Abstract
Purpose: To systematically review the psychometric properties of outcome measures used in stroke self-management interventions (SMIs) to (1) inform researchers, clinicians and commissioners about the properties of the measures in use and (2) make recommendations for the future development of self-management measurement in stroke. Methods: Electronic databases, government websites, generic internet search engines and hand searches of reference lists. Abstracts were selected against inclusion criteria and retrieved for appraisal and systematically scored, using the COSMIN checklist. Results: Thirteen studies of stroke self-management originating from six countries were identified. Forty-three different measures (mean 5.08/study, SD 2.19) were adopted to evaluate self-SMIs. No studies measured self-management as a discreet concept. Six (46%) studies included untested measures. Eleven (85%) studies included at least one measure without reported reliability and validity in stroke populations. Conclusions: The use of outcome measures which are related, indirect or proxy indicators of self-management and that have questionable reliability and validity, contributes to an inability to sensitively evaluate the effectiveness of stroke self-SMIs. Further enquiry into how the concept of self-management in stroke operates, would help to clarify the nature and range of specific self-management activities to be targeted and aid the selection of existing appropriate measures or the development of new measures.
Implications for Rehabilitation
The evaluation of complex interventions such as self-management interventions is aided by clear outcome expectations and valid and reliable measurement.
This review demonstrates a lack of outcome measures that specifically measure self-management of stroke. A minority of outcome measures that were used as proxy indicators for SM fulfill some of the criteria for quality outlined in the COSMIN checklist.
Clinicians should select measures which appropriately reflect expected outcomes, giving due consideration to the theoretical underpinnings of the intervention. Further work is required to establish which measures currently in use, if any, accurately reflect stoke self-management.
In the meantime, researchers should seek to develop psychometrically sound measures of stroke self-management to assist effective evaluation of such interventions in stroke.
Introduction
Stroke is a major cause of death and disability world-wide [Citation1]. By 2020 stroke, together with coronary-artery disease, are predicted to be the leading causes of global lost healthy life-years [Citation2]. Stroke represents an often devastating disruption to life [Citation3], the majority of survivors experiencing some degree of impairment requiring additional care or support 1 year post-stroke [Citation4].
Stroke is an acute event, but may result in significant long-term impact for the individual, such as social isolation, mood disturbance, communication difficulties and reduction in mobility and life roles [Citation5,Citation6]. Recovery following stroke is complex and multidimensional [Citation3,Citation7,Citation8], encompassing bio-medical, psychological and sociological elements [Citation9–11]. Engagement in self-management practices by individuals with long-term conditions has been suggested as key to promoting recovery [Citation12] and is cited as a means of empowerment and facilitator of improved health outcomes [Citation13,Citation14].
Self-management is a prominent issue in UK health policy [Citation15–17] and has been identified as a key priority for health by organisations independent of the UK government [Citation18,Citation19]. Self-management can be defined as the “active management by individuals of their treatment, symptoms, lifestyle, physical and psychological consequences inherent with living with a chronic condition” [Citation20]. Self-management is an attractive initiative in managing the increasing burden on health and social care resources and reducing associated costs; the assumption being that effective self-management by an individual reduces their healthcare utilisation [Citation21–23]. Healthcare professionals are well placed to promote the effective self-management of stroke [Citation9,Citation24,Citation25].
Self-management interventions (SMIs) are designed to enable people to manage their health more effectively. Evaluation therefore must consider two key areas; firstly whether people develop the skills to manage their own health and secondly, if this consequently results in better health. SMIs operate over multiple dimensions and within different contexts. As such evaluation is complex, not least due to variation in delivery, culture of the sponsoring healthcare organisation and anticipated goals and outcomes [Citation26,Citation27].
The UK Medical Research Council advocates establishing the theoretical basis of an intervention as a first step in estimating its possible outcomes [Citation27]. Currently, evidence suggests that the mediators of change and theoretical premises in SMIs are unclear [Citation28–30]. This poses difficulty in the evaluation and operation of interventions for two key reasons. Firstly, doubt exists regarding the appropriate outcome(s) to monitor to assist evaluation of the intervention and aid determination of cost-effectiveness and clinical impact. Secondly, if the theoretical premises underpinning the intervention are uncertain, intervention fidelity is difficult to monitor and maintain. Questions then exist regarding what influences change and how this can be appropriately measured and SMIs evaluated.
SMIs may be evaluated by examining the effect on health outcomes that potentially change as a consequence of better self-management. Using patient reported outcome measures (PROMs) (e.g. functional status, symptom control, mood and health-related quality of life) is an important way of to ensuring evaluation considers outcomes important to patients. Preliminary investigation of self-management suggests that effective self-management corresponds with positive changes in health behaviour [Citation20,Citation28]. More recently there has been a focus upon measuring attitudes since these are thought to modify health behaviour [Citation29–31]. Additionally, measurement may facilitate understanding of the relationships between attitudes and behaviour [Citation32,Citation33]. PROMs endeavor to capture information that is not directly observable and unmediated by healthcare professionals; consequently accurate measurement is contingent on the extent that the PROM is an accurate reflection of the variable in question [Citation34]. Therefore, it is vital to evaluate whether the measures adopted in SMI studies provide legitimate information to evaluate self-management, both the process and obtaining of skills to better manage health and subsequent potential improvements in health.
Before using an outcome measure in research or clinical practice, it should be assessed and considered to possess adequate psychometric properties. Despite the recognised value of reliable and valid outcome measures and the increasing importance of identifying effective SMIs in stroke, we know of no review that has systematically evaluated international research for the quality of outcome measures used in stroke self-management. The purpose of this article is to systematically review outcome measures used in stroke self-SMIs, with the aim of informing researchers, healthcare professionals and policy-makers and making recommendations for the design of future outcome measures suitable for use in stroke self-management.
Methods
This review seeks to systematically examine the outcome measures adopted in stroke SMIs in terms of the methodology adopted in their development and subsequent strength of their psychometric properties for use with stroke populations. Differing criteria have been adopted to evaluate the psychometric properties of outcome measures [Citation34,Citation35]. Often the methodology adopted in reviews of outcome measures, use differing assessment standards, creating confusion for researchers and clinicians [Citation36].
A recent international Delphi study of 57 experts (63% response rate) resulted in a tool to assess the methodological quality of studies on measurement properties, referred to as the COnsensus-based Standards for the selection of health status Measurement INstruments (COSMIN) checklist [Citation37]. The COSMIN list has good inter-rater agreement and reliability [Citation38] and represents the first critical appraisal tool that is based on the consensus of experts in psychometric theory. COSMIN has been used in other systematic reviews examining the measurement properties of outcome measures in a range of health conditions [Citation39–41]. The Delphi study consisted of four rounds and sought to reach consensus on the terminology and definitions to be adopted with regard to psychometrics. This consensus offers both researchers and clinicians guidance with regard to some of the complexities of measurement properties. COSMIN also addresses modern psychometric theory methodology, such as Item Response Theory, as well as Classical Test Theory. Further information on COSMIN can be accessed via www.cosmin.nl
The properties examined in this systematic review are defined by COSMIN [Citation42] and consist of nine items: Internal consistency; Reliability; Measurement error; Content Validity; Structural validity; Hypothesis testing; Cross-cultural validity; Criterion Validity and Responsiveness.
