Abstract
Background: While limited data from clinical trials show differences in efficacy between topical acne combination products and monotherapies, the impact in clinical practice is not explored. Purpose: To evaluate the effectiveness in clinical practice of prescribing topical acne combination treatments compared to monotherapy product(s) in minimizing treatment failures. Methods: Patients diagnosed with acne (ICD-9: 706.1) by a dermatologist between January 2009 and September 2011 and initially prescribed a topical acne treatment were identified. Multivariate Cox proportional hazards models were employed to compare the hazards of treatment failure for patients initially prescribed a combination product versus one monotherapy or multiple monotherapies. Subgroup analyses were also performed. Results: Three hundred and thirty-five patients were initially prescribed topical product(s) exclusively. The hazards of treatment failure for those prescribed a combination product compared to those prescribed one monotherapy product was HR = 0.91 (p = 0.65) and compared to those prescribed multiple monotherapy products was HR = 0.73 (p = 0.17). Limitations: Disease severity and treatments prescribed outside of the hospital system were not available. Conclusions: Treatment failures were consistent for patients prescribed combination product(s) or monotherapies.
Introduction
Adherence is a significant obstacle in the way of successful treatment of acne (Citation1). Nonadherence, regardless of cause, can appear as patients not responding to specific medications leading to “treatment failures.” In the dermatological setting, adherence may be improved with more frequent office visits and/or simplifying regimens by means of fewer doses, and combining multiple medications (Citation2,3). Combination products can help simplify treatment while maintaining (or improving on) the efficacy of a multiple medication approach (Citation2).
While existing studies on the relative efficacy of acne treatments are based on clinical trials, the purpose of this study is to evaluate the effectiveness of prescribing combination treatments in comparison to monotherapy product(s) regimens in minimizing treatment failures and consequent regimen modifications in clinical practice. We hypothesized that the improved adherence associated with use of combination products would reduce the chances of a treatment failure as measured by regimen modification and time between medication changes. Survival analysis models were used in order to test this hypothesis.
Methods
This cohort study followed patients initially prescribed 1) a combination product, 2) a monotherapy, or 3) multiple products to determine the hazards of treatment failure for the three groups. After receiving IRB approval, the Wake Forest Baptist Hospital Transitional Data Warehouse database was queried to identify all patients that received a diagnosis for acne (ICD-9: 706.1) by a dermatologist and had mention of an acne-related prescription between 1 January 2009 and 1 September 2011. Prescription records were followed for up to 1 year based on the medical database records.
EC and KH reviewed all medications prescribed and identified those likely prescribed for acne. All medications which were not likely prescribed for acne were excluded from the analysis. We included the following topical treatments: retinoids, benzoyl peroxide with and without urea, azelaic acid, clindamycin, dapsone, erythromycin, salicylic acid, sulfacetamide with and without sulfur, and combinations of these products. For oral treatments, we included clindamycin, doxycycline, erythromycin, isotretinoin, minocycline, sulfamethoxazole/trimethoprim, tetracycline, vitamin A, azithromycin, and hormonal treatments. Medications were not restricted by dosage as this analysis sought to see if any initial prescription for a topical combination product compared to any topical monotherapy or multiple products would lengthen the time to treatment failure.
Treatment failure was defined as when a physician prescribed another acne-related medication for the patient. As topical products are typically prescribed as first line treatments for mild to moderate acne, only patients that were prescribed a topical treatment(s) for their initial treatment plan were included in the analyses. Exposures were defined as initial prescriptions for a single combination product, and no other products; a monotherapy product, and no other products; and two or more topical products.
Between groups, comparisons were made using ANOVA test for continuous data and Fisher's exact test for categorical data. Treatment failure data were analyzed using Cox proportional hazards models. Compared to a chosen reference, hazard ratios (HR) < 1 suggests a lower likelihood of treatment failure occurring while the opposite is true for HR > 1. Crude and multivariate models were implemented to determine the hazards of treatment failure given initial prescription of combination or monotherapy product(s). Patient sex, age in years at first visit, and race were included as potential confounders. Robust sandwich covariance estimates were used to estimate 95% confidence intervals (CI). Secondary Cox proportional hazards model analyses compared prescriptions for specific combination products to any other topical prescription, after controlling for potential confounders. Data were analyzed as a complete case analysis.
