Abstract
The transmembrane domains of fusion proteins are known to be important for their fusogenic activity. In an effort to systematically investigate the structure/function relationships of transmembrane domains we had previously designed LV-peptides that mimic natural fusion protein TMDs in their ability to drive fusion after incorporation into liposomal membranes. Here, we investigate the impact of different structural features of LV-peptide TMDs on inner and outer leaflet mixing. We find that fusion driven by the helical peptides involves a hemifusion intermediate as previously seen for natural fusion proteins. Helix backbone dynamics enhances fusion by selectively promoting outer leaflet mixing. Furthermore, the hydrophobic length of the peptides as well as covalent attachment of long acyl chains affects outer and inner leaflet mixing to different extents. Different structural features of transmembrane domains thus appear to differentially influence the rearrangements of lipids in fusion initiation and the hemifusion-to-fusion transition. The relevance of these findings in respect to the function of natural fusion proteins is discussed.
| Abbreviations: | ||
| CD, | = | circular dichroism |
| (d)TFE, | = | (mono-deuterated) 2,2,2-trifluoroethanol |
| DHX, | = | deuterium/hydrogen-exchange |
| GPI, | = | glycosylphosphatidylinositol |
| NBD-PE, | = | N-(7-nitro-2,1,3-benzoxadiazol-4-yl)hexadecylphosphatidylethanolamine |
| DOPE, | = | di-oleoyl-phosphatidylethanolamine |
| DOPS, | = | di-oleoyl-phosphatidylserine |
| OL, | = | outer leaflet |
| IL, | = | inner leaflet |
| P/L, | = | peptide/lipid |
| POPC, | = | palmitoyl-oleoyl-phosphatidylcholine |
| Rh-PE, | = | N-(lissamine rhodamin B sulfonyl)hexadecylphosphatidylethanolamine |
| SNARE, | = | soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptor |
| TMD, | = | transmembrane domain |
Introduction
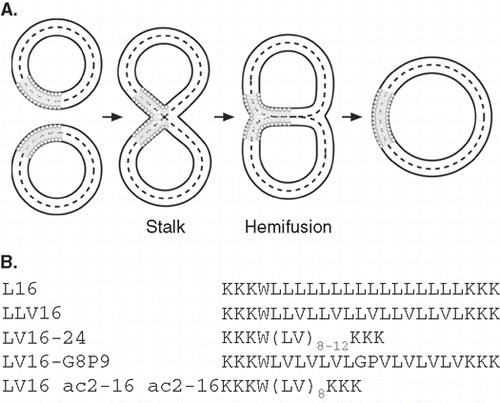
Biological membrane fusion is central to cellular secretion and endocytosis, infection of eukaryotic host cells by enveloped viruses, cell-cell fusion etc. [Citation1–4]. Fusion is thought to proceed in two sequential steps [Citation5–7]: (i) Merger of the contacting outer leaflet (OL) results in hemifusion via a stalk intermediate, and (ii) merger of the inner leaflet (IL) results in full fusion (). Most intracellular fusion events are driven by soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptors (SNAREs) and virus/host cell fusion depends on fusogenic viral envelope proteins [Citation1,Citation8]. Both types of fusogens have single transmembrane domains (TMDs) that are required for lipid mixing since substitution for lipid anchors, truncation, and point mutations affect different steps of the fusion process (reviewed in ref. [Citation2]). Specifically, there is evidence that outer leaflet mixing is compromised after replacing the TMD of Vesicular Stomatitis virus G-protein [Citation9], of influenza hemagglutinin [Citation10], or of flipped presynaptic SNAREs [Citation11] by a glycosylphosphatidylinositol (GPI)-anchor. Inner leaflet mixing is abrogated with GPI-anchored hemagglutinin [Citation12,Citation13] or flipped SNAREs [Citation11]. Similarly, mutating [Citation14,Citation15] or truncating viral fusion protein [Citation10] or SNARE [Citation16] TMDs partially arrested bilayer mixing at hemifusion. Hemifusion is therefore assumed to be an on-pathway intermediate of membrane fusion whose completion requires proteinaceous TMDs.
Figure 1. (A) Lipid topology in liposome fusion. The simplified schematic depicts the hypothetical lipid arrangements along the reaction pathway from unfused liposomes via the stalk and hemifusion intermediates to complete lipid mixing. (B) Design of LV-peptides used in this study. The hydrophobic cores are flanked by Lys residues and a Trp is added for quantification of peptides.
Many SNAREs and fusogenic viral envelope proteins are acylated within or adjacent to their TMDs [Citation17–22]. While the impact of acylation on SNARE fusogenicity is currently unclear, acylation of viral envelope proteins has been reported to affect fusion at different stages. Depending on the type of protein and assay system used, acylation influenced fusion pore formation [Citation23,Citation24], the hemifusion-to-full fusion transition [Citation25], or syncytium formation [Citation26]. Thus, acylation near the TMDs influences the function of viral fusogenic proteins although it is not clear yet which fusion subreaction is most affected.
