Newton P, et al. A specific antidote for ticagrelor. J Am Coll Cardiol 2015;65:A253
- Brandt JT, Payne CD, Wiviott SD, Weerakkody G, Farid NA, Small DS, Jakubowski JA, Naganuma H, Winters KJ. A comparison of prasugrel and clopidogrel loading doses on platelet function: Magnitude of platelet inhibition is related to active metabolite formation. Am Heart J 2007;153:e9–e16
- Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, Koutsogiannis N, Damelou A, Tsigkas G, Davlouros P, Hahalis G. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2012;5:797–804
- Morton AC, Hossain R, Ecob R, Walker J, Gunn J. Morphine delays the onset of action of prasugrel in patients with prior history of St Elevation Myocardial Infarction. Circulation 2013;128:A11449
- Parodi G, Bellandi B, Xanthopoulou I, Capranzano P, Capodanno D, Valenti R, Stavrou K, Migliorini A, Antoniucci D, Tamburino C, Alexopoulos D. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-Elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv 2015;8:e001593
- Parodi G, Xanthopoulou I, Bellandi B, Gkizas V, Valenti R, Karanikas S, Migliorini A, Angelidis C, Abbate R, Patsilinakos S, et al. Ticagrelor crushed tablets administration in STEMI patients: The MOJITO study. J Am Coll Cardiol 2015;65:511–512
- Angiolillo DJ, Schneider DJ, Bhatt DL, French WJ, Price MJ, Saucedo JF, Shaburishvili T, Huber K, Prats J, Liu T, et al. Pharmacodynamic effects of cangrelor and clopidogrel: The platelet function substudy from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION) trials. J Thromb Thrombolysis 2012;34:44–55
- Steg PG, Bhatt DL, Hamm CW, Stone GW, Gibson CM, Mahaffey KW, Leonardi S, Liu T, Skerjanec S, Day JR, et al. Effect of cangrelor on periprocedural outcomes in percutaneous coronary interventions: A pooled analysis of patient-level data. Lancet 2013;382:1981–1992
- Welsh RC, Rao SV, Zeymer U, Thompson VP, Huber K, Kochman J, McClure MW, Gretler DD, Bhatt DL, Gibson CM, et al., on behalf of the INNOVATE-PCI Investigators. A randomized, double-blind, active-controlled phase 2 trial to evaluate a novel selective and reversible intravenous and oral P2Y12 inhibitor elinogrel versus clopidogrel in patients undergoing nonurgent percutaneous coronary intervention: The INNOVATE-PCI trial. Circ Cardiovasc Interv 2012;5:336–346
- Steinbuhl SR, Oh JJ, Oestreich JH, Ferraris S, Charnigo R, Akers W. Transitioning patients from cangrelor to clopidogrel: Pharmacodynamic evidence of a competitive effect. Thromb Res 2008;121:527–534
- Schneider DJ, Seecheran N, Raza SS, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coron Artery Dis 2015;26:42–48
- Dovlatova NL, Jakubowski JA, Sugidachi A, Heptinstall S. The reversible P2Y antagonist cangrelor influences the ability of the active metabolites of clopidogrel and prasugrel to produce irreversible inhibition of platelet function. J Thromb Haemost 2008;6:1153–1159
- Cattaneo M, Faioni EM. Why does ticagrelor induce dyspnea? Thromb Haemost 2012;108:1031–1036
- Armstrong D, Summers C, Ewart L, Nylander S, Sidaway JE, van Giezen JJJ. Characterization of the adenosine pharmacology of ticagrelor reveals therapeutically relevant inhibition of equilibrative nucleoside transporter 1. J Cardiovasc Pharmacol Ther 2014;19:209–219
- Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, et al. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol 2014;63:872–877
- Wittfeldt A, Emanuelsson H, Brandrup-Wognsen G, van Giezen JJJ, Jonasson J, Nylander S, Gan L-M. Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. J Am Coll Cardiol 2013;61:723–727
- Varenhorst C, Alström U, Braun OO, Storey RF, Mahaffey KW, Bertilsson M, Cannon CP, Scirica BM, Himmelmann A, James SK, et al. Causes of mortality with ticagrelor compared with clopidogrel in acute coronary syndromes. Heart 2014;100:1762–1769
- Roe MT, Armstrong PW, Fox KAA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med 2012;367:1297–1309
- James SK, Roe MT, Cannon CP, Cornel JH, Horrow J, Husted S, Katus H, Morais J, Steg PG, Storey RF, et al., PLATO Study Group. Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: Substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ 2011;342:d3527
- Hamm CW, Bassand J-P, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2999–3054
- Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernández-Avilés F, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;32:2569–2619
- Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand S-LT, Braunwald E, Wiviott SD, Cohen DJ, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155–2166
- Navarese EP, Andreotti F, Schulze V, Ko odziejczak M, Buffon A, Brouwer M, Costa F, Kowalewski M, Parati G, Lip GYH, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: Meta-analysis of randomised controlled trials. BMJ 2015;350:h1618
- Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, Magnani G, Bansilal S, Fish MP, Im K, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015 372:1791–1800
- Montalescot G, Bolognese L, Dudek D, Goldstein P, Hamm C, Tanguay J-F, Berg ten JM, Miller DL, Costigan TM, Goedicke J, Silvain J, et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N Engl J Med 2013;369:999–1010
- Montalescot G, van't Hof AW, Lapostolle F, Silvain J, Lassen JF, Bolognese L, Cantor WJ, Cequier A, Chettibi M, Goodman SG, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med 2014;371:1016–1027
- Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, Husted SE, Cannon CP, Becker RC, Steg PG, et al. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets 2014;25:517–525
- Thomas MR, Storey RF. Impact of aspirin dosing on the effects of P2Y12 inhibition in patients with acute coronary syndromes. J Cardiovasc Transl Res 2014;7:19–28
- Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, et al. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med 2012;366:20–33
- Heptinstall S, Espinosa DI, Manolopoulos P, Glenn JR, White AE, Johnson A, Dovlatova N, Fox SC, May JA, Hermann D, et al. DG-041 inhibits the EP3 prostanoid receptor – a new target for inhibition of platelet function in atherothrombotic disease. Platelets 2008;19:605–613
- Iyú D, Glenn JR, White AE, Fox SC, Dovlatova N, Heptinstall S. P2Y12 and EP3 antagonists promote the inhibitory effects of natural modulators of platelet aggregation that act via cAMP. Platelets 2011;22:504–515
- Iyú D, Glenn JR, White AE, Johnson A, Heptinstall S, Fox SC. The role of prostanoid receptors in mediating the effects of PGE3 on human platelet function. Thromb Haemost 2012;107:797–799
- Glenn JR, White AE, Iyú D, Heptinstall S. PGE(2) reverses G(s)-mediated inhibition of platelet aggregation by interaction with EP3 receptors, but adds to non-G(s)-mediated inhibition of platelet aggregation by interaction with EP4 receptors. Platelets 2012;23:344–351
- Fox SC, May JA, Johnson A, Hermann D, Strieter D, Hartman D, Heptinstall S. Effects on platelet function of an EP3 receptor antagonist used alone and in combination with a P2Y12 antagonist both in-vitro and ex-vivo in human volunteers. Platelets 2013;24:392–400
- Tilly P, Charles A-L, Ludwig S, Slimani F, Gross S, Meilhac O, Geny B, Stefansson K, Gurney ME, Fabre J-E. Blocking the EP3 receptor for PGE2 with DG-041 decreases thrombosis without impairing haemostatic competence. Cardiovasc Res 2014;101:482–491