Results
Study population
From 1 January 2009 through 1 September 2011, there were 2305 individuals that received a diagnosis for acne by a dermatologist. Of these, 335 initially were prescribed exclusively topical treatment(s): with 61 mentioning only one combination product, 160 mentioning only one monotherapy, and 114 mentioning two or more products as initial prescriptions. Of the 61 initially prescribed one combination product, 36 were for clindamycin/benzoyl peroxide, 21 were for adapalene/benzoyl peroxide, 2 were for clindamycin/tretinoin, and 2 were for erythromycin/benzoyl peroxide. Age, sex, and race distributions were consistent between the three groups (p = 0.11, p = 0.54, and p = 0.14; ).
Table I. Demographic and treatment duration information by exposure group.
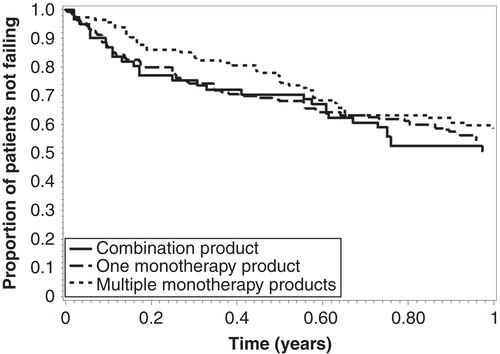
Treatment duration and follow-up
The ranges of follow-up time were 7–365 days for those initially prescribed a combination product, 1–365 days for those initially prescribed one monotherapy product, and 3–365 days for those initially prescribed multiple monotherapy products (p = 0.36; ). There were 116 (42%) treatment failures among all patients, with survival rates consistent between the three groups (Log-Rank: p = 0.47; ). Time to treatment failure ranged from 7 to 355 days for the combination agent group, 1 to 350 days for the single monotherapy agent group, and 3 to 364 days for the multiple monotherapy agents group (p = 0.51).
Cox proportional hazards models
In the unadjusted analysis, compared to patients initially prescribed one combination product, the HR for treatment failure were 0.91 for patients prescribed one monotherapy and 0.77 for patients prescribed two or more monotherapies (p = 0.67 and p = 0.25; ). After adjusting for patient age, sex, and race/ethnicity, the HR of treatment failure were 0.91 for patients prescribed one monotherapy and 0.73 for patients prescribed two or more monotherapies, compared to patients prescribed a combination product (p = 0.65 and p = 0.17).
Table II. Cox proportional hazards estimates for hazards of changing acne treatment.
Subgroup analyses
Patients initially prescribed adapalene/benzoyl peroxide (n = 21) compared to other topical products had a 15% lower hazard of switching medications (HR 0.85, p = 0.61), after controlling for age, race, and sex of patients (). Patients initially prescribed clindamycin/benzoyl peroxide (n = 36) compared to other products had a 29% increased hazard of switching medications (HR 1.29, p = 0.33), after controlling for potential confounders. As there were only two people, respectively, prescribed tretinoin/benzoyl peroxide and erythromycin/benzoyl peroxide, no subgroup analyses were performed for these products.
Discussion
Combination therapy is used to increase efficacy, reduce adverse events, and improve adherence to treatment regimens (Citation4–7). What is specifically appealing about combination products is the synergistic effects affording increased efficacy without significantly affecting safety or tolerability (Citation8–11). In this small observational study, the hazards of treatment failure resulting in treatment modifications were consistent between patients initially prescribed a single product, multiple products, or a combination project. There was no marked worsening of treatment effectiveness in practice by prescribing combination products compared to monotherapies.
The combination of a retinoid and an anti-infective is efficacious for the treatment of acne vulgaris (Citation8). While under-powered, our data also suggest consistent results regarding treatment effectiveness, and thus non-treatment failure, when patients were initially prescribed adapalene/benzoyl peroxide compared to all other products. However, a larger study would be necessary to further evaluate the magnitude and direction of the observed protective effect.
These findings are generalizable to patients who refer to a dermatology clinic for acne treatment – mainly those presenting with mild to moderate acne as we restricted our cohort to patients initially prescribed topical product(s). While we cannot state that patients with more severe acne were initially treated with combination therapy, by excluding those initially prescribed oral medications, we hoped to limit our study population to those with milder forms of acne. Disease severity, which could modulate adherence, was not determinable from the data in this retrospective analysis. Some ascertainment bias may be present because of over-the-counter medications not being captured; this would have underestimated the proportion of patients initially prescribed a topical monotherapy. For patients that did not “fail” treatment, the reasons for not failing (i.e., loss to follow-up, satisfaction with current treatment) could not be ascertained. However, differential loss to follow-up was not expected. If anything, we may expect that patients prescribed monotherapies alone experienced more side effects and choose to no longer visit their dermatologist for care. Overall, the results of this study are limited due to being underpowered. However, they highlight trends that support previous literature (Citation4–11).