Synthetic peptides that correspond to fusion protein TMDs mimic the basic function of fusion proteins in that they drive liposome fusion in a sequence-specific way. Fusogenic activity was demonstrated for membrane-integrated TMD peptides of synaptic [Citation27] and yeast vacuolar [Citation28] SNAREs and of the Vesicular Stomatitis Virus G-protein [Citation14,Citation29]. In the absence of the soluble SNARE coiled coil domains, it is assumed that the presence of the TMD helices destabilizes the membranes such that random liposome-liposome collisions result in a higher number of productive OL mixing reactions. This, in turn, suggests a membrane-perturbing activity of the TMDs. An impact of fusogenic model TMDs on lipid phase has indeed been shown previously [Citation30] by solid-state NMR. TMD peptide fusogenicity is related to conformational backbone dynamics of the transmembrane helices as the helix-destabilizing β-branched amino acids Val and Ile are overrepresented in SNARE TMDs [Citation27,Citation31]. Deuterium/hydrogen-exchange (DHX) experiments indeed demonstrated that synaptic SNARE TMDs form helices with conformationally dynamic backbones and mutating β-branched residues reduced dynamics and fusogenicity [Citation32].
Inspired by the overrepresentation of Ile/Val in SNARE TMDs we had previously designed a set of low-complexity membrane-fusogenic TMD-peptides de novo, termed LV-peptides. LV-peptides contain hydrophobic core sequences that are composed of Leu and helix-destabilizing Val, Gly, or Pro residues (, ref [Citation33]) and flanked by Lys triplets. Indeed, increased contents of helix-destabilizing core residues enhanced the fusogenicity of LV-peptides [Citation33] and the dynamics of the helix backbone as assessed by deuterium/hydrogen-exchange experiments in isotropic solution [Citation34]. In the membrane, the purely aliphatic LV-peptides L16, LLV16, and LV16 form mainly α-helices while LV16-G8P9 exhibits only a minor fraction of α-helix and a major β-sheet component [Citation35].
Here, we deconstructed total fusion kinetics of LV-peptide-driven liposome fusion into OL and IL mixing kinetics. We show that fusion proceeds via a hemifusion intermediate. Further, sequence and length of the hydrophobic core, covalent attachment of various acyl chains as well as peptide concentration differentially affect the efficiency of fusion initiation and the hemifusion-to-fusion transition.
Materials and methods
Peptide synthesis
Peptides were synthesized by Boc chemistry (PSL, Heidelberg, Germany) and were > 90% pure as judged by mass spectrometry. Concentrations were determined via tryptophan absorbance using an extinction coefficient of 5,600 M-1cm-1.
Preparation of liposomes
Liposomes (final lipid concentration 3 mM) were made by lyophilizing mixtures of palmitoyl-oleoyl-phosphatidylcholine (POPC), di-oleoyl-phosphatidylethanolamine (DOPE) and di-oleoyl-phosphatidylserine (DOPS) at a 3:1:1 molar ratio with or without peptides followed by hydration and sonication [Citation33] in 10 mM Tris/HCl, pH 7.4, 10 mM NaCl for CD spectroscopy, or in 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.1 mM EDTA for fusion assays. Peptide concentrations and peptide/lipid (P/L)-ratios were determined as described [Citation33].
CD spectroscopy
CD spectra were measured with a Jasco J-710 automatic recording spectral polarimeter. Spectra of liposomes (1.5 mM lipid containing peptides at P/L ≈ 0.01) were recorded from 200–240 nm in a 1-mm dichroically neutral quartz cuvette at 20°C by using a time constant of 4 sec, a scan speed of 100 nm/min, and a sensitivity of 100 millidegrees per cm. Spectra represent the signal-averaged accumulation of 10 scans. Baseline spectra were recorded with pure liposomes and subtracted from the spectra of peptide-containing liposomes. All spectra were converted to mean residue ellipticity (θmr), and secondary structures were calculated using the CDNN/PEPFIT algorithm that is based on a user-defined set of peptide-based reference spectra [Citation36].
Liposome fusion
Liposomes with or without integrated peptides were prepared as described above and their fusion was determined using a well-established fluorescence dequenching assay upon rapidly shifting the temperature to 37°C as described [Citation33]. For inner leaflet mixing kinetics, the NBD chromophore located within the outer leaflet was quenched by incubation of donor liposomes with 20 mM Na-dithionite (DTN) for 20 min on ice. Excess DTN was removed via gel-filtration on Sephadex G50 columns. All values were corrected for the peptide-independent, spontaneous fusion of pure liposomes (extent of fusion after 1 h < ∼ 5%) and for detergent quenching of NBD fluorescence (< 3% of the total values with unbleached samples and ∼ 16% in case of dithionite-treated liposomes). The initial rates of fusion were calculated by fitting the first 10 min of the kinetics by a polynomial function and determining its first derivative, extents of fusion after 1 h were obtained by averaging the last 5 min of kinetics (Origin Software). The percentages of hemifusion seen at 1 h were calculated with the equation:
where PT is the percentage of total lipid mixing and PI is that of inner leaflet mixing. Outer leaflet mixing kinetics were calculated by subtraction of inner leaflet kinetics from those of total fusion [Citation37].
