Abstract
Background: Newer therapies provide high levels of skin clearance in patients with moderate to severe psoriasis. However, insufficient evidence exists on the impact of total skin clearance from the patient's perspective.
Objectives: To examine effects of total skin clearance on health-related quality of life (HRQoL) and psoriasis symptom severity in subjects with moderate to severe psoriasis.
Methods: Pooled data from a phase 2 dose-ranging trial in psoriasis using brodalumab (antibody to interleukin-17 receptor A) were used to compare subjects with static physician global assessment (sPGA) 1 versus sPGA 0 and subjects with Psoriasis Area and Severity Index (PASI) 75 to <100 versus PASI 100 at week 12 based on no impairment in Dermatology Life Quality Index (DLQI = 0) and no psoriasis symptoms (Psoriasis Symptom Inventory = 0).
Results: Of subjects with sPGA 0 (clear) and 1 (almost clear), 61.4% and 45.7% had a DLQI = 0 (p = 0.15), and 65.5% and 32.6% had a Psoriasis Symptom Inventory = 0 (p = 0.001), respectively. Significantly more subjects with sPGA 1 continued to report itching, redness, scaling, and flaking compared to subjects with sPGA 0. Similar results were observed based on PASI score.
Conclusions: A higher proportion of subjects with total skin clearance reported no impairment in HRQoL and no psoriasis symptoms than those who were almost clear.
Introduction
Psoriasis is a symptomatic disease that significantly impacts health-related quality of life (HRQoL) outcomes, with impairments in physical functioning and well-being (Citation1). HRQoL outcomes improve with successful treatment of psoriasis (Citation2–4); however, subjects that respond to therapy but continue to have residual disease may continue to have negative impacts on HRQoL. Clinical measures such as psoriasis-affected body surface area (BSA), the Psoriasis Area Severity Index (PASI) (Citation5), and the static Physician Global Assessment (sPGA) (Citation6) are frequently used to evaluate psoriasis severity and improvement with treatment. Subjects with a 75% improvement in PASI (PASI 75) or a sPGA score of 0 or 1 are considered responders to a psoriasis therapy, yet these subjects may continue to have residual disease, and evidence on the association among higher levels of skin clearance, symptom improvement, and HRQoL outcomes is limited.
Some previous studies have demonstrated significant improvements in Dermatology Life Quality Index (DLQI) and symptom scores with increasing PASI improvement (Citation3,Citation7,Citation8). In reports by Shikiar et al. (Citation7,Citation8), the greatest improvements in HRQoL were seen in patients who achieved a PASI 75, but no attempt was made to examine and compare groups achieving greater levels of symptom improvement. Revicki et al. found that the greatest improvements in DLQI total scores (decreases > 10 points) were observed in patients in the PASI 90 to <100 and PASI 100 groups (Citation3). In addition, a greater percentage of patients reported a DLQI score of 0 in the PASI 90 to <100 and PASI 100 groups compared with the PASI 75 to <90 group and the other PASI response groups. Comparable findings were seen based on patient and clinician global assessments of disease activity (Citation3).
Newer biologic agents provide high levels of skin clearance in patients with moderate to severe psoriasis. The impact of achieving higher levels of clearance and in particular, total skin clearance, from the patient's perspective has not been well characterized. As a secondary analysis of clinical trial data, we examined the effect of total skin clearance on dermatology-specific HRQoL, measured by the DLQI (Citation9), and psoriasis symptom severity, measured by the Psoriasis Symptom Inventory (Citation10,Citation11), in subjects with moderate to severe psoriasis following 12 weeks of treatment. We specifically compared dermatology-specific HRQoL and psoriasis symptom severity between subjects with total skin clearance (defined as sPGA score of 0 or 100% improvement in PASI score [PASI 100]) and those who were almost clear (defined as sPGA of 1 or 75% to <100% improvement in PASI score). The DLQI and Psoriasis Symptom Inventory are disease specific, and were selected because they are used to evaluate patients with moderate to severe psoriasis and are more sensitive compared to generic patient-reported outcome (PRO) instruments.