The purpose of the review is not to make a judgement on the quality of the SMI studies, or to synthesize findings to answer questions regarding the effectiveness of the interventions in the review. Instead the review focuses upon the value of the outcome measures adopted within stroke SMI studies according to their methodological quality, reliability and validity for stroke populations as outlined by COSMIN [Citation42].This is vital since judgments about the results and impact attributed to SMIs are dependent on valid and reliable measurement.
In order to examine the properties of the outcome measures used in stroke SMIs, it was first necessary to identify which measures were used to evaluate the SMIs. Stroke self-management literature was systematically searched on the following electronic databases by one author (E.J.B.): Medline, PsychInfo, Science Direct, Web of Science and CINAHL. The following terms were used to identify existing stroke SMI studies:
self-management
self-care
intervention*
program* AND stroke
rehabilitation
outcome*
education*
Search terms were chosen to represent concepts often linked to self-management (education, rehabilitation), however, studies were excluded unless they specifically stated their purpose was to enhance self-management. Article reference lists, website of UK government health department, generic internet search engines, and stroke-specific organisations were also searched. Dissertations and conference abstracts were excluded, however, searches for publications by dissertation or conference abstract authors were conducted. Selected articles described either (1) stroke-SMI development and/or implementation or (2) presented outcomes of stroke SMIs. Identified interventions and associated outcome measures were extracted and tabulated. Authors screened each abstract to eliminate articles that were not relevant, based on the following inclusion criteria:
(1) the study was published in English;
(2) the article addressed self-management specific to stroke; and
(3) was published between January 1990 and June 2011, to examine the current and most relevant evidence for practice.
One reviewer (E.J.B.) assessed all relevant full text articles obtained and a second reviewer (S.D.) assessed 10% to check reliability. Outcome measures included in studies were then identified and recorded (the version adopted by the SMI was selected for review). A second literature search then sought to find evidence for the measurement properties (as outlined by COSMIN) of those outcome measures identified. The following electronic databases were used: Medline, PsychInfo, Science Direct, Web of Science and CINAHL. The following search terms were used in conjunction with the title of the identified outcome measure:
Valid*
Reliable AND stroke
Responsive*
Sensitive
For example, for a search on the validity of the Geriatric Depression Scale, the search terms “Stroke” AND “Validity” AND “geriatric depression scale” was performed. Search terms were chosen to represent the properties of outcome measure quality as outlined by the COSMIN checklist. No time limitations were set as advocated by COSMIN, since older literature on measurement properties is still relevant. Searches were conducted to specifically find evidence of those properties with stroke populations. This is crucial since a measure’s reliability and validity are on-going properties, dependent upon the context and population with which it is used [Citation34,Citation43]. For example, a measure developed to assess quality of life with a traumatic brain injury population will not necessarily possess acceptable content validity for stroke populations, since the issues faced by both populations may have similarities and differences.
Article reference lists from the originating studies and generic internet search engines were also searched. Discussion between the authors sought to clarify any issues regarding terminology and interpretation of the COSMIN checklist and preceded scoring of the identified measurement tools. Studies were excluded if they investigated postal or proxy reliability and validity unless this was how they were used in the SMIs. For outcome measures with more than one result per COSMIN criteria, the article stating the most robust results was reviewed. Where it was not clear that the study populations were specifically stroke, articles were excluded. One reviewer (E.J.B.) assessed all relevant full text articles using a standard data extraction form advocated by COSMIN. To ensure consistency of interpretation and scoring, a second reviewer (S.D.) independently scored a random 10% of the articles, with discussion between the two reviewers regarding the scores attained. Disagreement regarding interpretation of COSMIN terminology was resolved through consensus meetings. Agreement between scores was consistent.
Identified interventions and associated outcome measures were extracted and tabulated. Paper authors were contacted for further details where the study reported on early phases, or cited unpublished work. Outcome measures included in identified stroke SMIs were rated using the COSMIN checklist [Citation37]. COSMIN consists of four steps and 12 items with different categories for scoring. Ten items are used to assess whether a study meets the standard for good methodological quality. Two items contain general requirements for articles in which Item Response Theory (IRT) methods and general requirements for the generalisability of the results are applied. Where a published paper does not report on a COSMIN item, the item is not scored. For example, if the responsiveness of the measure has yet to be determined, this item is not scored. Each item is rated as excellent (++++), good (+++), fair (++) or poor (+). Full details of the scoring system are available at www.cosmin.nl. The overall score per item is determined by the category with the lowest score.
Results
Search results
Eighty nine records for possible stroke SMIs were identified. Of those, 43 abstracts were identified as potentially relevant studies and were screened (46 duplicate records were excluded). From these, 19 articles were retrieved and reviewed for inclusion criteria and data extraction (studies were excluded because they did not meet the detailed criteria or if they reported on an earlier phase of the same study). Outcome measures within each study were then identified and grouped conceptually into different themes, using content analysis. A total of 13 studies met the eligibility criteria ().
Table I. Summary of included studies.
Profiles of stroke SMIs
All studies included participants over 18 years of age who had experienced a stroke. Three studies of stroke-SMIs originated from the UK; three from Australia; two from Canada; two from the USA; two from Sweden and one from Hong Kong. Nine studies reported upon interventions aimed at community-dwelling participants; two upon the acute recovery phase (<3 months post-stroke); one upon recovery for care home residents and one study did not report details of the setting. Four studies delivered individualised interventions; three utilised workbook interventions; four tested group SMIs designed specifically for stroke and two tested existing self-management programs adapted for stroke. Interventions were delivered primarily; by Allied Health Professionals (six); Nurse specialists (four); researcher (two) and lay experts (one).
Concepts measured
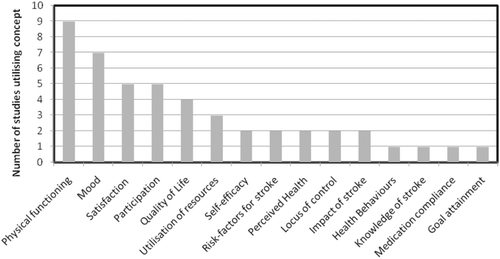
Four studies identified primary outcomes; Health-related Quality of Life [Citation44]; self-efficacy [Citation45]; physical functioning [Citation46] and feasibility [Citation47]. Although all studies focused upon stroke self-management, none measured stroke self-management as a discrete concept. Instead, a range of concepts were measured which presumably were selected to reflect the expected outcomes or process of self-management. Evaluation relies upon judgements concerning the process of the SMI and the outcome expected following participation in the SMI. The majority of measures used sought to measure health outcomes e.g. physical functioning, mood, quality of life (). Attitudes were also measured which could be considered to more readily reflect the process of self-management e.g. healthcare utilization, medication compliance although the theoretical mechanisms linking self-management to these concepts was not elucidated by the authors.