Results
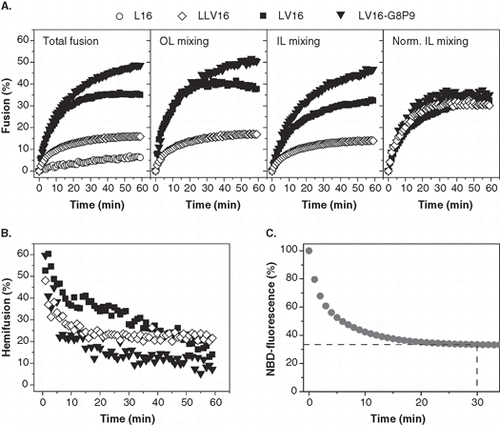
In order to identify structural features that influence OL and IL mixing we tested a series of LV-peptide TMDs with different hydrophobic core sequences as well as two sets of novel derivatives with altered core length or covalently attached acyl chains (). Total liposome fusion was detected by a standard fluorescence dequenching assay. This assay is based on decreasing fluorescence resonance energy transfer between the membrane-bound fluorophores NBD-PE and Rh-PE upon fusion of fluorescence-labelled donor liposomes with unlabelled acceptor liposomes containing the TMDs. The extent of fusion at each time point is calculated by normalizing the respective fluorescence values to maximal fluorescence dequenching seen upon detergent lysis of the liposomes. Since the fluorescent lipids are equally distributed in the outer and inner leaflets, different total fusion kinetics induced by different TMDs can either result from different OL or IL mixing efficiencies, or from both. Reduced IL mixing would indicate impaired transition of a hemifusion intermediate to complete bilayer mixing. IL mixing can be distinguished from total fusion after dithionite treatment of the fluorescent donor liposomes. Dithionite converts the NBD moiety present in the outer membrane leaflet to a non-fluorescent derivative while leaving NBD of the inner leaflet unaffected. Accordingly, only inner leaflet mixing can now result in fluorescence dequenching [Citation38]. Under our conditions, bleaching with dithionite reproducibly eliminates about two-thirds of NBD fluorescence which reaches a plateau after 30 min (). This reveals preferential bleaching of NBD present in the outer monolayer that contains about two-thirds of the total lipid of our unilamellar liposomes having a mean diameter of ∼35 nm [Citation27]. OL mixing kinetics and the percentage of hemifusion as a function of reaction time are calculated from total fusion and IL mixing as detailed in Materials and methods.
Figure 2. Dependence of liposome fusion elicited by LV-peptides on primary structure. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 4–8 experiments recorded at P/L = 0.0037–0.0061 and scaled to P/L = 0.005 for better comparability. (B) The percentage of hemifusion as a function of reaction time as calculated from total and IL mixing kinetics given in part A. (C) Kinetics of dithionite bleaching. The decrease of NBD-fluorescence upon incubation of donor liposomes with 20 mM Na-dithionite on ice to about two-thirds of the original value after ∼ 20 min indicates preferential bleaching of NBD-PE within the outer leaflet.
The sequence of the hydrophobic TMD core primarily affects OL mixing
Previously, LV-peptides with different primary structures of the hydrophobic core () were [Citation33] determined to induce total membrane fusion in the order LV16-G8P9 > LLV16 > LV16 > L16 as shown in (left panel) for P/L-ratios ≈ 0.005. Fusion approaches saturation after ∼ 20 min with LLV16 and LV16 but continues with LV16-G8P9 beyond this time point which may indicate that LV16-G8P9 drives more rounds of fusion than LV16. The total fusion kinetics of the three fusogenic TMDs, LLV16, LV16 and LV16-G8P9, were then deconstructed into OL and IL mixing (, center panels). Apparently, OL and uncorrected IL mixing follow the same rank order as total fusion. Since IL mixing can only proceed after successful OL mixing, we normalized IL mixing to OL mixing. This enables us to investigate the impact of our TMDs on IL mixing independent from prior OL mixing. The result (, right panel) shows that normalized IL mixing is comparable for the three TMDs. Furthermore, transforming total fusion and IL mixing kinetics into hemifusion kinetics reveals that all TMDs induce mainly hemifusion at early time points that progressively declines in favour of full fusion (). This decline of hemifusion with time suggests that the mixing of inner leaflets is delayed relative to that of outer leaflets, thus confirming hemifusion as an on-pathway intermediate in LV-peptide-mediated fusion.
