Sirs,
Off-label use of topical pimecrolimus is increasing for the treatment of inflammatory skin diseases other than atopic dermatitis in daily practice (Citation1).
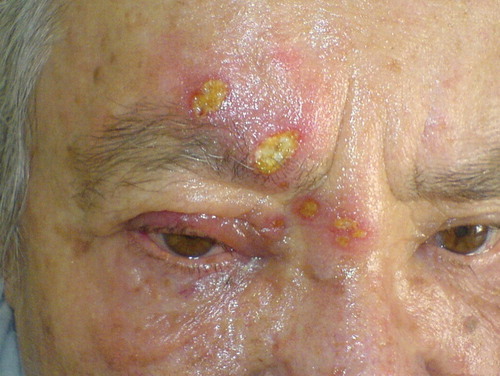
A 58-year-old female patient admitted to our outpatient clinic with complaints of painful swelling, erythema, ulcerations, vesicles, and blisters around her right eye for 2 days (). Ten years previously she had been given a diagnosis of subacute cutaneous lupus erythematosus (SCLE) and was using topical steroids and sun-care products intermittently. The last time she had used topical steroids was 2 months previously. At the time of admission, she was undergoing topical pimecrolimus cream therapy twice daily for 20 days. She had no history of systemic lupus erythematosus (SLE) or any other autoimmune disease, immunosuppressive drug usage, skin or internal malignancy, immune deficiency syndromes or trauma, which are the known risk factors for the development of herpes zoster (Citation2). After the clinical diagnosis of herpes zoster, pimecrolimus treatment was stopped and 125 mg brivudine was started once a day. All herpes zoster lesions were cleared after 15 days. No complications, including post-herpetic neuralgia, were observed in the following 6 months.
SCLE is a subtype of cutaneous lupus erythematosus (CLE) presenting with annular or papulosquamous lesions, generally over the sun-exposed areas and not infrequently associated with SLE (Citation3).
Topical steroids are widely used for the treatment of SCLE (Citation4,5). Since long-term use of topical steroids causes skin atrophy and telangiectasias, topical preparations including topical calcineurin inhibitors are tried for the treatment of SCLE (Citation4,5).
Topical calcineurin inhibitors (pimecrolimus and tacrolimus) specifically inhibit T-cell activation and interleukin (IL)-2, IL-3, IL-4, tumor necrosis factor (TNF)-α, granulocyte/macrophage colony-stimulating factor (GM-CSF) and interferon-γ production by interacting with immunophylins (Citation4,5).
Clinical experiences with the use of topical calcineurin inhibitors in the treatment of CLE were mostly based on tacrolimus use in discoid lupus erythematosus (DLE) treatment (Citation4,5). Pimecrolimus was found to be as effective and safe as betamethasone 17-valerate for the treatment of DLE lesions (Citation6). Use of pimecrolimus decreased the clinical severity of DLE lesions by 52% compared with baseline severity (Citation7). Pimecrolimus is accepted as being more effective in the treatment of cutaneous lesions of SLE than in SCLE and DLE lesions and its relatively reduced effect in the latter lesions was explained by the unresponsiveness of skin infiltrating cells to the drug or because of limited penetration of the drug, especially in hypertrophic lesions (Citation4). Little is known about the efficacy and safety of pimecrolimus use for the treatment of SCLE (Citation8).
Herein, we present a herpes zoster case during pimecrolimus therapy in a patient with SCLE lesions which did not respond well to treatment. Although caution about the risk of occurrence of acute viral infections during pimecrolimus therapy was mentioned in the pimecrolimus package insert, use of the drug did not increase the incidence of viral infections (Citation9–11). In another study, it was detected that although no statistical difference was present between the groups, there was a slight increase of viral skin infections in the group prescribed pimecrolimus. Interferons play a central role against viral infections. Inhibition of interferon production by pimecrolimus may predispose the occurrence of viral infections (Citation12). On the other hand, it is known that infection risk is increased in SLE patients, even when they do not receive immunosuppressive treatments and herpes zoster is the most common viral infection seen in SLE patients (Citation13,Citation14).
Consequently, since there is no available study comparing pimecrolimus with a well-known treatment agent such as topical steroids in SCLE treatment, it can be said that the efficacy and safety of pimecrolimus in SCLE treatment is still not known completely. In conclusion, pimecrolimus should be prescribed very carefully for the treatment of SCLE.
References
- Sehgal VN, Pahwa M. Pimecrolimus, yet another intriguing topical immunomodulator. J Dermatolog Treat. 2007;18:147–150.
- Liesegang TJ. Herpes zoster ophthalmicus natural history, risk factors, clinical presentation, and morbidity. Ophthalmology. 2008;115(2 suppl):S3–S12.
- Sontheimer RD. Subacute cutaneous lupus erythematosus: 25-year evolution of a prototypic subset (subphenotype) of lupus erythematosus defined by characteristic cutaneous, pathological, immunological, and genetic findings. Autoimmun Rev. 2005;4:253–263.
- Tzellos TG, Kouvelas D. Topical tacrolimus and pimecrolimus in the treatment of cutaneous lupus erythematosus: An evidence-based evaluation. Eur J Clin Pharmacol. 2008;64:337–341.
- Wollina U, Hansel G. The use of topical calcineurin inhibitors in lupus erythematosus: An overview. J Eur Acad Dermatol Venereol. 2008;22:1–6.
- Barikbin B, Givrad S, Yousefi M, Eskandari F. Pimecrolimus 1% cream versus betamethasone 17-valerate 0.1% cream in the treatment of facial discoid lupus erythematosus: A double-blind, randomized pilot study. Clin Exp Dermatol. Epub 2009 May 18.
- Tlacuilo-Parra A, Guevara-Gutiérrez E, Gutiérrez-Murillo F, Soto-Ortiz A, Barba-Gómez F, Hernández-Torres M, Pimecrolimus 1% cream for the treatment of discoid lupus erythematosus. Rheumatology (Oxford). 2005;44:1564–1568.
- Kreuter A, Gambichler T, Breuckmann F, Pawlak FM, Stücker M, Bader A, Pimecrolimus 1% cream for cutaneous lupus erythematosus. J Am Acad Dermatol. 2004;51:407–410.
- Luger TA, Lahfa M, Fölster-Holst R, Gulliver WP, Allen R, Molloy S, Longterm safety and tolerability of pimecrolimus cream 1% and topical corticosteroids in adults with moderate to severe atopic dermatitis. J Dermatolog Treat. 2004;15:169–178.
- Ashcroft DM, Dimmock P, Garside R, Stein K, Williams HC. Efficacy and tolerability of topical pimecrolimus and tacrolimus in the treatment of atopic dermatitis: Meta analysis of randomized controlled trials. BMJ. 2005;330:516.
- Paul C, Cork M, Rossi AB, Papp KA, Barbier N, de Prost Y. Safety and tolerability of 1% pimecrolimus cream among infants: Experience with 1133 patients treated for up to 2 years. Pediatrics. 2006;117:118–128.
- Mossman KL. Activation and inhibition of virus and interferon: The herpes virus story. Viral Immunol. 2002;15:3–15.
- Kang I, Park SH. Infectious complications in SLE after immunosuppressive therapies. Curr Opin Rheumatol. 2003;15:528–534.
- Ishikawa O, Abe M, Miyachi Y. Herpes zoster in Japanese patients with systemic lupus erythematosus. Clin Exp Dermatol. 1999;24:327–328.