Unreported measures
The term “unreported” is used in this review to describe an outcome measure that has not, at time of writing, been published in peer reviewed publicly available media. Unreported measures relate to those developed either by the study authors or through modifications made to existing measures without examination to ensure such assumptions or modifications were valid. Therefore, it is not possible to determine if unreported measures meet any of the COSMIN criteria. Six studies adopted unreported measures of concepts presumably (although the theoretical links were not explicitly stated by the authors), relating to the process of self-management, that had unknown reliability, validity, responsiveness.
Allen et al. [Citation48] included an unreported measure to assess condition management and patient and carer satisfaction with the intervention. Two studies used 10 point visual analogue rating scales (VASs) to assess usefulness and intelligibility [Citation49] and satisfaction [Citation50] related to the intervention. Whilst VASs are brief and simple to administer and minimal in terms of respondent burden, without established reliability or validity relating to the underlying construct purported to be measured, they remain of limited value. Ljungberg and colleagues [Citation51] designed four questions to assess life satisfaction pre- and post-participation in the SMI and Sit and colleagues [Citation52] modified an existing stroke knowledge scale; details of the modifications were absent in the paper. Marsden and colleagues [Citation44] used a measure of stroke knowledge test, but stated clearly they were not basing inferences from the data obtained using this measure.
Quality of outcome measures
Forty-three different outcome measures were adopted by studies in this review of measures used in stroke SMIs. Of these, 21 measures (49%) demonstrated some properties in stroke populations, according to the COSMIN checklist [Citation42] (). For the remaining measures no evidence could be found for any of the COSMIN properties in stroke populations (n = 16, 39%), or the measures were observer-based assessments (n = 5, 12%).
Table II. COSMIN property scores in stroke populations of outcome measures used in stroke self-management interventions.
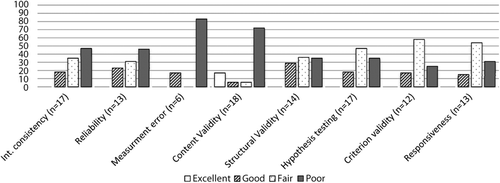
A summary of how the measures included in the stroke SMI studies scored according to COSMIN, when examined for their measurement properties with stroke populations, is shown in . Not every measure scored in each category on COSMIN. Of 21 measures, none scored in every category of COSMIN. Where more than one paper addressed a COSMIN category, the article which stated the most robust results was scored. The majority of measures scored either “fair” or “poor” in each category. The only category to obtain an “excellent” rating was content validity. Three measures scored “excellent” in this category as follows; the Stroke Adapted Sickness Impact Profile (SA-SIP30); the Stroke Self-Efficacy Questionnaire (SSEQ) and the Subjective Index of Physical and Social Outcome (SIPSO).
Discussion
This review examined the methodological quality of studies determining the psychometric properties of outcome measures, used in stroke SMIs according to criteria outlined by the COSMIN checklist. Consistent with measurement theory we explored the validity and reliability of these measures for use in people with stroke, not their general use in broader populations. To our knowledge, this is the first review to systematically appraise and summarize the evidence on the quality of outcome measures used in stroke SMIs. Since no study adopted a measure of stroke self-management attitudes or behaviours, the theoretical concepts utilised by studies in the review to measure self-management will first be addressed.
Theoretical concepts of self-management
The range and number of different published outcome measures adopted by studies in this review [Citation42] may suggest a current lack of consensus regarding the appropriate measures to assist evaluation of stroke SMIs. Alternatively, the use of heterogeneous measures may be reflective of recognition by researchers that self-management embraces a range of differing concepts. The current absence of consensus may in part reflect an underlying lack of consensus about the concept and operation of self-management in stroke. In addition, most SMIs have been developed for generic audiences, which may partly explain the lack of specific measures developed for stroke self-management. An argument exists for research to investigate the conceptual properties of stroke self-management, to examine which measurement concepts currently being used, if any, are appropriate.
A range of concepts were measured. Some captured health outcomes, such as physical functioning, which the study authors anticipated may be affected by the SMI; others attempted to capture behaviours, such as resource utilisation or attitudes, such as changes in self-efficacy thought to be associated with self-management processes (). However, how the concepts measured align with the patient experience of stroke self-management remain unknown. Physical function (PF) was most often used as an indicator of effective self-management. Of the 21 measures possessing at least one property of the COSMIN checklist in this review, 11 related to PF (52%). This is potentially suggestive of an assumption that effective self-management results in improved PF, or that improved PF is a desired outcome. PF appears to remain a dominant concept within stroke rehabilitation, despite increasing evidence of the role of psychosocial factors in recovery [Citation53–55]. For example, the measurement of PF is of limited value in studies that target speech disorder, depression, social participation or cognitive function [Citation56], debatably all factors in effective stroke self-management. Questions regarding the differing priorities of rehabilitation between healthcare professionals and those affected by stroke have been raised before [Citation57]. Effective self-management extends beyond the ability to perform certain tasks, encompassing decision making and choices regarding health and behaviour [Citation14]. The role of PF in stroke self-management requires further clarification before it can be adopted as a robust indicator of effective self-management.
Six studies collected information on health behaviours and healthcare resource utilisation. However, issues of potential greater importance to patients, for example a change in confidence or increased awareness about how to manage fatigue, may not be captured in measures focused upon management of health behaviours or resource utilisation. There is a need to further conceptualise stroke self-management to ensure that self-management strategies pertinent to people recovering from stroke are captured in existing or new outcome measures.
Eight of the studies in this review explicitly cited a theoretical basis to the intervention adopted in the study (). The most commonly cited theory was psychologist Albert Bandura’s Social Cognition Theory and the concept of Self-Efficacy (n = 4 studies). Self-efficacy can be described as the belief in one’s capabilities to organise and execute the course of action required, to produce given achievements[Citation58,Citation59]. The validity of outcome measures is contingent upon using them for the purpose they were intended for.
Of the four studies citing self-efficacy as a theoretical premise underpinning the intervention, only two studies utilised outcome measures to reflect change attributed to this theoretical concept in the interventions [Citation45,Citation60]. The measure adopted by Kendall and colleagues, The Self-Efficacy Scale [Citation61], has unknown psychometric properties in stroke populations, and therefore requires further examination to establish its validity for use in these populations. The Stroke Self-efficacy Scale adopted by Jones and Colleagues [Citation45] was developed with stroke populations. However questions exist concerning the relevance of the sample. Data were generated with people a relatively short time frame since stroke (mean duration was 4.2 weeks and 16 days post-stroke for two of the development phases). This may not represent sufficient time since stroke for individuals to adequately appraise their situation, especially as some were still in hospital.
The relationship to self-management of any of the measures in this review was not explicitly stated by any of the study authors. This suggests that further clarification is required to determine the extent to which they reflect the process or outcomes of self-management. Whilst potential theoretical bases for the self-management of long-term conditions, such as self-efficacy, have gained increasing acknowledgement, the role in stroke self-management remains unclear. This is in part due to a lack of robust outcome measures and, in addition, a lack of clarity regarding the purported theoretical foundations of stroke self-management [Citation20,Citation62].