One might argue that transbilayer movement of NBD-labeled lipid from the inner to the outer leaflet (flop) would create a situation where apparent IL mixing is confounded by OL mixing. Thus, lipid flop might lead to an overestimation of hemifusion. LV-peptides indeed induce transbilayer movement of up to ∼ 15% NBD-PE across the liposomal bilayer within one hour (ML and DL, unpublished). Assuming that subsequent to dithionite bleaching even a fraction of 20% of all NBD-PE moves from the IL (where it is originally present at 0.8 mol% in total) to the OL in the fusion experiment this would result in the presence of 0.08 mol% NBD-PE in the OL that has twice the area of the IL. To assess whether dequenching of 0.08 mol% NBD-PE in the OL could significantly influence the signal resulting from IL mixing, we have done the following control experiment: Maximal dequenching of NBD-PE fluorescence was induced by detergent lysis and compared after incorporation of 0.08 mol% (= flop control) or 0.8 mol% (= fusion assay) into liposomal membranes along with 0.8 mol% Rhodamine-PE under fusion conditions. The result (Supplementary Figure 1, online version only) clearly shows that very little NBD fluorescence dequenching is seen with 0.08 mol% NBD-PE as compared with the strong dequenching seen with 0.8% NBD-PE. This is due to the fact that the NBD-Rhodamine distance at the lower NBD-PE concentration is much longer than the respective Foerster distance. In other words, even if we assume that more than the maximum amount of NBD-PE, that can flop within one hour, is already present in the OL at the start of the fusion reaction, the dequenching of flopped NBD-PE would add only insignificantly to the signal generated by true IL mixing.
In summary, the different efficiencies of LLV16, LV16, and LV16-G8P9 in inducing total fusion are primarily ascribed to different efficiencies in OL mixing.
The length of the hydrophobic TMD core affects OL mixing and IL mixing
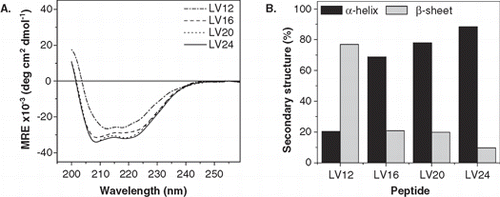
Here, we studied the effect of various lengths of the hydrophobic peptide core, ranging from 12–24 residues, on OL and IL mixing. First, the secondary structure of the peptides in liposomal membranes was examined by CD spectroscopy. The spectra are shown in and their quantitative evaluation in terms of percent α-helix and β-sheet gave the values depicted in . LV16, LV20, and LV24 shared strong helicities (70–90%). Surprisingly, shortening the core to 12 residues (LV12) resulted in predominating β-sheet.
Figure 3. Secondary structure of LV-peptides with different hydrophobic length in liposomal membranes determined by CD spectroscopy. (A) The curve shapes of the CD spectra recorded at at P/L ≈ 0.01 indicate α-helical structures for LV16, LV20, and LV24 and a predominating β-sheet for LV12. Curves are averaged from 2–5 independent measurements. (B) Deconvolution of the spectra quantitates helix and sheet contents. The remaining secondary structure is accounted for by random coil and turn elements.
Second, we monitored the effect of core length on total fusion as well as on OL and IL mixing at P/L ≈ 0.005. reveals that the extents of total fusion after 1 h follow the rank order LV16 > LV12 >> LV20 ≈ LV24. A similar order is seen for OL mixing, except that LV12 here is somewhat less active relative to LV16. In contrast, the rank order of normalized IL mixing (LV12 ≥ LV16 >> LV20 > LV24) shows that LV12 represents the most potent peptide in IL fusion. OL and IL mixing kinetics were also evaluated by calculating the corresponding initial fusion rates. The initial OL mixing rate reflects the probability by which the outer leaflets of randomly colliding liposomes fuse. Although this rate is also influenced by the distance-dependence of fluorescence transfer, this latter factor is identical for all peptides. The initial rate of normalized IL mixing corresponds to the probability by which a hemifused liposome converts to a fully fused one. confirms that LV16 is most efficient in inducing OL mixing while the IL mixing rate is highest with LV12 and decreases with core length. Length-dependent IL mixing is also reflected by the derived hemifusion kinetics (). LV20 and LV24 elicit strong initial hemifusion which only slowly declines with time and remains at high levels after 1 h. In contrast, hemifusion is barely detectable of LV12 at any time point thus confirming the high IL mixing efficiency of this peptide.
Figure 4. Kinetics of liposome fusion elicited by LV-peptides with different hydrophobic lengths. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 4–8 experiments recorded at P/L = 0.0034–0.0056 and scaled to P/L = 0.005 for better comparability. (B) The initial rates of OL and normalized IL mixing kinetics as obtained by fitting the data presented in part A. (C) The percentage of hemifusion as a function of reaction time as calculated from total and IL mixing kinetics given in part A.