Materials and methods
Patients and data source
A secondary analysis was performed using data from a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study that evaluated safety, tolerability, and efficacy of brodalumab in subjects with moderate to severe psoriasis (Citation12). The phase 2 study comprised 198 subjects at 23 study sites worldwide randomized to one of five treatment arms in a 1:1:1:1:1 ratio (brodalumab 70 mg, 140 mg, or 210 mg every 2 weeks [Q2W] with a loading dose at week 1, brodalumab 280 mg every 4 weeks [Q4W], or placebo for 12 weeks). All subjects provided written informed consent and the study was approved by the institutional review board of each participating site. Subjects with PASI and sPGA data available at baseline and week 12 (n = 188) were pooled from all treatment arms and classified by clinical outcomes based on sPGA at week 12 (sPGA score/status of 0/clear, 1/almost clear, and ≥2/mild to severe) and PASI response (PASI < 50, 50 to <75, 75 to <100, and 100).
Outcome measures
DLQI
The DLQI is a 10-item, subject-completed, dermatology-specific PRO instrument designed to assess the impact of skin disease on HRQoL. Items rated on a 4-point Likert scale were summed to create a DLQI total score ranging from 0 to 30, with the lower scores indicating better HRQoL. Total scores of the DLQI are interpreted as 0–1 = no effect at all on patient's life, 2–5 = small effect on patient's life, 6–10 = moderate effect on patient's life, 11–20 = very large effect on patient's life, 21–30 = extremely large effect on patient's life (Citation13).
Psoriasis Symptom Inventory
The Psoriasis Symptom Inventory is an 8-item, subject-completed, psoriasis-specific PRO instrument designed to assess the severity of the participant's psoriasis symptoms (itch, redness, scaling, burning, stinging, cracking, flaking, and pain) (Citation11). The severity of each symptom rated on a 5-point Likert-type rating scale was summed to create the Psoriasis Symptom Inventory total score ranging from 0 to 32, with the lower scores indicating less symptom severity. A 0 score for any item indicates that the subject reported that the symptom was “not at all severe;” qualitative research with patients with psoriasis shows that they equate “not at all severe” psoriasis symptoms with experiencing “no symptoms.”
Static physician's global assessment
The sPGA is the physician's global assessment of the subject's psoriasis based on severity of induration, scaling, and erythema. The sPGA is a scale from 0 to 5 where 0 indicates clear and 5 indicates severe disease.
Statistical analyses
Descriptive analyses based on pooled data across all treatment groups from the brodalumab phase-2 clinical trial were conducted. Residual disease was assessed by the percentage of psoriasis-affected BSA and PASI response at week 12. Differences in proportion of DLQI 0 scores and Psoriasis Symptom Inventory 0 scores between sPGA of 0 versus 1 and sPGA of 0 versus ≥2 and between PASI 75 and <100 response versus PASI 100 response were assessed based on Cochran–Mantel–Haenszel test stratified by baseline body mass index (BMI) (≤35 kg/m2, >35 kg/m2) and adjusted for baseline PRO score. Sample size limitations prevented a statistical analysis involving subjects categorized based on further stratification of the PASI 75 to <100 group to PASI 75 to <90 (n = 15) and PASI 90 to <100 (n = 33). Thus, to assess the effect of total skin clearance compared with achievement of a lower level of improvement, subjects with PASI 75 to <100 were compared with subjects with PASI 100. Numeric results for the PASI 75 to <90 group are reported as a sensitivity analysis although no statistical comparisons were made. Differences in mean BSA, PASI response, DLQI total scores, and Psoriasis Symptom Inventory total scores between sPGA categories were assessed based on a linear-mixed model adjusting for heterogeneity of variance among the efficacy groups stratified by baseline BMI (≤35 kg/m2, >35 kg/m2) and adjusted for baseline PASI, as appropriate. Analyses were conducted on observed data for subjects who were randomized and had a valid measurement at week 12.
To determine the psoriasis symptoms that persisted in subjects who were almost clear (sPGA 1) and the resulting impact on HRQoL, we determined the percentage of subjects with DLQI subscale and item scores of 0 and Psoriasis Symptom Inventory item scores of 0 by sPGA category.
Results
Subjects
Of the 188 subjects included in the analysis, 36% were women and 89% were white (). At week 12, subjects who were almost clear (sPGA 1) had significantly more residual disease than subjects who achieved total skin clearance (sPGA 0). Compared with mean (standard deviation; SD) BSA of 0% (0.1) and mean absolute PASI score of 0 (0.0) in subjects with sPGA of 0, subjects with sPGA of 1 had 3.8% (4.0) BSA (p < 0.0001) and PASI score of 2.3 (2.0) (p < 0.0001) (). Mean (SD) percentage change in PASI from baseline was 100.0% (0.1) in subjects with sPGA 0 compared with 86.0% (14.0) in subjects with sPGA 1 (p < 0.0001).