Quality of outcome measures
A paucity of measures scored “excellent” or “good” for quality according to the criteria outlined by COSMIN (). The COSMIN checklist does not advocate summarising the quality criteria into one overall quality score, as is often the case in other systematic reviews. An overall quality score would assume that all measurement properties are of equal importance. Since measurement properties are in part affected by the context in which they have been determined, this approach would be misleading. For example in our review, no measure was scored on cross-cultural validity, since the purpose of the review was not to assess how well a measure had been developed and validated in other languages or cultures.
Outcome measures should be developed with involvement of the target population to identify what is meaningful from their perspective and hence enhance content validity and clinical utility [Citation61,Citation63,Citation64]. Three measures scored “excellent” in the content validity category (SSEQ; SIPSO; SA-SIP30). Measures that did not include involvement of users in the development of the measure scored “poor” on the COSMIN checklist, regardless of other aspects of the content validity process which may have been classed “fair” “good” or “excellent”. This is partly as a result of the COSMINs scoring method in which the lowest score in any given category counts, but is also indicative of the importance of involving potential users in measurement development. Arguably, measures developed without user-involvement have questionable meaning and other types of validity, since without steps in the design to capture the experience of the population to be measured, the context of the measure remains largely that of the measure developers [Citation65,Citation66]. More recently techniques such as cognitive interviewing [Citation67], have been used by researchers [Citation68,Citation69] to ensure the content validity of new measures is optimal.
Difficulty exists in determining which measures used in the stroke SMIs in this review reflect self-management with validity since most measures did not score well according to the COSMIN criteria.
Several studies included unreported measures, designed specifically by the authors for the purpose of the SMI study [Citation44,Citation48–52]. With the exception of Marsden et al., studies utilised data from unreported measures as indicators of outcomes. An absence of psychometric data confounds the ability to draw reliable inferences from studies adopting those measures. In addition, a lack of information regarding the development or modification of unreported measures limits the ability to make judgments upon the validity and appropriateness of the measure. A further possible limitation on interpreting data from unreported measures may be a tendency for reporting positive results [Citation70]. Without establishment of reliability and validity the outcome measure is little more than a collection of items that have meaning to the developer alone [Citation33,Citation71]. Given the lack of consensus of how stroke self-management operates in the literature, and a lack of consensus upon the theoretical premises grounding stroke SMIs, the assumptions underpinning unreported measures remain speculative. Researchers and clinicians should exercise caution in considering findings from studies adopting unreported measures.
Of note is that 11 studies (85%) within this review adopted at least one outcome measure without reported validity and reliability with stroke populations. The reporting of minimal, or non-significant, observed changes following stroke SMIs in those studies including measures without established psychometric properties in stroke populations may be indicative of a lack of relevance and meaningfulness of those measures to stroke populations. Problems exist in using unreported measures when determining whether change occurred as a result of an ineffective intervention or due to imprecise measures.
Measures developed with intended user populations, facilitate the gaining of information about health, illness and the effects of health-care interventions from the perspective of the patient [Citation72,Citation73]. As well as enhancing content validity, this can also facilitate shared decision making with healthcare professionals. This is of particular relevance to those involved in promoting self-management and increasing patient autonomy, such as nurses and therapists.
Responsiveness is a necessary property of instruments intended for measuring clinically meaningful change, such as in stroke self-SMIs [Citation74,Citation75]. Involvement of users in the development of outcome measures promotes the responsiveness of measures. Arguments exist that responsiveness should focus upon detecting change that is valued by the person rather than the clinician or researcher [Citation72]. This is of particular relevance to self-management.
Change attributed to an intervention is an important aspect of evaluating clinical effectiveness. In this review, 13 measures included information on responsiveness in stroke populations. None of these measures scored “excellent” for this property, and only 15% scored “good”. Aside from inadequate sample sizes, a common finding was that studies often were not clear about what happened to study populations between testing. Additionally, authors often did not specify how missing items from respondents were handled. The result is that judgments regarding responsiveness data were difficult to substantiate. This also affected how well measures scored for reliability and other areas of validity. There is, therefore, a need for future measurement developers to specify these overlooked aspects of development more clearly in subsequent reporting.
The majority of SMI study populations within this review experienced stroke <24 months previously, with a number of studies using populations experiencing stroke no more than 6 months previously [Citation48,Citation50,Citation51,Citation76]. This may be a result of sampling to reduce the influence of additional factors upon study outcomes, such as the development of unhelpful coping behaviours, the likelihood of which might increase over time, and out of an assumption that more change may be observed in those early in their recovery. However, in reality the number of people living in the community and recovering from stroke extends beyond those who are 6–24 months post-stroke. As engagement in self-management activities varies during recovery, particularly following adjustment to stroke as a long-term condition, there is a future need to consider outcome measures sensitive to change(s) at different durations since stroke.
The role of PROMs, developed using rigorous investigation with the population to be measured extends beyond validating patient experience [Citation77]. PROMs may improve the quality of interactions between health professionals and patients, assess levels of health and need, and provide evidence of outcomes of services, for the purposes of audit, quality assurance and comparative performance evaluation [Citation78,Citation79]. This is of particular importance when trying to capture the essence of self-management, since the experience of clients is vital in determining what is valued from their perspective. There is a need to focus upon the development of measures of self-management developed with people recovering from stroke.
Limitations of review
This review focused upon the current state of measurement in stroke self-management. Consensus between reviewers was used to determine eligibility and inclusion of SMI articles. Whilst we were in agreement, there is the possibility for selection bias. Our aim was not to make judgments on the quality of the SMIs identified. However, the use of a standardized critical appraisal tool may assist the selection of articles for future reviews. Where interpretation of the COSMIN criteria differed, agreement was reached by discussion and consensus. Additional reviewers may have further validated this process however the criteria within COSMIN are explicitly stated and differences were quickly resolved. Data extraction was facilitated by a standardized tool advocated by COSMIN, with extraction and scoring checked in a random 10% of articles.We acknowledge that checking of 10% may be viewed as a limitation of this review, however, assert that a systematic process using a standard data extraction tool was followed throughout.
That COSMIN operates a “lowest score counts” scoring system may account for the lack of measures scoring well in the measurement property criteria. Some studies used otherwise appropriate methodologies, but were rated as “poor” due to inadequate sample sizes for analyses. For example, for a measure to be rated as “good” for reliability, measurement error, criterion validity and responsiveness, a sample size of n = 50–99 is required. To score “good” for internal consistency and structural validity, the sample size required increased to five times the number of items within a measure (and ≥100 OR 5–7* #items but <100). Therefore, if those studies were repeated with larger sample sizes, their ratings according to COSMIN could change dramatically. The tendency for measures to score poorly may be reflective of a floor effect of the COSMIN checklist. COSMIN was developed following consensus of experts in health measurement, therefore if its stringent criteria is to be adopted this is indicative of a need to debate the rigorous methods for measure development required. It is fair to comment that some of the measures in this review were developed before the focus upon involving potential users in measure development. It may be that the measures examined in this review require further development and investigation to establish adequate measurement properties for use with stroke populations.