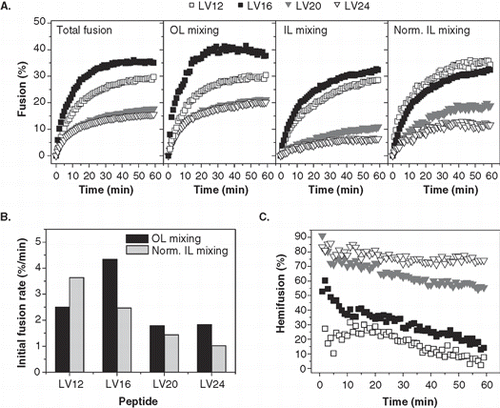
In summary, elongating the hydrophobic cores of the helical peptides beyond 16 residues affects OL and IL mixing; shortening it to 12 residues results in mainly β-sheet conformation with diminished OL, but increased IL, mixing efficiency.
Covalent attachment of acyl chains primarily affects OL mixing
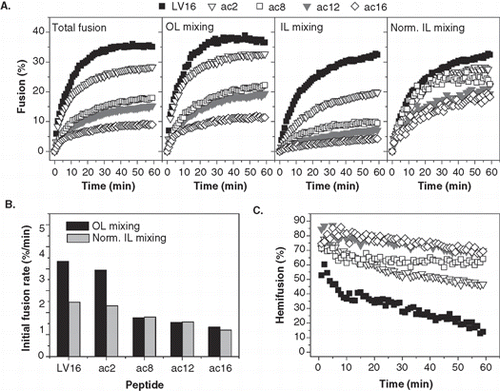
Here, we investigated the impact of attaching saturated acyl chains containing from 2–16 carbons to the N-terminus of LV16 (). The fusion kinetics () recorded at P/L-ratios ≈ 0.005 reveal that total fusion and OL mixing were reduced by the acyl chains in the order LV16 > LV16ac2 >> LV16ac8 > LV16ac12 > LV16ac16. By comparison, acylation had a smaller impact on normalized IL mixing (LV16 ≥ LV16ac2 ≥ LV16ac8 ≥ LV16ac12 ≥ LV16ac16). Similar results were obtained by comparing the initial rates which confirm that acetylation (ac2) has a minor impact on OL and IL mixing and that ac8-ac16 chains affect OL mixing more strongly than IL mixing (). The derived hemifusion kinetics show that fusion induced by all acylated peptides proceeds through initial hemifusion; hemifusion decreases during the reaction but remains at substantial levels after 1 h which is in line with the decreased IL mixing efficiencies ().
Figure 5. Kinetics of liposome fusion elicited by LV-peptides with covalently attached acyl chains. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 5–8 experiments recorded at P/L = 0.0036–0.0059 and scaled to P/L = 0.005 for better comparability. (B) The initial rates of OL and normalized IL mixing kinetics as obtained by fitting the data presented in part A.
Discussion
By deconstructing total liposome fusion kinetics into OL and IL mixing we identify structural features of LV-peptides that differentially influence both fusion subreactions.
We have previously shown that fusogenicity of LV-peptides increases with the conformational dynamics of the helix backbone as increasing the Val/Leu ratio increases the rate and extent of total liposome fusion [Citation33] as well as the rate of deuterium/hydrogen exchange kinetics [Citation34]. Furthermore, the backbone dynamics of hydrophobic regions close to the helix termini is more relevant for fusogenicity than that of central domains [Citation34]. Our current results reveal that helix backbone dynamics influence primarily the efficiency of OL mixing. Thus, we propose that a dynamic helix influences the structure of the surrounding bilayer such as to facilitate fusion initiation, i.e., stalk formation, upon random liposome-liposome collision. The biological significance of this finding is borne out by the previous observation that SNARE TMDs display an overabundance of helix-destabilizing Ile and Val residues [Citation27]. The fact that TMD-mimicking peptides alone can be fusogenic is taken for evidence that the TMDs by themselves play an important role in the fusion process. It has initially been surprising that the macroscopic fusion kinetics observed with the peptides [Citation27] is similar to the kinetics observed with full-length SNARE proteins (e.g., refs [Citation39,Citation40]). At first glance, this result would seem incompatible with the SNARE hypothesis which posits that formation of a soluble coiled-coil complex between syntaxin and SNAP-25 on one membrane and synaptobrevin on the other one, which leads to vesicle docking, is required for subsequent fusion. Some more recent experiments resolve this conundrum. It has been shown that the macroscopic liposome fusion kinetics observed with reconstituted full-length SNAREs is limited by the frequency of diffusive collisions [Citation40], similar to our peptide model. Furthermore, the probability by which a random collision results in a trans-SNARE complex followed by fusion is dramatically increased by stabilization of the syntaxin/SNAP-25 complex at a 1:1 stoichiometry by addition of a synaptobrevin-derived soluble peptide [Citation41]. Under these conditions, macroscopic fusion kinetics seen with full-length SNAREs is indeed much faster than that of the peptide-driven fusion kinetics revealing that interaction of protein domains in trans strongly enhances the probability by which a random liposome collision turns into fusion.