The effectiveness of topical medications is dependent on many factors other than the medication itself; side effects, adherence to treatment, and patient disease characteristics, among others, play a large role in determining the effectiveness of dermatological treatment. Combination therapy can improve adherence and increase efficacy due to synergistic effects (Citation6). Our findings suggest this may be true in practice; however, an analysis of a larger cohort is needed to confirm our findings and enforce the generalizability of the findings.
Declaration of interest
The Center for Dermatology Research is supported by an unrestricted educational grant from Galderma Laboratories, L.P. S Feldman is a consultant and speaker for Galderma, Stiefel/GlaxoSmithKline, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, and Bristol Myers Squibb. S Feldman has received grants from Galderma, Astellas, Abbott Labs, Warner Chilcott, Janssen, Amgen, Photomedex, Genentech, BiogenIdec, Coria/Valeant, Pharmaderm, Ortho Pharmaceuticals, Aventis Pharmaceuticals, Roche Dermatology, 3M, Bristol Myers Squibb, Stiefel/GlaxoSmithKline, Novartis, Medicis, Leo, HanAll Pharmaceuticals, Celgene, Basilea, and Anacor and has received stock options from Photomedex. S Feldman is the founder and holds stock in Causa Research. K Huang and Samuel Evan Carstensen have no conflicts to disclose. These findings have not been previously published.
References
- Zaghloul SS, Cunliffe WJ, Goodfield MJ. Objective assessment of compliance with treatments in acne. Br J Dermatol. 2005;152:1015–1021.
- Yentzer BA, Ade RA, Fountain JM, Clark AR, Taylor SL, Fleischer AB Jr, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis. 2010;86:103–108.
- Lott R, Taylor SL, O'Neill JL, Krowchuk DP, Feldman SR. Medication adherence among acne patients: a review. J Cosmet Dermatol. 2010;9:160–166.
- Eichenfield LE, Jorizzo JL, Dirschka T, Taub AF, Lynde C, Graeber M, et al. Treatment of 2,453 acne vulgaris patients aged 12–17 years with the fixed-dose adapalene-benzoyl peroxide combination topical gel: efficacy and safety. J Drugs Dermatol. 2010;9:1395–1401.
- Dutil M. Benzoyl peroxide: enhancing antibiotic efficacy in acne management. Skin Therapy Lett. 2010;15:5–7.
- Kircik LH. Doxycycline and minocycline for the management of acne: a review of efficacy and safety with emphasis on clinical implications. J Drugs Dermatol. 2010;9:1407–1411.
- Thiboutot DM, Weiss J, Bucko A, Eichenfield L, Jones T, Clark S, et al. Adapalene-benzoyl peroxide, a fixed-dose combination for the treatment of acne vulgaris: results of a multicenter, randomized double-blind, controlled study. J Am Acad Dermatol. 2007;57:791–799.
- Feneran AN, Kaufman WS, Dabade TS, Feldman SR. Retinoid plus antimicrobial combination treatments for acne. Clin Cosmet Investig Dermatol. 2011;4:79–92.
- Leyden JJ, Berger RS, Dunlap FE, Ellis CN, Connolly MA, Levy SF. Comparison of the efficacy and safety of a combination topical gel formulation of benzoyl peroxide and clindamycin with benzoyl peroxide, clindamycin and vehicle gel in the treatments of acne vulgaris. Am J Clin Dermatol. 2001;2:33–39.
- Lookingbill DP, Chalker DK, Lindholm JS, Katz HI, Kempers SE, Huerter CJ, et al. Treatment of acne with a combination clindamycin/benzoyl peroxide gel compared with clindamycin gel, benzoyl peroxide gel and vehicle gel: combined results of two double-blind investigations. J Am Acad Dermatol. 1997;37:590–595.
- Eichenfield LF, Alio Saenz AB. Safety and efficacy of clindamycin phosphate 1.2%-benzoyl peroxide 3% fixed-dose combination gel for the treatment of acne vulgaris: a phase 3, multicenter, randomized, double-blind, active- and vehicle-controlled study. J Drugs Dermatol. 2011;10:1382–1396.