Our review points to existing limitations in the evaluation of stroke self-SMIs. Our recommendations for clinicians and researchers seeking to evaluate such interventions would be firstly to clarify the theoretical premise of the intervention in question, as advocated elsewhere [Citation27,Citation80,Citation81]. Without this step, it is difficult to identify the mechanisms by which the intervention may influence outcomes, and thus difficult to select an outcome measure which appropriately captures the potential outcome. Potential outcome measures should be selected on the basis that they appropriately reflect and capture the expected outcome change.
This review highlights that the reported theoretical drivers within stroke SMIs are unclear, not least because they are often not explicitly stated by researchers. The heterogeneity of the outcome measures utilised by SMIs in this review may indicate a difficulty in determining the expected outcomes of stroke SMIs. A systematic review demonstrated that interventions with specific aims, such as reduced systolic blood pressure in Hypertension or glycosylated haemoglobin levels in Diabetes, produced greater effect sizes than those without defined outcomes [Citation82]. Further work is therefore warranted to conceptualise stroke self-management and examine the theoretical premises supporting such interventions, and expected outcomes so that appropriate outcome measures which accurately reflect the concept can be selected and/or developed. Until such clarification, researchers and clinicians should, where possible, select outcome measures with reliability and validity data in the population to be tested in the intervention. The selection of outcome measures developed with involvement from the target population is also advocated. This ensures that what is meaningful to the patient is more likely to be captured appropriately, thus enhancing content validity [Citation83].
In the meantime, researchers must support clinicians by conducting further work to examine the concept and theoretical premises of self-management and developing appropriate measures if required.
Conclusion
This is the first systematic review of international research on outcome measures used and selected in stroke self-SMI studies. We have identified important limitations in the measures used to evaluate the effectiveness of stroke self-SMIs, which has significant implications for the inferences we are currently able to draw about the evidence base. None of the measures used in studies of stroke SMIs, purported to specifically measure self-management as a discrete concept. This is indicative of the difficulty in conceptualisation and operation of this concept, a view expressed elsewhere [Citation13,Citation84]. Further work is required to determine how the measures identified in this review, align with the concept of self-management. The range of outcomes adopted, the lack of observed changes in outcomes following stroke SMIs and the lack of consensus surrounding which outcome measures to utilise, indicates that the causal mechanisms of stroke SMIs remain imprecise. Stroke SMIs have raced ahead of the evidence to support their theoretical basis, operation and effective evaluation [Citation85,Citation86]. Work to conceptualise stroke self-management is required to help identify which outcomes are most appropriate for evaluating interventions, to further inform the theoretical basis for SMIs [Citation87] and to assist the development of interventions. There is a need for studies to explore the theoretical underpinnings of SMI in stroke and for the development of robust outcome measures to enable evaluation of stroke SMIs.
Declaration of Interest: This work was supported by a University of Southampton research studentship. The authors report no declarations of interest.
References
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009;8:355–369.
- NAO, National Audit Office. Reducing brain damage: faster access to better stroke care. London: Stationary Office, 2005
- Ellis-Hill C, Payne S, Ward C. Using stroke to explore the life thread model: an alternative approach to understanding rehabilitation following an acquired disability. Disabil Rehabil 2008;30:150–159.
- Horgan NF, O’Regan M, Cunningham CJ, Finn AM. Recovery after stroke: a 1-year profile. Disabil Rehabil 2009;31:831–839.
- McKevitt C, Redfern J, Mold F, Wolfe C. Qualitative studies of stroke: a systematic review. Stroke 2004;35:1499–1505.
- Young J, Murray J, Forster A. Review of longer-term problems after disabling stroke. Rev Clin Gerontol 2003;13:55–65.
- Ch’ng A, French D, McLean N. Coping with the challenges of recovery from stroke: long term perspectives of stroke support group members. J Health Psychol 2008;13:1136–1146.
- Mukherjee D, Levin RL, Heller W. The cognitive, emotional, and social sequelae of stroke: psychological and ethical concerns in post-stroke adaptation. Top Stroke Rehabil 2006;13:26–35.
- Dowswell G, Lawler J, Dowswell T, Young J, Forster A, Hearn J. Investigating recovery from stroke: a qualitative study. J Clin Nurs 2000;9:507–515.
- Ellis-Hill CS, Payne S, Ward C. Self-body split: issues of identity in physical recovery following a stroke. Disabil Rehabil 2000;22:725–733.
- Vanhook P. The domains of stroke recovery: a synopsis of the literature. J Neurosci Nurs 2009;41:6–17.
- de Ridder D, Geenen R, Kuijer R, van Middendorp H. Psychological adjustment to chronic disease. Lancet 2008;372:246–255.
- Battersby M, Lawn S, Pols R. Conceptualisation of self-management. In: Kralik D, Paterson B, Coates V (eds). Translating chronic illness research into practice. Chichester: John Wiley and Sons Ltd., 2010.
- Kralik D, Koch T, Price K, Howard N. Chronic illness self-management: taking action to create order. J Clin Nurs 2004;13:259–267.
- DoH. White paper ‘Equity and excellence: liberating the NHS’. Health, Do. (ed). London: Department of Health, 2010.
- DoH. White paper. healthy lives, healthy people: our strategy for public health in England. London: The Stationary Office, 2010.
- DoH. The health and social care bill. Health, Do. (ed). London: Department of Health, 2011.
- Imison C. et al. Transforming our health care system: Top ten priorities for commissioners. London: The King’s Fund, 2011.
- Foundation H. Evidence: helping people help themselves. A review of the evidence considering whether it is worthwhile to support self-management. London: The Health Foundation, 2011.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 2003;26:1–7.
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA 2002;288:2469–2475.
- Glasgow RE, Funnell MM, Bonomi AE, Davis C, Beckham V, Wagner EH. Self-management aspects of the improving chronic illness care breakthrough series: implementation with diabetes and heart failure teams. Ann Behav Med 2002;24:80–87.
- Rogers A, Bury M, Kennedy A. Rationality, rhetoric, and religiosity in health care: the case of England’s Expert Patients Programme. Int J Health Serv 2009;39:725–747.
- Robinson-Smith G. Self-efficacy and quality of life after stroke. J Neuroscience Nursing 2002;34:91–98.
- Western H. Altered living: coping, hope and quality of life after stroke. Br J Nurs 2007;16:1266–1270.
- Du S, Yuan C. Evaluation of patient self-management outcomes in health care: a systematic review. Int Nurs Rev 2010;57:159–167.
- MRC. A framework for development and evaluation of RCTs for complex interventions to improve health. London: Medical Research Council, 2000.
- Lorig KR, Sobel DS, Stewart AL, Brown BW Jr, Bandura A, Ritter P, Gonzalez VM, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care 1999;37:5–14.
- Ajzen I, Fishbein M. The influence of attitude on behaviour. In: The Handbook of Attidues. Albarracín D, Johnson B, Zanna M (eds.). New Jersey: Lawrence Erlbaum Ltd., 2005, 173–221.
- Hardeman W et al. Application of the theory of planned behaviour in behaviour change interventions: a systematic review. Psychology & Health 2002;17:123–158.
- Hirsche RC, Williams B, Jones A, Manns P. Chronic disease self-management for individuals with stroke, multiple sclerosis and spinal cord injury. Disabil Rehabil 2011;33:1136–1146.