Peptide LV16-G8P9 forms a mixture of helical and predominating sheet structures that are likely to arise from backfolding of β-strands at the central Gly-Pro pair [Citation35]. It remains unresolved whether or not the fusogenicity of LV16-G8P9 is entirely accounted for by a highly dynamic helix or whether its β-sheet content contributes to fusion.
Furthermore, our current results show that liposome fusion induced by most LV-peptide TMDs proceeds through a transient hemifusion intermediate. In this respect, LV-peptides behave like the synthetic TMD of the yeast vacuolar SNARE Vam3p [Citation28] and full-length SNAREs [Citation11,Citation16,Citation42,Citation43]. Interestingly, no hemifusion intermediate was detectable for LV12 which forms mainly β-sheet in the membrane. Sheet formation is ascribed to negative hydrophobic mismatch since the calculated length of a 12 residues hydrophobic helix (∼ 1.8 nm) is too short to span the acyl chain region of a membrane with a lipid composition used here (2.6–2.9 nm, ref. [Citation44]). In agreement with this notion, we find that LV12 is predominantly α-helical (BP and DL, unpublished data) in DMPC membranes (2.3 nm hydrophobic core, ref. [Citation44]). The apparent absence of a hemifusion intermediate may indicate that fusion driven by the sheet-forming LV12 directly proceeds from a stalk to full fusion. Indeed, molecular modelling of liposome fusion has previously suggested that the fusion process branches at the stalk stage into a reaction directly leading to complete fusion and into a pathway involving a hemifusion diaphragm [Citation45].
Our results obtained with TMDs of different hydrophobic length suggest that negative hydrophobic mismatch may promote OL and IL mixing along the hemifusion pathway. A 16-residue hydrophobic core would form a ∼ 2.4 nm helix that may generate some hydrophobic mismatch in our membranes. A small extent of hydrophobic mismatch can lead to snorkelling of the terminal Lys residues [Citation46] and/or to a reduced width of the membrane containing non-bilayer forming lipids, like PE [Citation47]. No negative hydrophobic mismatch is predicted for LV20 and LV24. Since these longer TMD helices are less efficient in both OL and IL mixing, it is likely that minor negative hydrophobic mismatch with the possible consequence of local membrane deformation promotes the fusion process at both stages. This view is consistent with the previous finding that LV-peptide fusogenicity critically depends on positively charged terminal residues. His-flanked derivatives induced liposome fusion only at acidic pH values where His is protonated and thus potentially generates mismatch [Citation48]. It must be borne in mind however, that fusion also depends on helix backbone dynamics as the stable L16 is virtually non-fusogenic.
Covalent acylation also modulates LV-peptide fusogenicity by affecting OL and IL mixing. The strong impact of ac8–ac16 chains cannot be ascribed to a mere N-capping effect since acetylation had only a minor effect. It is currently not clear how acylation exerts its effect. Presumably, the long acyl chains may interfere with the lipid rearrangement that is required for fusion close to the TMDs. Acylation does not significantly affect deuterium/hydrogen exchange kinetics of these peptides thus excluding its influence via altered helix backbone dynamics although acylation has been shown to stabilize an intrinsically flexible helix globally [Citation49].
Is regulation of LV-peptide-mediated fusion by acylation relevant for natural fusogenic proteins? Many membrane-spanning yeast SNARE proteins are acylated [Citation19–21] and the synaptic SNARE synaptobrevin is acylated within the TMD in a developmentally regulated way [Citation22]. While the impact of acylation on SNARE fusogenicity is currently unclear, there is ample evidence that acylation of viral envelope proteins affects virus-cell fusion. For example, influenza HA has three highly conserved palmitoylated acylation sites and mutating them affected fusogenicity [Citation50], fusion pore formation [Citation23,Citation24], the hemifusion-to-full fusion transition [Citation25], or syncytium formation [Citation26]. Acylation at sites within the TMD of the Coronavirus spike protein also appears to affect fusogenicity [Citation51] whereas mutation of acylation sites within the TMD of Sindbis virus envelope glycoprotein had no discernible effect [Citation17]. Thus, acylation of TMDs or flanking regions appears to modulate the function of different viral fusogenic proteins although the situation is currently confounded by strain specificity of the effects [Citation52]. It seems clear, however, that our current data collected with acylated LV-peptides support the hypothesis that attachment of long acyl chains to fusion protein TMDs regulates the efficiency of OL and IL mixing.
In conclusion, different structural properties of LV-peptides, a simplified model of fusion protein TMDs, including helix backbone dynamics, hydrophobic length, and acylation can have pronounced effects on the initiation of fusion as well as on the hemifusion-to-fusion reaction.
Supplementary Material
Download PDF (59.6 KB)Acknowledgements
This work was supported by the Munich Center for Integrative Protein Sciences (CIPSM) and the State of Bavaria.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Söllner TH. 2004. Intracellular and viral membrane fusion: A uniting mechanism. Curr Opin Cell Biol 16:429–435.