Figure 1. Residual disease at week 12. Mean values for (A) BSA, (B) PASI total score, (C) DLQI total score, and (D) Psoriasis Symptom Inventory total score for subjects with sPGA score of ≥2 (black bars), 1 (gray bars), or 0 (white bars) at week 12 of treatment are shown. Error bars represent standard deviations. *p < 0.05; †p ≤ 0.001; ‡p ≤ 0.0001 for comparison with sPGA of 0. BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; SD, standard deviation; sPGA, static physician global assessment.
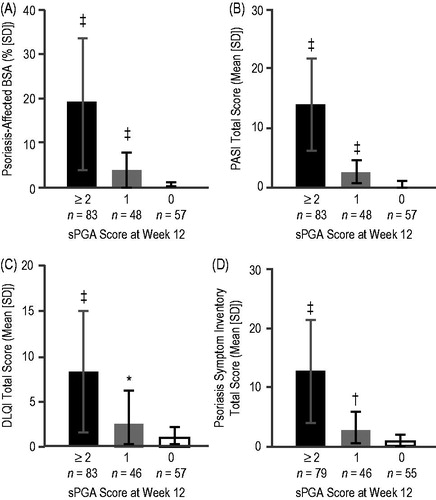
Table 1. Demographics and clinical characteristics at baseline.
Differences in HRQoL and psoriasis symptoms based on sPGA response categories
When evaluating the proportion of subjects with no impairment on HRQoL, 61.4% of subjects with sPGA of 0 at week 12 achieved a DLQI of 0 compared with 45.7% of subjects with sPGA of 1 (p = 0.15) (). There was a statistically significant difference between sPGA 0 and sPGA 1 in mean DLQI total scores (0.8 [1.3] versus 2.5 [3.7]; p = 0.0039) ().
Table 2. Clinical response and patient-reported outcome improvements from baseline to week 12.
When evaluating the proportion of subjects who experienced no symptoms, significantly more subjects with sPGA of 0 (65.5%) had a Psoriasis Symptom Inventory total score of 0 compared with subjects with sPGA of 1 (32.6%; p = 0.001) (). Statistically significant differences were also found between sPGA 0 and sPGA 1 groups in mean [SD] improvement in Psoriasis Symptom Inventory total score (16.5 [7.7] versus 14.7 [7.4]; p = 0.0002) and Psoriasis Symptom Inventory total score at week 12 (0.7 [1.2] versus 2.7 [3.3]; p = 0.0002) ().
Differences in HRQoL and psoriasis symptoms based on PASI response categories
To further examine the effect of total skin clearance on HRQoL and psoriasis symptoms, we evaluated the relationship between PASI responses and PROs. When evaluating the proportion of subjects with no impact on HRQoL, 60.7% of subjects with a PASI 100 response at week 12 had a DLQI score of 0 compared with 53.2% of subjects with a PASI 75 to <100 response (p = 0.5) (). The majority of subjects who achieved total skin clearance (PASI 100) had a Psoriasis Symptom Inventory total score of 0, representing a significant difference compared to subjects with a PASI 75 to <100 response (p = 0.04) ().
Mean (SD) improvement in DLQI at week 12 was 9.9 (7.1) in subjects with a PASI 75 to <100 response and 9.8 (6.3) in those with a PASI 100 response (p = 0. 0409). There was a statistically significant difference between subjects with a PASI 100 response and PASI 75 to <100 response in mean [SD] improvements Psoriasis Symptom Inventory total score (16.6 [7.8] versus 15.6 [6.3]; p = 0.0055)
Results from a sensitivity analysis comparing subjects with a PASI 75 to <90 response and with a PASI 100 response showed greater numeric differences. Of subjects with a PASI 75 to <90 response, 35.7% (5/14) achieved a DLQI score of 0 compared with 60.7% (34/56) of subjects with a PASI 100 response. Of subjects with a PASI 75 to <90 response, 21.4% (3/14) achieved a Psoriasis Symptom Inventory total score of 0 compared with 64.8% (35/54) of subjects with a PASI 100 response.