- Fazio RH, Olson MA. Implicit measures in social cognition. research: their meaning and use. Annu Rev Psychol 2003;54:297–327.
- Oppenheim A. Questionnaire design, interviewing and attitude measurement. 2nd edn., 2000, London: Pinter.
- Lohr KN, Aaronson NK, Alonso J, Burnam MA, Patrick DL, Perrin EB, Roberts JS. Evaluating quality-of-life and health status instruments: development of scientific review criteria. Clin Ther 1996;18:979–992.
- Valderas JM, Ferrer M, Mendívil J, Garin O, Rajmil L, Herdman M, Alonso J; Scientific Committee on “Patient-Reported Outcomes” of the IRYSS Network. Development of EMPRO: a tool for the standardized assessment of patient-reported outcome measures. Value Health 2008;11:700–708.
- Mokkink LB, Terwee CB, Stratford PW, Alonso J, Patrick DL, Riphagen I, Knol DL, et al. Evaluation of the methodological quality of systematic reviews of health status measurement instruments. Qual Life Res 2009;18:313–333.
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 2010;19:539–549.
- Mokkink LB, Terwee CB, Gibbons E, Stratford PW, Alonso J, Patrick DL, Knol DL, et al. Inter-rater agreement and reliability of the COSMIN (COnsensus-based Standards for the selection of health status Measurement Instruments) checklist. BMC Med Res Methodol 2010;10:82.
- Elbers RG, Rietberg MB, van Wegen EE, Verhoef J, Kramer SF, Terwee CB, Kwakkel G. Self-report fatigue questionnaires in multiple sclerosis, Parkinson’s disease and stroke: a systematic review of measurement properties. Qual Life Res 2012;21:925–944.
- Schellingerhout JM, Heymans MW, Verhagen AP, de Vet HC, Koes BW, Terwee CB. Measurement properties of translated versions of neck-specific questionnaires: a systematic review. BMC Med Res Methodol 2011;11:87.
- Smit S, Lamping D, Maclaine G. Measuring health-related quality of life in diabetic peripheral neuropathy: A systematic review. Diabetes Res Clin Practice, 2012;96:261–270.
- Terwee CB, Mokkink LB, Knol DL, Ostelo RW, Bouter LM, de Vet HC. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual Life Res 2012;21:651–657.
- DeVellis R. Scale development. Theory and applications. Bickman L, Rog D (eds.). Applied social research methods series. Vol. 26. 2nd edn., 2003, Sage Publications: Thousand Oaks.
- Marsden D, Quinn R, Pond N, Golledge R, Neilson C, White J, McElduff P, Pollack M. A multidisciplinary group programme in rural settings for community-dwelling chronic stroke survivors and their carers: a pilot randomized controlled trial. Clin Rehabil 2010;24:328–341.
- Jones F, Mandy A, Partridge C. Changing self-efficacy in individuals following a first time stroke: preliminary study of a novel self-management intervention. Clin Rehabil 2009;23:522–533.
- Sackley C, Wade DT, Mant D, Atkinson JC, Yudkin P, Cardoso K, Levin S, et al. Cluster randomized pilot controlled trial of an occupational therapy intervention for residents with stroke in UK care homes. Stroke 2006;37:2336–2341.
- Cadilhac DA, Hoffmann S, Kilkenny M, Lindley R, Lalor E, Osborne RH, Batterbsy M. A phase II multicentered, single-blind, randomized, controlled trial of the stroke self-management program. Stroke 2011;42:1673–1679.
- Allen K, et al. Improving stroke outcomes: implementation of a postdischarge care management model. J Clin Outcomes Manag 2004;11:707–714.
- Frank G, et al. Perceived control and recovery from functional limitations: Preliminary evaluation of a workbook-based intervention for discharged stroke patients. British J Health Psychol 2000;5:413–420.
- Johnston M, Bonetti D, Joice S, Pollard B, Morrison V, Francis JJ, Macwalter R. Recovery from disability after stroke as a target for a behavioural intervention: results of a randomized controlled trial. Disabil Rehabil 2007;29:1117–1127.
- Ljungberg C, Hanson E, Lovgren M. A home rehabilitation program for stroke patients: a pilot study. Scandinavian J Caring Sciences 2001;15:44–53.
- Sit JW, Yip VY, Ko SK, Gun AP, Lee JS. A quasi-experimental study on a community-based stroke prevention programme for clients with minor stroke. J Clin Nurs 2007;16:272–281.
- Chau JP, Thompson DR, Twinn S, Chang AM, Woo J. Determinants of participation restriction among community dwelling stroke survivors: a path analysis. BMC Neurol 2009;9:49.
- Saxena SK, Ng TP, Koh G, Yong D, Fong NP. Is improvement in impaired cognition and depressive symptoms in post-stroke patients associated with recovery in activities of daily living? Acta Neurol Scand 2007;115:339–346.
- Whyte EM, Mulsant BH, Vanderbilt J, Dodge HH, Ganguli M. Depression after stroke: a prospective epidemiological study. J Am Geriatr Soc 2004;52:774–778.
- Quinn T, Langhorne P, Stott D. Barthel index for stroke trials: development, properties, and application. Stroke 2011;42:1146–1151.
- Cott C, Wiles R, Devitt R. Continuity, transition and participation: preparing clients for life in the community post-stroke. Disabil Rehabil 2007;29:1566–1574.
- Jerant A, von Friederichs-Fitzwater M, Moore M. Patients’ percieved barriers to active self-management of chronic conditions. Patient Education and Counseling 2005;57:300–307.
- Bandura A. The nature and structure of self-efficacy. In: Bandura A (ed). Self-efficacy: The exercise of control, New York: WH Freeman and Company, 1997, pp 3–5.
- Kennedy A, Reeves D, Bower P, Lee V, Middleton E, Richardson G, Gardner C, et al. The effectiveness and cost effectiveness of a national lay-led self care support programme for patients with long-term conditions: a pragmatic randomised controlled trial. J Epidemiol Community Health 2007;61:254–261.
- Bowling A. What things are important in people’s lives? A survey of the public’s judgements to inform scales of health related quality of life. Soc Sci Med 1995;41:1447–1462.
- Bury M, Newbould J, Taylor D. A rapid review of the current state of knowledge regarding lay-led self-management of chronic illness. London: National Institute for Health and Clinical Excellence, 2005.
- Vogt D, King D, King L. Focus groups in psychological assessment: enhancing content validity by consulting members of the target population. Psychol Assess, 2004;16:231–243.
- McDowell I, Newell C. Measuring health: a guide to rating scales and questionnaires. 2nd edn, New York: Oxford University Press, 1996.
- Switzer GE, Wisniewski SR, Belle SH, Dew MA, Schultz R. Selecting, developing, and evaluating research instruments. Soc Psychiatry Psychiatr Epidemiol 1999;34:399–409.
- Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, Bouter LM, de Vet HC. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60:34–42.
- Willis GL, Moore C, Armstrong SM. Breaking away from dopamine deficiency: an essential new direction for Parkinson’s disease. Rev Neurosci 2012;23:403–428.