- Langosch D, Hofmann MW, Ungermann C. 2007. The role of transmembrane domains in membrane fusion. Cell Mol Life Sci 64:850–864.
- Sudhof TC, Rothman JE. 2009. Membrane fusion: Grappling with SNARE and SM proteins. Science 323:474–477.
- Yoon TY, Shin YK. 2009. Progress in understanding the neuronal SNARE function and its regulation. Cell Mol Life Sci 66:460–469.
- Chernomordik LV, Kozlov MM. 2003. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem 72:175–207.
- Tamm LK, Crane J, Kiessling V. 2003. Membrane fusion: A structural perspective on the interplay of lipids and proteins. Curr Opin Struct Biol 13:453–466.
- Ungermann C, Langosch D. 2005. Functions of SNAREs in intracellular membrane fusion and lipid bilayer mixing. J Cell Sci 118:3819–3828.
- Jahn R, Scheller RH. 2006. SNAREs – engines for membrane fusion. Nature Rev Mol Cell Biol 7:631–643.
- Odell D, Wanas E, Yan J, Ghosh HP. 1997. Influence of membrane anchoring and cytoplasmic domains on the fusogenic activity of vesicular stomatitis virus glycoprotein G. J Virol 71:7996–8000.
- Armstrong RT, Kushnir AS, White JM. 2000. The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol 151:425–437.
- Giraudo CG, Hu C, You DQ, Slovic AM, Mosharov EV, Sulzer D, Melia TJ, Rothman JE. 2005. SNAREs can promote complete fusion and hemifusion as alternative outcomes. J Cell Biol 170:249–260.
- Kemble GW, Danieli T, White JM. 1994. Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell 76:383–391.
- Nüssler F, Clague MJ, Herrmann A. 1997. Meta-stability of the hemifusion intermediate induced by glycosylphosphatidylinositol-anchored influenza hemagglutinin. Biophys J 73:2280–2291.
- Cleverley DZ, Lenard J. 1998. The transmembrane domain in viral fusion: Essential role for a conserved glycine residue in vesicular stomatitis virus G protein. Proc Natl Acad Sci USA 95:3425–3430.
- Melikyan GB, Markosyan RM, Roth MG, Cohen FS. 2000. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol Biol Cell 11:3765–3775.
- Xu Y, Zhang F, Su Z, McNew JA, Shin Y-K. 2005. Hemifusion in SNARE-mediated membrane fusion. Nat Struct Mol Biol 12:417–422.
- Smit JM, Bittman R, Wilschut J. 2001. Deacylation of the transmembrane domains of Sindbis virus envelope glycoproteins E1 and E2 does not affect low-pH-induced viral membrane fusion activity. Febs Lett 498:57–61.
- Lang T, Halemani ND, Rammner B. 2008. Interplay between lipids and the proteinaceous membrane fusion machinery. Prog Lipid Res 47:461–469.
- Couve A, Protopopov V, Gerst JE. 1994. Yeast synaptobrevin homologs are modified posttranslationally by the addition of palmitate. Proc Natl Acad Sci USA 92:5987–5991.
- Roth AF, Wan JM, Bailey AO, Sun BM, Kuchar JA, Green WN, Phinney BS, Yates JR, Davis NG. 2006. Global analysis of protein palmitoylation in yeast. Cell 125:1003–1013.
- Valdez-Taubas J, Pelham H. 2005. Swf1-dependent palmitoylation of the SNARE Tlg1 prevents its ubiquitination and degradation. EMBO J 24:2524–2532.
- Veit M, Becher A, Ahnert-Hilger G. 2000. Synaptobrevin 2 is palmitoylated in synaptic vesicles prepared from adult, but not from embryonic brain. Mol Cell Neurosci 15:408–416.
- Melikyan GB, Jin H, Lamb RA, Cohen FS. 1997. The role of the cytoplasmic tail region of influenza virus hemagglutinin in formation and growth of fusion pores. Virology 235:118–128.
- Wagner R, Herwig A, Azzouz N, Klenk HD. 2005. Acylation-mediated membrane anchoring of avian influenza virus hemagglutinin is essential for fusion pore formation and virus infectivity. J Virol 79:6449–6458.
- Sakai T, Ohuchi R, Ohuchi M. 2002. Fatty acids on the A/USSR/77 influenza virus hemagglutinin facilitate the transition from hemifusion to fusion pore formation. J Virol 76:4603–4611.
- Fischer C, Schroth-Diez B, Herrmann A, Garten W, Klenk H-D. 1998. Acylation of the influenza hemagglutinin modulates fusion activity. Virology 248:284–294.
- Langosch D, Crane JM, Brosig B, Hellwig A, Tamm LK, Reed J. 2001. Peptide mimics of SNARE transmembrane segments drive membrane fusion depending on their conformational plasticity. J Mol Biol 311:709–721.
- Hofmann MW, Peplowska K, Rohde J, Poschner B, Ungermann C, Langosch D. 2006. Self-interaction of a SNARE transmembrane domain promotes the hemifusion-to-fusion transition in lipid mixing. J Mol Biol 364:1048–1060.