Impact of residual psoriasis in subjects who were almost clear
The effect of residual disease in subjects that responded to treatment was assessed by comparing the proportion of subjects with sPGA of 0 to subjects with sPGA of 1 who reported no impact on the dimensions of the DLQI (). Of subjects with an sPGA of 0, 71.9% reported no impact on symptoms and feelings compared with 47.8% of subjects with sPGA of 1 (p = 0.02). Similarly, 91.2% of subjects with sPGA of 0 reported no impact on leisure compared with 73.9% of subjects with sPGA of 1 (p = 0.03). In contrast, there was no statistically significant difference between subjects with sPGA 0 and sPGA 1 in the proportion of subjects reporting no residual impact of psoriasis on daily activities (84.2% versus 67.4%; p = 0.07). Subjects with residual disease reported being embarrassed and also reported having to alter their clothing compared to those who were clear ().
Figure 2. Residual psoriasis symptoms. The percentage of subjects with sPGA score of ≥2 (black bars), 1 (gray bars), or 0 (white bars) at week 12 of treatment who achieved (A) a DLQI subscale score of 0, (B) DLQI item score, or (C) a Psoriasis Symptom Inventory item score of 0 at week 12 are shown. Error bars represent 95% confidence intervals (CIs). *p < 0.05; †p < 0.01; ‡p < 0.001 for comparison with sPGA of 0. DLQI, Dermatology Life Quality Index; sPGA, static physician global assessment.
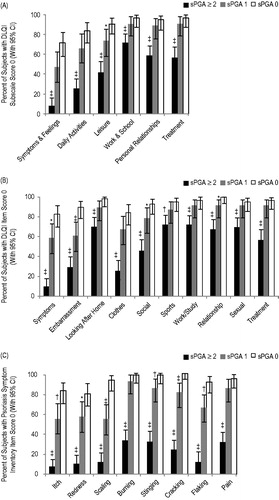
When individual Psoriasis Symptom Inventory item scores of 0 were compared between subjects with sPGA 1 and sPGA 0, residual disease was associated with significantly fewer subjects reporting no psoriasis symptoms for scaling (54.3% versus 93.0%; p < 0.001), flaking (66.0% versus 91.2%; p = 0.002), itching (55.3% versus 82.5%; p = 0.004), and redness (57.4% versus 80.4%; p = 0.02) ().
Discussion
The majority of subjects who were clear at week 12 (sPGA of 0 or PASI 100 response) also had a DLQI total score of 0. Similarly, the majority of subjects who were rated as clear at week 12 also had a Psoriasis Symptom Inventory total score of 0, which was a significant improvement over subjects with a PASI 75 to <100 response or those with an sPGA of 1. Mean DLQI total score and Psoriasis Symptom Inventory total score were statistically significantly different in subjects who achieved total skin clearance compared with those who were almost clear. These results suggest that total skin clearance represents a meaningful level of improvement for subjects, reflected in the experience of no psoriasis symptoms and, correspondingly, no impairment at all on their HRQoL.
Although subjects with a PASI 75 or a sPGA score of 0 or 1 are often considered responders, these subjects may continue to have residual disease that has a negative impact on their HRQoL. Subjects with sPGA of 1 had residual BSA involvement, impairment in HRQoL, as measured by the DLQI, and persistent psoriasis symptoms, as measured by the Psoriasis Symptom Inventory. A recent study by Dommasch et al. suggested that an improvement of at least 6% in affected BSA identified a clinically significant improvement as reported by the patient (Citation14). In our study, approximately 20% of subjects with an sPGA of 1 had ≥6% affected BSA at week 12 (data not shown). Based on this interpretation of BSA improvement, these subjects could have a clinically meaningful benefit if they became clear (0% affected BSA). The mean DLQI of subjects with sPGA of 1 at week 12 in our study was 2.5, indicating that even when almost clear, the residual amount of psoriasis has a persistent negative impact on the patients' lives. As indicated by the individual DLQI item scores, subjects with remaining psoriasis were embarrassed and had to alter their clothing more often than those who were clear (). The responses to the individual Psoriasis Symptom Inventory item scores indicated that they also experienced more severe scaling, redness, itching, and flaking than those who were clear. Together, these results suggest that even small areas of residual psoriasis have a negative impact on patients' lives as compared to experiencing total skin clearance.