- Beck SL, Towsley GL, Berry PH, Brant JM, Smith EM. Measuring the quality of care related to pain management: a multiple-method approach to instrument development. Nurs Res 2010;59:85–92.
- Rosal M, Carbone E, Goins K. Use of cognitive interviewing to adapt measurement instruments for low-literate Hispanics. Diabetes Educ 2003;29:1006–1017.
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. Br J Psychiatry 2000;176:249–252.
- Raykov T, Marcoulides GA. Introduction to psychometric theory. Taylor & Francis, New York, USA, 2010.
- Fitzpatrick R, et al. Evaluating patient-based outcome measures for use in clinical trials. Health Technol Assess 1998;2:1–74.
- O’Donnell AB, Lutfey KE, Marceau LD, McKinlay JB. Using focus groups to improve the validity of cross-national survey research: a study of physician decision making. Qual Health Res 2007;17:971–981.
- Beaton DE, Bombardier C, Katz JN, Wright JG. A taxonomy for responsiveness. J Clin Epidemiol 2001;54:1204–1217.
- Liang MH, Lew RA, Stucki G, Fortin PR, Daltroy L. Measuring clinically important changes with patient-oriented questionnaires. Med Care 2002;40:II45–II51.
- Kendall E, Catalano T, Kuipers P, Posner N, Buys N, Charker J. Recovery following stroke: the role of self-management education. Soc Sci Med 2007;64:735–746.
- Greenhalgh J, Long AF, Flynn R. The use of patient reported outcome measures in routine clinical practice: lack of impact or lack of theory? Soc Sci Med 2005;60:833–843.
- Darzi A. Our NHS our future: High quality care for all in NHS next stage review final report. London: Department of Health, 2008.
- Jenkinson C, Gibbons E, Fitzpatrick R. A structured review of patient-reported outcome measures in relation to stroke. Oxford: Department of Public Health University of Oxford: P.-r.O.M. Group, 2009.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, Tyrer P. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694–696.
- Redfern J, McKevitt C, Wolfe CD. Development of complex interventions in stroke care: a systematic review. Stroke 2006;37:2410–2419.
- Warsi A, Wang PS, LaValley MP, Avorn J, Solomon DH. Self-management education programs in chronic disease: a systematic review and methodological critique of the literature. Arch Intern Med 2004;164:1641–1649.
- Lasch KE, Marquis P, Vigneux M, Abetz L, Arnould B, Bayliss M, Crawford B, Rosa K. PRO development: rigorous qualitative research as the crucial foundation. Qual Life Res 2010;19:1087–1096.
- Blakeman T, Bower P, Reeves D, Chew-Graham C. Bringing self-management into clinical view: a qualitative study of long-term condition management in primary care consultations. Chronic Illn 2010;6:136–150.
- Bury M, Pink D. The HSJ debate. Self-management of chronic disease doesn’t work. Health Serv J 2005;115:18–9, 1.
- Jones F, Riazi A. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil 2011;33:797–810.
- Cano SJ, Hobart JC. The problem with health measurement. Patient Prefer Adherence 2011;5:279–290.
- Wagner E. Care of older people with chronic illness. In: Calkins E et al. (eds). New ways to care for older people: building systems based on evidence. Springer: New York, 1999.
- Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr 1966;80:1–28.
- Schwarzer R. Self-efficacy in the adoption and maintenance of health behaviours: Theoretical approaches and a new model. In: Schwarzer R (ed). Self-efficacy: Thought control of action. Hemisphere: London, 1992, pp. 217–243.
- Guidetti S, Ytterberg C. A randomised controlled trial of a client-centred self-care intervention after stroke: a longitudinal pilot study. Disabil Rehabil 2011;33:494–503.
- Huijbregts MP, Myers AM, Streiner D, Teasell R. Implementation, process, and preliminary outcome evaluation of two community programs for persons with stroke and their care partners. Top Stroke Rehabil 2008;15:503–520.
- Huijbregts M, McEwen S, Taylor D. Exploring the Feasibility and Efficacy of a Telehealth Stroke Self-Management Programme: A Pilot Study. Physiotherapy Canada 2009;61:210–220.
- Orem D. Nursing: concepts of practice. St Louis, USA: Mosby, 1995.
- Carr J, Sheppard R. A motor relearning programme for stroke. 2nd edn. London: Heinemann, 1987.
- Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci 1995;50A:M28–M34.
- Botner E, Miller W, Eng J. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil 2005;27:156–163.
- Hawthorne G, Richardson J, Osborne R. The Assessment of Quality of Life (AQoL) instrument: a psychometric measure of health-related quality of life. Qual Life Res 1999;8:209–224.
- Sturm JW, Osborne RH, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Brief comprehensive quality of life assessment after stroke: the assessment of quality of life instrument in the north East melbourne stroke incidence study (NEMESIS). Stroke 2002;33:2888–2894.
- Mahoney FI, Barthel DW. Functional evaluation: The Barthel Index. Md State Med J 1965;14:61–65.
- Green J, Forster A, Young J. A test-retest reliability study of the Barthel Index, the Rivermead Mobility Index, the Nottingham Extended Activities of Daily Living Scale and the Frenchay Activities Index in stroke patients. Disabil Rehabil 2001;23:670–676.
- Jacob-Lloyd H. et al., Effective measurement of the functional progress of stroke clients. British J Occupat Ther 2005;68:253–259.
- Hsueh IP, Wang CH, Sheu CF, Hsieh CL. Comparison of psychometric properties of three mobility measures for patients with stroke. Stroke 2003;34:1741–1745.
- Duncan PW, Samsa GP, Weinberger M, Goldstein LB, Bonito A, Witter DM, Enarson C, Matchar D. Health status of individuals with mild stroke. Stroke 1997;28:740–745.
- van Hartingsveld F, Lucas C, Kwakkel G, Lindeboom R. Improved interpretation of stroke trial results using empirical Barthel item weights. Stroke 2006;37:162–166.
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. Int Disabil Stud 1988;10:61–63.
- Hsueh I, Lee M, Hsieh C. Psychometric characteristics of the Barthel activities of daily living index in stroke patients. J Formos Med Assoc 2001;100:526–532.
- Berg K. Measuring balance in the elderly: preliminary development of an instrument. Physiotherapy Canada 1989;41:304–311.
- Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health 1992;83 Suppl 2:S7–11.
- Berg K, Wood-Dauphinee S, Williams JI. The Balance Scale: reliability assessment with elderly residents and patients with an acute stroke. Scand J Rehabil Med 1995;27:27–36.
- English CK, Hillier SL, Stiller K, Warden-Flood A. The sensitivity of three commonly used outcome measures to detect change amongst patients receiving inpatient rehabilitation following stroke. Clin Rehabil 2006;20:52–55.
- Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Aust J Physiother 2001;47:29–38.
- Salbach NM, Mayo NE, Hanley JA, Richards CL, Wood-Dauphinee S. Psychometric evaluation of the original and Canadian French version of the activities-specific balance confidence scale among people with stroke. Arch Phys Med Rehabil 2006;87:1597–1604.
- Radloff L. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401.
- Agrell B, Dehlin O. Comparison of six depression rating scales in geriatric stroke patients. Stroke 1989;20:1190–1194.