- Langosch D, Brosig B, Pipkorn R. 2001. Peptide mimics of the Vesicular Stomatitis virus G-protein transmembrane segment drive membrane fusion in vitro. J Biol Chem 276:32016–32021.
- Agrawal P, Kiihne S, Hollander J, Hulsbergen F, Hofmann M, Langosch D, de Groot H. 2007. Solid state NMR investigation of the interaction between biomimetic lipid bilayers and de novo designed fusogenic peptides. ChemBiochem 8:493–496.
- Langosch D, Arkin IT. 2009. Interaction and conformational dynamics of membrane-spanning protein helices. Protein Sci 18:1343–1358.
- Stelzer W, Poschner BC, Stalz H, Heck AJ, Langosch D. 2008. Sequence-specific conformational flexibility of SNARE transmembrane helices probed by hydrogen/deuterium exchange. Biophys J 95:1326–1330.
- Hofmann MW, Weise K, Ollesch J, Agrawal A, Stalz H, Stelzer W, Hulsbergen F, deGroot H, Gerwert K, Reed J, Langosch D. 2004. De novo design of conformationally flexible transmembrane peptides driving membrane fusion. Proc Natl Acad Sci USA 101:14776–14781.
- Poschner BC, Quint S, Hofmann M, Langosch D. 2009. Sequence-specific conformational dynamics of model transmembrane domains determines their membrane fusogenic function. J Mol Biol 386:733–741.
- Ollesch J, Poschner BC, Nikolaus J, Hofmann MW, Herrmann A, Gerwert K, Langosch D. 2008. Secondary structure and distribution of fusogenic LV-peptides in lipid membranes. Eur Biophys J 37:435–445.
- Poschner B, Reed J, Langosch D, Hofmann MW. 2007. An automated application for deconvolution of Circular Dichroism spectra of small peptides. Analyt Biochem 363:306–308.
- Lu XB, Zhang F, McNew JA, Shin YK. 2005. Membrane fusion induced by neuronal SNAREs transits through hemifusion. J Biol Chem 280:30538–30541.
- Hoekstra D, Düzgünes N. 1993. Lipid mixing assay to determine fusion in liposome systems. Methods Enzymol 220:15–32.
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. 1998. SNAREpins: Minimal machinery for membrane fusion. Cell 92:759–772.
- Schuette CG, Hatsuzawa K, Margittai M, Stein A, Riedel D, Küster P, König M, Seidel C, Jahn R. 2004. Determinants of liposome fusion mediated by synaptic SNARE proteins. Proc Natl Acad Sci USA 101:2858–2863.
- Pobbati AV, Stein A, Fasshauer D. 2006. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science 313:673–676.
- Reese C, Heise F, Mayer A. 2005. Trans-SNARE pairing can precede a hemifusion intermediate in intracellular membrane fusion. Nature 436:410–415.
- Jun Y, Wickner W. 2007. Assays of vacuole fusion resolve the stages of docking, lipid mixing, and content mixing. Proc Natl Acad Sci USA 104:13010–13015.
- de Planque MRR, Killian JA. 2003. Protein-lipid interactions studied with designed transmembrane peptides: Role of hydrophobic matching and interfacial anchoring. Mol Membr Biol 20:271–284.
- Kasson PM, Kelley NW, Singhal N, Vrljic M, Brunger AT, Pande VS. 2006. Ensemble molecular dynamics yields submillisecond kinetics and intermediates of membrane fusion. PNAS 103:11916–11921.
- de Planque MR, Kruijtzer JA, Liskamp RM, Marsh D, Greathouse DV, Koeppe RE 2nd, de Kruijff B, Killian JA. 1999. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane alpha-helical peptides. J Biol Chem 274:20839–20846.
- Killian JA. 2003. Synthetic peptides as models for intrinsic membrane proteins. FEBS Lett 555:134–138.
- Hofmann MW, Poschner BC, Hauser S, Langosch D. 2007. pH-activated fusogenic transmembrane LV-peptides. Biochemistry 46:4204–4209.
- Poschner BC, Langosch D. 2009. Stabilization of conformationally dynamic helices by covalently attached acyl chains. Protein Sci 18:1801–1805.
- Naeve CW, Williams D. 1990. Fatty-acids on the a-Japan-305-57 influenza-virus hemagglutinin have a role in membrane-fusion. Embo J 9:3857–3866.
- Bos ECW, Heijnen L, Luytjes W, Spaan WJM. 1995. Mutational analysis of the murine coronavirus spike protein: Effect on cell-to-cell fusion. Virology 214:453–463.
- Chen BJ, Takeda M, Lamb RA. 2005. Influenza virus hemagglutinin (H3 Subtype) requires palmitoylation of its cytoplasmic tail for assembly: M1 proteins of two subtypes differ in their ability to support assembly. J Virol 79:13673–13684.