These findings extend those observed by Shikiar et al. (Citation7,Citation8) regarding the impact of achieving PASI improvements greater than 90%. The improved HRQoL scores seen in the current study are comparable to those observed in Revicki et al. (Citation3). In general, improvements in clinician-rated symptoms and disease activity assessments are strongly associated with improvements in patient-reported HRQoL and symptom severity scores. The greatest improvements in these PROs are seen in subjects achieving total skin clearance (either sPGA clear or PASI response of 100%). Analyses based on differences in proportions of subjects with DLQI 0 and the Psoriasis Inventory Score of 0 showed that the magnitude of the difference was higher based on the Psoriasis Symptom Inventory (19.1% for PASI comparisons; 32.9% for sPGA) than based on DLQI (7.5% for PASI; 15.7% for sPGA). Regardless of the method of analysis, subjects achieving total skin clearance report benefits of experiencing no symptoms and having no impairment in HRQoL.
A limitation of this study included the small sample sizes for the discrete PASI and sPGA categories. In particular, the number of subjects in the PASI 75 to <90 group did not allow for statistical comparisons to be conducted. The analysis of PASI 75 to <100 compared to PASI 100 reported in this manuscript is a more conservative analysis. It is not known if comparisons with the PASI 75 to < 90 and PASI 90 to <100 group would result in stronger associations between PASI score and HRQoL. Results from this analysis may not be generalizable to broader populations, as the subject population was from a controlled clinical trial rather than from real-world practice. Further research is needed to confirm these findings in patients with moderate to severe psoriasis treated in community practice settings.
In conclusion, subjects with moderate to severe psoriasis who achieved total skin clearance reported greater HRQoL improvements than those who were responders, as measured by PASI or sPGA but were not clear, as demonstrated by a higher proportion of subjects having no impairment in dermatology-specific HRQoL (DLQI score of 0) and experiencing no psoriasis symptoms (Psoriasis Symptom Inventory total score of 0). Achieving total skin clearance provides significant benefits to patients with moderate to severe psoriasis in terms of improvements in HRQoL and psoriasis symptom severity.
Declaration of interest
HV, DC, CM, WY, NE, and PK are employees and shareholders of Amgen Inc. DAR has received research funding and provided consulting services for Amgen Inc. This study was funded by Amgen Inc., Jon Nilsen of Amgen Inc. and Julia R Gage on behalf of Amgen Inc. provided medical writing support. Assistance with formatting the manuscript to meet journal specifications was provided by Shobana Ganesan, CACTUS Communications, on behalf of Amgen Inc.
References
- de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: a systematic literature review. J Investig Dermatol Symp Proc. 2004;9:140–7
- Feldman SR, Kimball AB, Krueger GG, et al. Etanercept improves the health-related quality of life of patients with psoriasis: results of a phase III randomized clinical trial. J Am Acad Dermatol. 2005;53:887–9
- Revicki DA, Willian MK, Menter A, et al. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology. 2008;216:260–70
- Shikiar R, Heffernan M, Langley RG, et al. Adalimumab treatment is associated with improvement in health-related quality of life in psoriasis: patient-reported outcomes from a phase II randomized controlled trial. J Dermatolog Treat. 2007;18:25–31
- Fredriksson T, Pettersson U. Severe psoriasis – oral therapy with a new retinoid. Dermatologica. 1978;157:238–44
- Bonifati C, Berardesca E. Clinical outcome measures of psoriasis. Reumatismo. 2007;59:64–7
- Shikiar R, Bresnahan BW, Stone SP, et al. Validity and reliability of patient reported outcomes used in psoriasis: results from two randomized clinical trials. Health Qual Life Outcomes. 2003;1:53
- Shikiar R, Willian MK, Okun MM, et al. The validity and responsiveness of three quality of life measures in the assessment of psoriasis patients: results of a phase II study. Health Qual Life Outcomes. 2006;4:71
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–16
- Martin ML, McCarrier KP, Chiou CF, et al. Early development and qualitative evidence of content validity for the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure of psoriasis symptom severity. J Dermatolog Treat. 2013;24:255–60
- Bushnell DM, Martin ML, McCarrier K, et al. Validation of the Psoriasis Symptom Inventory (PSI), a patient-reported outcome measure to assess psoriasis symptom severity. J Dermatolog Treat. 2013;24:356–60
- Papp KA, Leonardi C, Menter A, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9
- Hongbo Y, Thomas CL, Harrison MA, et al. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125:659–64
- Dommasch ED, Shin DB, Troxel AB, et al. Reliability, validity and responsiveness to change of the Patient Report of Extent of Psoriasis Involvement (PREPI) for measuring body surface area affected by psoriasis. Br J Dermatol. 2010;162:835–42