- Shinar D, Gross CR, Price TR, Banko M, Bolduc PL, Robinson RG. Screening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke 1986;17:241–245.
- Kim JH, Park EY. Rasch analysis of the Center for Epidemiologic Studies Depression scale used for the assessment of community-residing patients with stroke. Disabil Rehabil 2011;33:2075–2083.
- Wilde B. Quality of care: models, instruments and empirical results among elderly. In: Department of Geriatric Medicine. University of Gothernburg, Gothenburg, Sweden, 1994.
- Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, Sanford J, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993;24:58–63.
- Huijbregts M, Gowland C, Gruber R. Measuring clinically important change with the Activity Inventory of the Chedoke-McMaster Stroke Assessment. Physiotherapy Canada, 2000;52:295–304.
- Holbrook M, Skilbeck CE. An activities index for use with stroke patients. Age Ageing 1983;12:166–170.
- Wade D, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med 1985;7:176–181.
- Piercy M, Carter J, Mant J, Wade DT. Inter-rater reliability of the Frenchay activities index in patients with stroke and their careers. Clin Rehabil 2000;14:433–440.
- Schepers VP, Ketelaar M, Visser-Meily JM, Dekker J, Lindeman E. Responsiveness of functional health status measures frequently used in stroke research. Disabil Rehabil 2006;28:1035–1040.
- Pedersen PM, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Comprehensive assessment of activities of daily living in stroke. The Copenhagen Stroke Study. Arch Phys Med Rehabil 1997;78:161–165.
- Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke 1993;24:1173–1177.
- Hsueh IP, Lin JH, Jeng JS, Hsieh CL. Comparison of the psychometric characteristics of the functional independence measure, 5 item Barthel index, and 10 item Barthel index in patients with stroke. J Neurol Neurosurg Psychiatr 2002;73:188–190.
- Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch Phys Med Rehabil 1993;74:531–536.
- Cavanagh SJ, Hogan K, Gordon V, Fairfax J. Stroke-specific FIM models in an urban population. J Neurosci Nurs 2000;32:17–21.
- Daving Y, Andrén E, Nordholm L, Grimby G. Reliability of an interview approach to the Functional Independence Measure. Clin Rehabil 2001;15:301–310.
- Segal ME, Schall RR. Determining functional/health status and its relation to disability in stroke survivors. Stroke 1994;25:2391–2397.
- Brock K, Goldie P, Greenwood K. Evaluating the effectiveness of stroke rehabilitation: choosing a discriminative measure. Arch Phys Med Rehabil 2002;83:92–99.
- Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil 1996;77:1226–1232.
- Sheikh, J, Yesavage J. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin Gerontologist, 1986;5:165–172.
- Wancata J, Alexandrowicz R, Marquart B, Weiss M, Friedrich F. The criterion validity of the Geriatric Depression Scale: a systematic review. Acta Psychiatr Scand 2006;114:398–410.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361–370.
- Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics 2002;43:386–393.
- Johnston M, Pollard B, Hennessey P. Construct validation of the hospital anxiety and depression scale with clinical populations. J Psychosom Res 2000;48:579–584.
- Lennon S, Johnson L. The modified rivermead mobility index: validity and reliability. Disabil Rehabil 2000;22:833–839.
- Johnson, L, Selfe J. Measurement of mobility following stroke: a comparison of the Modified Rivermead Mobility Index and the Motor Assessment Scale. Physiotherapy, 2004;90:132–138.
- Hsieh C, Hsueh I, Mao M. Validity and responsiveness of the Rivermead Mobility Index in stroke patients. Scand J Rehab Med, 2000;32:140–142.
- Partridge C, Johnston M. Perceived control of recovery from physical disability: measurement and prediction. Br J Clin Psychol 1989;28 (Pt 1):53–59.
- Johnston M et al., Perceived control, coping and recovery from disability following stroke. Psychology & Health, 1999;14:181–192.
- Wood-Dauphinee SL, Opzoomer MA, Williams JI, Marchand B, Spitzer WO. Assessment of global function: The Reintegration to Normal Living Index. Arch Phys Med Rehabil 1988;69:583–590.
- Daneski K, Coshall C, Tilling K, Wolfe CD. Reliability and validity of a postal version of the Reintegration to Normal Living Index, modified for use with stroke patients. Clin Rehabil 2003;17:835–839.
- Stark SL, Edwards DF, Hollingsworth H, Gray DB. Validation of the Reintegration to Normal Living Index in a population of community-dwelling people with mobility limitations. Arch Phys Med Rehabil 2005;86:344–345.
- Whiting, S, Lincoln N. An ADL assessment for stroke patients. British J Occup Ther 1980;43:44–46.
- Lincoln NB, Edmans JA. A re-validation of the Rivermead ADL scale for elderly patients with stroke. Age Ageing 1990;19:19–24.
- Nouri, F, Lincoln N. An extended activities of daily living scale for stroke patients. Clin Rehabil 1987;1:301–305.
- Rossier P, Wade DT, Murphy M. An initial investigation of the reliability of the Rivermead Extended ADL index in patients presenting with neurological impairment. J Rehabil Med 2001;33:61–70.
- van Straten A, de Haan RJ, Limburg M, Schuling J, Bossuyt PM, van den Bos GA. A stroke-adapted 30-item version of the Sickness Impact Profile to assess quality of life (SA-SIP30). Stroke 1997;28:2155–2161.
- van de Port I et al., Monitoring the functional health status of stroke patients: the value of the Stroke-Adapted Sickness Impact Profile-30. Disability & Rehabilitation, 2004;26:635–640.
- van Straten A, de Haan RJ, Limburg M, van den Bos GA. Clinical meaning of the Stroke-Adapted Sickness Impact Profile-30 and the Sickness Impact Profile-136. Stroke 2000;31:2610–2615.
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke 1999;30:2131–2140.
- Duncan PW, Bode RK, Min Lai S, Perera S; Glycine Antagonist in Neuroprotection Americans Investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil 2003;84:950–963.
- Lin KC, Fu T, Wu CY, Wang YH, Liu JS, Hsieh CJ, Lin SF. Minimal detectable change and clinically important difference of the Stroke Impact Scale in stroke patients. Neurorehabil Neural Repair 2010;24:486–492.
- Trigg R, Wood VA. The Subjective Index of Physical and Social Outcome (SIPSO): a new measure for use with stroke patients. Clin Rehabil 2000;14:288–299.
- Trigg R, Wood VA. The validation of the Subjective Index of Physical and Social Outcome (SIPSO). Clin Rehabil 2003;17:283–289.
- Kersten P, Ashburn A, George S, Low J. The subjective index for physical and social outcome (SIPSO) in stroke: investigation of its subscale structure. BMC Neurol 2010;10:26.
- Jones F, Partridge C, Reid F. The Stroke Self-Efficacy Questionnaire: measuring individual confidence in functional performance after stroke. J Clin Nurs 2008;17:244–252.
- Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke 1999;30:1362–1369.
- Williams LS, Weinberger M, Harris LE, Biller J. Measuring quality of life in a way that is meaningful to stroke patients. Neurology 1999;53:1839–1843.